Abstract
Background
The prognostic significance of ambulatory blood pressure (ABP) has not been established in patients with type 2 diabetes (T2DM).
Methods
To clarify the impact of ABP on cardiovascular prognosis in patients with or without T2DM, we performed ABP monitoring (ABPM) in 1268 subjects recruited from nine sites in Japan, who were seen for the evaluation of hypertension. The mean age was 70.4±9.9 years, and 301 had diabetes; they were followed for 50±23 months. Incident cardiovascular disease (CVD) were related to different measures of ABP, including three categories of awake systolic BP (SBP <135, 135-150, and >150 mmHg), sleep SBP (<120, 120-135, and >135 mmHg), and nocturnal BP dipping (dippers, non-dippers, and risers). Cox regression models controlling for classic risk factors, were performed.
Results
Higher awake and sleep SBP predicted higher incidence of CVD in both diabetes and non-diabetes groups. In multivariable analyses, elevated awake and sleep SBP predicted increased risk of CVD more closely than clinic BP in both groups. The relationships between ABP level and CVD were similar in both groups. In Kaplan-Meier analyses, the incidence of CVD in non-dippers was similar to dippers, but risers experienced the highest risk of CVD in both groups (ps<0.01). The riser pattern was associated with approximately a 150% increase in risk of CVD, in both groups.
Conclusions
These findings suggest that ABPM improves the prediction of cardiovascular risk, over and above clinic BP, as much in patients with type 2 diabetes as it does in patients without diabetes.
Keywords: type 2 diabetes, ambulatory blood pressure monitoring, non-dipper, riser, cardiovascular disease
Introduction
Type 2 diabetes is one of the most important predisposing factors for the development of cardiovascular disease,1 particularly when associated with hypertension,2,3 and aggressive blood pressure reduction has been shown to be very beneficial in patients with diabetes.2,4,5 Therefore, recent international guidelines for the management of hypertension recommend that blood pressure (BP) in diabetes should be lowered to <130/80mmHg.5,6 Nevertheless, in data from the United States National Health and Nutrition Examination Surveys (NHANES), the percentage of diabetic patients meeting the JNC VI BP goal (<130/85mmHg) was only 35.9 % in 2001-2002.7
There have been several studies showing that ambulatory BP (ABP) is a better predictor of risk than traditional clinic or office BP measurement in hypertensive patients,8-12 but none have described whether the same is true for patients with diabetes. In addition, variations in the normal diurnal rhythm of BP such as the non-dipping or rising pattern during the night,8-10 nocturnal hypertension,9-11 and morning hypertension13 have been reported to be high-risk phenotypes of hypertension. Because of its limited reproducibility, some have reported that only 65.9% of non-dippers are reproducible when ABPM was repeated without any intervention. Again, most of the subjects in these studies were non-diabetic.8-12 ABPM has been widely advocated for improving the estimation of cardiovascular risk in hypertensive patients by organizations such as the American Society of Hypertension14 and the International Society of Hypertension,15 but the American Diabetes Association has made no recommendations on the use of out-of-office monitoring, on the grounds that there are insufficient published data in patients with diabetes. Nevertheless, ABP, and particularly the non-dipping pattern, has been reported to be associated more closely than clinic BP with diabetic microvascular disease, such as nephropathy,16-21 retinopathy,22 and neuropathy,23 but the prognostic significance of ABP in type 2 diabetes has rarely been reported.24,25 There is some uncertainty as to whether the adverse prognosis associated with the non-dipping pattern reported in mostly non-diabetic hypertensive subjects applies to non-dippers in general, or is limited to the inverted dippers (also known as risers), as has been suggested by the only two studies that have examined the prognosis of risers and the “true” non-dippers (who show an absent or diminished fall of nocturnal BP, but not an increase) separately.9,11 Therefore, the hypotheses tested in this study were that: ABP in patients with type 2 diabetes predicts cardiovascular disease (CVD) better than clinic BP; the association of ABP in patients with type 2 diabetes on the subsequent incidence of CVD is stronger than in non-diabetes; and risers, but not true non-dippers, have an increased incidence of CVD when compared with dippers.
Methods
This prospective study was performed in a sample of 1268 asymptomatic patients with (n=301) or without (n=967) type 2 diabetes who were seen for the evaluation of hypertension in general internal medicine clinics at 9 participating institutes in Japan: 3 clinics, 2 hospitals and one hypertension clinic of a university hospital (the Jichi Medical School- JMS ABPM Study Wave 1); and from 1 clinic and 2 hospitals in the Karatsu-Nishiarita Study.26-28
Subjects and definitions
During the period of recruitment, 1990-1998 for the JMS ABPM Study Wave 1 sample and 1996-2002 for the Karatsu-Nishiarita Study, hypertensive or possible hypertensive subjects were enrolled consecutively in the clinic, and agreed to undergo ABP monitoring (ABPM). The patients included many patients who came to the clinics first time for the evaluation of their BP. The mean age was 70.4 ± 9.9 years (range 33-97 years); there were 483 men and 785 women; 94 % of subjects were hypertensive. At least two clinic BP readings were taken on each of two separate occasions after at least 5 min of rest in the sitting position, which included before and after being fitted with an ABPM in subjects who stopped medication for ABPM. Hypertension was diagnosed when the clinic systolic BP (SBP) was ≥140 and/or diastolic BP (DBP) was ≥90 mmHg on at least two occasions according to current guidelines,29 or by a previous diagnosis of hypertension with current antihypertensive medication use. Subjects who were taking medications (N=680, 54%) stopped antihypertensive medications for 14 days preceding the ABPM study except for certain subjects who were not willing to stop medications long period or considered to be high risk (e.g. family history of CVD, high clinic BP, and mild renal insufficiency). Type 2 diabetes was diagnosed according to the guidelines of the American Diabetes Association30 or a previous diagnosis and currently taking anti-diabetic medication. We excluded patients with type 1 or secondary diabetes, renal dysfunction (serum creatinine >1.9 mg/dl), hepatic damage, ischemic heart disease or other cardiac diseases, congestive heart failure, arrhythmias (including atrial fibrillation), stroke (including transient ischemic attacks), or other major concomitant non-cardiovascular diseases. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Smoking was defined as current smoking. This study was approved by the Institutional Review Board of each participating hospital or clinic. All the subjects studied were ambulatory and gave informed consent for the study.
Ambulatory BP monitoring
Noninvasive ABPM was performed on a weekday with an automatic system (either ABPM-630, {Nippon Colin. Co}, TM2421, or TM2425, {A&D, Tokyo}) which recorded BP using the oscillometric method, and pulse rate every 30 minutes for 24 hours. These devices have been previously validated.31,32 Awake and sleep time were defined based on patients’ written diaries recorded during ABPM. Mean awake and sleep levels of SBP and DBP were computed and the nocturnal BP fall (%) was calculated as (awake SBP–sleep SBP)/awake SBP. Nocturnal BP fall was classified as follows: dipper if the nocturnal BP fall was ≥10%, non-dipper if it was ≥0% but <10%, and riser if it was <0%.9,11,26 Three categories of awake SBP level were used with cutoff values of 135 and 150 mmHg (i.e. <135, 135-150, and >150 mmHg), and three categories of sleep SBP level with cutoff values of 120 and 135 mmHg (i.e. <120, 120-135, and >135 mmHg). These values (awake SBP 135 mmHg and sleep SBP 120 mmHg) are recommended in multiple Hypertension guidelines.6,29,33
Follow-up and events
The subjects’ medical records were reviewed periodically after ABPM for the purpose of identifying incident CVD. The follow up examination of 811 participants from JMS ABPM Study Wave 1 was performed from 1996 to 1998, and the 457 participants from the Karatsu-Nishiarita Study from March 2004 to October 2006. The mean follow periods were 5.7 years in the former and 9.7 years in the latter. We defined three outcomes: stroke, fatal or non-fatal myocardial infarction (MI), and sudden cardiac death. Participants who developed a CVD other than these three (n=27), a malignant disease (n=33), dementia or physical inactivity (n=8), died or suffered from non-cardiovascular causes such as infection, accident, neurologic disorders (n=13), and moved or changed their telephone number (n=4) were censored as of the time such events took place (totally 85 subjects). The average follow-up period was 50 ± 23 months (range: 1 to 116 months). When subjects did not visit the clinics, we interviewed them by telephone. Strokes and cardiac events were diagnosed by the physician caring for the patient at the time of the event, and independent neurologists or cardiologists reviewed the cases and confirmed the diagnosis by referrals or medical records. Stroke was diagnosed on the basis of sudden onset of a neurological deficit that persisted for >24 hours in the absence of any other disease process that could explain the symptoms. Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined types of stroke. We excluded transient ischemic attacks (n=4), in which the neurological deficit cleared completely in <24 hours.11 MI was diagnosed based on the AHA criterion of “definite” MI.34 Angina (n=8), congestive heart failure (n=9), end stage renal disease (n=2), peripheral artery disease (n=3), and arrhythmia needing permanent pacemaker (n=1) were not treated as endpoints.
Statistical analyses
All statistical analyses were carried out with SPSS/Windows, version 13.0 (SPSS Inc., Chicago, Illinois). The data are expressed as the mean (± SD) or percentage. The chi-square test was used to compare proportions. The independent samples t test was performed to test mean differences between groups. The log-rank statistic was used to test the differences among Kaplan-Meier survival curves. Adjusted hazard ratios (HRs) with 95% confidence intervals were based on multivariable Cox regression analysis. As a preliminary analysis, we performed Cox regression analysis using variables except for BP parameters: age, sex, BMI, smoking status, creatinine, cholesterol, triglycerides, and the use of antihypertensive medication. As a result, age, smoking, and creatinine survived, then we determined to use essential variables (age, sex, and BMI) and smoking status, and creatinine as non-BP variables. Analyses were initially performed separately for diabetes and non-diabetes groups. In order to test for group differences in the relationship of ABP to incident CVD, interaction terms (between the measures of ABP and the presence of diabetes) were tested in the full sample. The null hypothesis was rejected when two-tailed P<0.05.
Results
The BMI, 24-hour BP, and awake BP were similar in the diabetes and non-diabetes groups (Table1). There were, however, some differences: the percentage of males, current smoking, hematocrit, triglycerides, sleep SBP, 24-hour and sleep pulse rates, and the proportion of true non-dippers and risers were significantly higher in patients with diabetes than in non-diabetes. The age, serum creatinine, and clinic BP were significantly higher in patients with non-diabetes than in diabetes.
Table 1 (a).
Baseline characteristics of diabetic subjects
| Risers (n=27) |
Non-dippers (n=118) |
Dippers (n=156) |
P | |
|---|---|---|---|---|
| Age (years) | 71.3 ± 10.7 | 67.8 ± 9.6 | 67.2 ± 9.3 | 0.12 |
| Male sex (%) | 44 | 52 | 46 | 0.61 |
| Body mass index (kg/m2) | 22.6 ± 3.3 | 24.6 ± 4.1 | 24.1 ± 3.2 | 0.04 |
| Smoker (%) | 37 | 30 | 35 | 0.57 |
| Antihypertensive medication (%) | 70 | 51 | 60 | 0.11 |
| Hematocrit (%) | 37.4 ± 8.3 | 41.1 ± 4.0 | 40.6 ± 4.4 | 0.001 |
| Triglycerides (mg/dl) | 140 ± 66 | 147 ± 102 | 140 ± 86 | 0.83 |
| Serum creatinine (mg/dl) | 0.77 ± 0.24 | 0.81 ± 0.22 | 0.78 ± 0.26 | 0.48 |
| Cockcroft -Gault GFR (ml/min) | 68 ± 28 | 74 ± 30 | 76 ± 31 | 0.41 |
| Clinic SBP (mmHg) | 162 ± 26 | 151 ± 23 | 154 ± 20 | 0.054 |
| Clinic DBP (mmHg) | 85 ± 15 | 82 ± 14 | 85 ± 14 | 0.25 |
| 24-hour SBP (mmHg) | 151 ± 19 | 140 ± 19 | 139 ± 15 | 0.003 |
| 24-hour DBP (mmHg) | 83 ± 9 | 79 ± 11 | 79 ± 8 | 0.08 |
| Awake SBP (mmHg) | 147 ± 18 | 143 ± 20 | 148 ± 17 | 0.07 |
| Awake DBP (mmHg) | 83 ± 9 | 80 ± 11 | 84 ± 9 | 0.03 |
| Sleep SBP (mmHg) | 158 ± 20 | 134 ± 20 | 123 ± 14 | <0.001 |
| Sleep DBP (mmHg) | 83 ± 11 | 76 ± 11 | 70 ± 8 | <0.001 |
| 24-hour PR (beats/min) | 71 ± 9 | 70 ± 8 | 72 ± 9 | 0.20 |
| Awake PR (beats/min) | 75 ± 10 | 73 ± 9 | 76 ± 10 | 0.07 |
| Sleep PR (beats/min) | 64 ± 9 | 63 ± 9 | 62 ± 9 | 0.66 |
| White-coat hypertension (%) | 22.2 | 16.1 | 12.2 | 0.33 |
| Calcium channel blockers (%) | 44 | 31 | 37 | 0.39 |
| ARB (%) | 0 | 6 | 5 | 0.44 |
| ACE inhibitors (%) | 33 | 25 | 24 | 0.62 |
| Diuretics (%) | 7 | 7 | 3 | 0.20 |
| β-blockers (%) | 0 | 3 | 7 | 0.11 |
| α-blockers (%) | 7 | 2 | 3 | 0.25 |
During the follow-up of 50 ± 23 months, 100 cardiovascular events occurred. The incidence of CVD was 2.1/100 person-years in diabetes and 1.8/100 person-years in non-diabetes. The actual numbers of event (%) are listed in Table 2. As shown, the Risers had the highest event rates followed by non-dippers and dippers in both diabetics and non-diabetics. In univariate analyses, clinic SBP was significantly associated with CVD in both diabetes and non-diabetes, but the measures of ambulatory BP had better predictions (Table 3, A and B). In multivariable Cox regression analyses that controlled for age, sex, BMI, antihypertensive drugs, serum creatinine, and site, clinic SBP was independently associated with CVD in both diabetes and non-diabetes groups (Model 1, Table 3). However, when 24-hour SBP, awake SBP, sleep SBP, or both awake and sleep SBPs were entered into the models (Models 2 thru 5), clinic SBP was no longer a significant predictor of CVD. In Model 2, 24-hour SBP was independently associated with CVD in both diabetes (P<0.001) and non-diabetes (P=0.001) groups. When awake and sleep SBP were entered one by one in the same models (Models 3 and 4), they were independently associated with CVD in both diabetes and non-diabetes groups. Finally, when awake and sleep SBP were entered together (Model 5), awake SBP in diabetes and sleep SBP in non-diabetes were most closely associated with CVD, although neither was significantly better than the other measure.
Table 2.
Number of events in each dipping group
| Dippers | Non-dippers | Risers | P value | |
|---|---|---|---|---|
| Diabetes | 11/156 (7.1%) | 11/118 (9.3%) | 7/27 (25.9%) | 0.012 |
| Non-diabetes | 34/591 (5.8%) | 24/305 (7.9%) | 13/71 (18.3%) | 0.001 |
|
| ||||
| Overall | 45/747 | 35/423 | 20/98 | <0.001 |
Data are shown as number (%).
Table 3.
Multivariable Cox regression analysis predicting incident cardiovascular events
| A. Diabetes Covariates |
Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| Clinic SBP (10 mmHg) |
1.20* (1.02-1.41) |
0.95 (0.76-1.17) |
0.92 (0.74-1.16) |
1.01 (0.83-1.23) |
0.93 (0.74-1.16) |
1.20* (1.02-1.41) |
1.18 (1.00-1.40) |
| 24-hour SBP (10 mmHg) |
1.44** (1.15-1.80) |
||||||
| Awake SBP (10 mmHg) |
1.48** (1.16-1.89) |
1.29 (0.92-1.82) |
|||||
| Sleep SBP (10 mmHg) |
1.32** (1.10-1.58) |
1.15 (0.90-1.47) |
|||||
| Non-dipping† (yes=1, no=0) |
1.85 (0.85-4.05) |
||||||
| Non-dipper vs. dipper |
1.60 (0.67-3.83) |
||||||
| Riser vs. dipper | 2.55 (0.88-7.33) |
||||||
| −2log likelihood | 270.5 | 261.4 | 261.4 | 262.3 | 260.1 | 268.0 | 267.4 |
| χ 2 | - | 9.1 | 9.1 | 8.2 | 10.4 | 2.5 | 3.1 |
| P value | - | 0.003 | 0.003 | 0.004 | 0.006 | 0.11 | 0.08 |
| B. Non-diabetes Covariatess |
Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| Clinic SBP (10 mmHg) |
1.18** (1.05-1.33) |
1.03 (0.88-1.20) |
1.07 (0.92-1.25) |
1.09 (0.94-1.25) |
1.06 (0.91-1.23) |
1.18** (1.05-1.33) |
1.17* (1.04-1.32) |
| 24-hour SBP (10 mmHg) |
1.32** (1.10-1.58) |
||||||
| Awake SBP (10 mmHg) |
1.19* (1.01-1.40) |
1.10 (0.90-1.34) |
|||||
| Sleep SBP (10 mmHg) |
1.20* (1.03-1.39) |
1.14 (0.95-1.37) |
|||||
| Non-dipping† (yes=1, no=0) |
1.30 (0.79-2.11) |
||||||
| Non-dipper vs. dipper |
1.04 (0.61-1.80) |
||||||
| Riser vs. dipper | 2.39* (1.23-4.65) |
||||||
| −2log likelihood | 845.0 | 835.7 | 840.0 | 839.3 | 838.4 | 843.9 | 838.8 |
| χ 2 | - | 9.3 | 5.0 | 5.7 | 6.6 | 1.1 | 6.2 |
| P value | - | 0.002 | 0.03 | 0.02 | 0.04 | 0.29 | 0.01 |
Values are hazard ratios (95% CI), per 10 mmHg difference in SBP.
P<0.05,
P<0.01,
P<0.001
SBP indicates systolic blood pressure.
Non-dipping includes true non-dippers and risers. Each model was adjusted for age, sex, BMI, smoking, and serum creatinine. Comparison of log-likelihood functions, based on chi-square distribution are shown as −2 log likelihood and χ2 in the bottom of each model. The −2 log likelihood and χ2 indicate improvement vs. Model 1 for clinic SBP. The p-values indicate for the improvements in the model when awake and/or sleep ambulatory BP are added to the equations, which were highly significant, and their significance levels were essentially the same as those of the regression estimate for the ABP measure.
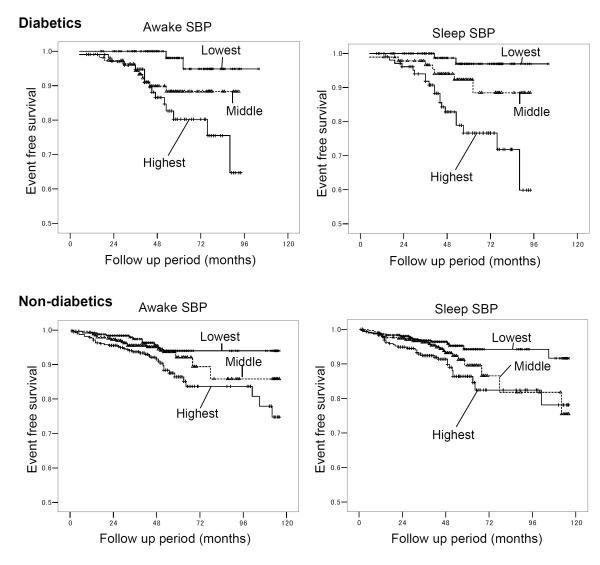
The Kaplan-Meier survival curves for the three categories of awake SBP (awake SBP<135-lowest, 135-150-middle, and >150 mmHg-highest) and sleep SBP (sleep SBP<120-lowest, 120-135-middle, and >135 mmHg-highest) are shown in Figure 1. The number of events in those awake and sleep SBP groups are shown in Table 4. In diabetes group, the highest and middle categories had a significantly higher incidence of CVD than the lowest category. However, the difference between the highest and the middle category was not statistically significant. In non-diabetes, the highest category had a higher incidence of CVD than the other two, but the difference between the middle and the lowest category was not significant. The highest sleep SBP category had a higher incidence of CVD than the others in both diabetes and non-diabetes groups. However, the difference was not significant between the middle and the lowest categories in either group, nor between the highest and the middle categories in non-diabetics.
Figure 1.
Event-free survival Kaplan-Meier curves for three categories of awake and sleep SBP. The numbers of subjects and events in each category are shown in the Table 4. Log-rank statistic between highest- vs. lowest-awake SBP is 11.2 (P=0.001) for diabetes and 8.4 (P=0.004) for non-diabetes groups, that of middle- vs. lowest-awake SBP is 4.5 (P=0.03) for diabetes and 1.0 (P=0.32) for non-diabetes groups. Log-rank statistic between highest- vs. middle- awake SBP is 1.8 (P=0.19) for diabetes and 4.0 (P=0.046) for non-diabetes groups. Log-rank statistic between highest- vs. lowest-sleep SBP is 16.3 (P<0.001) for diabetes and 11.3 (P=0.001) for non-diabetes, that of middle- vs. lowest-sleep SBP is 3.3 (P=0.07) for diabetes and 3.8 (P=0.05) for non-diabetes groups. Log-rank statistic between highest- vs. lowest-sleep SBP is 6.4 (P=0.01) for diabetes and 1.9 (P=0.17) for non-diabetes. SBP indicates systolic blood pressure.
Table 4.
Number of events in each SBP group stratified by awake and sleep SBP
| Awake SBP<135 | Awake SBP 135-150 | Awake SBP >150 | P value | |
|---|---|---|---|---|
| Diabetes | 2/91 (2.2%) | 11/108 (10.2%) | 16/102 (15.7%) | 0.002 |
| Non-diabetes | 16/274 (5.8%) | 19/335 (5.7%) | 36/358 (10.1%) | 0.03 |
|
| ||||
| Overall | 17/365 | 30/443 | 52/460 | |
| Sleep SBP≤120 | Sleep SBP 120-135 | Sleep SBP >135 | P value | |
|
| ||||
| Diabetes | 2/100 (2.0%) | 7/93 (7.5%) | 20/108 (18.5%) | <0.001 |
| Non-diabetes | 16/365 (4.4%) | 23/305 (7.5%) | 32/297 (10.8%) | 0.002 |
|
| ||||
| Overall | 18/465 | 30/398 | 52/405 | |
Data are shown as number (%).
In order to determine if the difference between diabetes and non-diabetes groups, we performed an additional multivariable analysis in the whole population to clarify the interactions between the measures of ABP and the presence of diabetes. However, none of the interactions between diabetes and the ABP measures (24-hour, awake, and sleep SBP, and non-dipper and risers) were statistically significant.
In Model 6 of Table 3, the non-dipping pattern (defined as riser plus true non-dipper) was not associated with increased risk of CVD in either diabetes or non-diabetes groups. In contrast, the riser pattern was a stronger independent predictor for CVD than the dipper and true non-dipper patterns in both groups (Model 7, Table 3) although the result was not statistically significant in diabetes group.
Discussion
It is established that ABP predicts CVD better than clinic BP in essential hypertensive patients,8,10,12 but the clinical significance of ABP as a predictor for CVD in diabetes has not yet been established.35 In the present study, which is the first to compare the prediction of cardiovascular events by ABPM and clinic BP in patients with diabetes and non-diabetics from the same cohort, we showed that 24-hour BP was independently associated with future cardiovascular events in both groups. There have been a few studies in diabetes showing associations of ABP with cardiovascular outcomes36 and all-cause mortality,25,37 but they had significant limitations: in one case ABPM was performed in a hospital setting 36; in another the study design was retrospective and the number of patients was small 25; and in the third only clinic and ambulatory PP were used in the analysis, and the contributions of ambulatory awake/sleep PPs or ambulatory SBP/DBP were not analyzed.37 As a result, the utility of ABPM in patients with diabetes is not yet accepted. In our study, although clinic SBP predicted cardiovascular risk, it was no longer significant in either group after 24-hour BP had been entered. Although the second hypothesis that the prediction of ABPM in diabetes is better than non-diabetes was not supported in our data, we could see the trends. These findings documenting the importance of ABPM for predicting CVD in patients with diabetes compared with non-diabetes in the same study have not been reported before.
ABPM can assess the “true” or “mean” BP levels, and also the diurnal rhythm of BP.38,39 The importance of BP control in patients with diabetes has been shown in many outcome trials,2,4 and review articles,3,40 but the BP has so far been evaluated only by clinic BP. Despite this lack of evidence, the International Diabetes Federation has recommended using ABPM and home BP monitoring as diagnostic tools for evaluation of BP.41
In patients with diabetes, it has been reported that sleep BP was better associated with target organ damage16,18,22,23 or incident vascular events24 than awake BP. In contrast, in our data, awake BP had a similar prediction of incident CVD to sleep BP. This result is partly in agreement with a paper showing that the awake BP was a significant marker of albuminuria in elderly subjects with diabetes.21 The exact reason for this finding is unknown, but it is possible that some diabetes-specific factors may be operating. Because ABPM in our subjects was performed in patients’ natural daily-life settings, the amount of physical activity was not restricted. Hypertensive patients with diabetes generally have more severe target organ damage than those without diabetes,28 and BP regulation may be impaired by autonomic neuropathy.42 Therefore BP increases occurring during daily life stress might trigger CVD events.
In most previous studies, the category of “non-dipping” combines those whose BP declines little during the night (the true non-dippers as defined in this study) and those whose BP actually rises (the risers). It has been reported that a blunted nocturnal BP fall is associated with CVD in hypertensive patients,8-12 and in diabetes it has been reported to be associated with diabetic microvascular complications such as nephropathy,16,18 retinopathy22 and autonomic neuropathy,23 which suggests that the non-dipping pattern should be associated with a higher incidence of CVD in patients with type 2 diabetes. Such a positive association between non-dipping and CVD in diabetes has been reported in one Japanese study,36 but the ABPM was performed in hospital. Furthermore, the daytime BP value may have been artificially low43 because the extent of physical activity is limited in the hospital setting, which probably led to some dippers being misclassified as non-dippers. There has been another study showing an association between non-dipping and all cause mortality in diabetes,25 but the sample size was too small to provide a definite conclusion. Therefore, these previous reports are inconclusive, and our report is the first to describe the relationship between the dipping pattern evaluated by out-of-hospital ABPM and cardiovascular prognosis in type 2 diabetics; we found that the event rate of non-dippers was not different from dippers. The classification of non-dipping is recognized as being generally not very reproducible,44 but this may not be the case in diabetics.45
Riser, or reverse pattern has been reported in patients with severe autonomic dysfunction and closely associated with adverse CV events. Only two previous studies have attempted to distinguish risers from true non-dippers, but both concluded that the increased risk associated with non-dipping is largely attributable to the risers, both for cardiovascular mortality9 and stroke events.11 And in one analysis using a continuous variable of the night-day ratio of SBP, the event rate was highest in the risers.10 As shown in Model 6 (Table 3), when risers and non-dippers are combined, the increase in risk associated with the non-dipping pattern is small and non-significant in both diabetes and non-diabetes; when the risers are differentiated from the non-dippers (Model 7), the risers experience approximately a 150% greater risk than the normal dippers, while the true non-dippers exhibit only a small increase in risk that does not approach statistical significance. This finding has at least two possible implications: first, it may explain the apparent paradox of why we did not confirm that the non-dipping pattern predicts risk in diabetics, and second, that other studies should pay greater attention to the distinction between true non-dippers and risers.
This study has some limitations. Our data were derived from an elderly and predominantly female population, and the BMI was lower than in western populations.1,2 Because the focus in this study was the predictive utility of ABPM for hypertensive patients with diabetes, and diabetes and non-diabetes groups were from the same cohort, we believe that these differences in population characteristics are not a problem. Because of the limited number of risers in the diabetes group, the risk prediction of ABP in diabetes was statistically not more predictive than that in non-diabetes and the riser pattern (Model 7, Table 2) was not statistically significant, although the HR was similar to that seen in non-diabetes group. Further study is needed to resolve this issue.
In conclusion, the ambulatory BP level, especially awake BP, was a strong predictor for cardiovascular events in patients with type 2 diabetes. The findings suggest that this may be different from the pattern seen in non-diabetes, where sleep BP appears to be more closely associated with cardiovascular events than awake BP. The risers, but not non-dippers, were at increased risk of cardiovascular events in both the diabetes and non-diabetes groups. Our findings provide strong support for the use of ABPM in the management of hypertensive patients with type 2 diabetes, and the fact that the awake BP was an equally important predictor to sleep BP raises the possibility that home BP monitoring might also have prognostic utility.
Table 1 (b).
Baseline characteristics of non-diabetic subjects
| Risers (n=71) |
Non-dippers (n=305) |
Dippers (n=591) |
P | |
|---|---|---|---|---|
| Age (years) | 75.2 ± 9.6 | 72.0 ± 9.8 | 70.2 ± 9.9 | <0.001 |
| Male sex (%) | 48 | 37 | 32 | 0.02 |
| Body mass index (kg/m2) | 22.6 ± 3.4 | 23.4 ± 3.7 | 24.2 ± 3.3 | <0.001 |
| Smoker (%) | 25 | 24 | 15 | 0.001 |
| Antihypertensive medication (%) | 49 | 52 | 53 | 0.86 |
| Hematocrit (%) | 39.6 ± 8.2 | 39.1 ± 4.5 | 39.8 ± 4.4 | 0.15 |
| Triglycerides (mg/dl) | 124 ± 61 | 132 ± 67 | 135 ± 70 | 0.38 |
| Serum creatinine (mg/dl) | 0.89 ± 0.19 | 0.88 ± 0.20 | 0.86 ± 0.23 | 0.32 |
| Cockcroft -Gault GFR (ml/min) | 52 ± 19 | 56 ± 19 | 61 ± 21 | <0.001 |
| Clinic SBP (mmHg) | 167 ± 19 | 162 ± 17 | 163 ± 18 | 0.06 |
| Clinic DBP (mmHg) | 86 ± 17 | 89 ± 13 | 91 ± 13 | 0.009 |
| 24-hour SBP (mmHg) | 142 ± 19 | 140 ± 16 | 137 ± 16 | 0.002 |
| 24-hour DBP (mmHg) | 79 ± 10 | 79 ± 10 | 78 ± 10 | 0.39 |
| Awake SBP (mmHg) | 139 ± 19 | 143 ± 17 | 147 ± 17 | <0.001 |
| Awake DBP (mmHg) | 78 ± 11 | 80 ± 10 | 83 ± 11 | <0.001 |
| Sleep SBP (mmHg) | 145 ± 19 | 135 ± 16 | 120 ± 15 | <0.001 |
| Sleep DBP (mmHg) | 80 ± 12 | 76 ± 10 | 69 ± 10 | <0.001 |
| 24-hour PR (beats/min) | 69 ± 7 | 69 ± 8 | 70 ± 7 | 0.45 |
| Awake PR (beats/min) | 73 ± 8 | 74 ± 9 | 76 ± 9 | 0.004 |
| Sleep PR (beats/min) | 60 ± 7 | 60 ± 8 | 60 ± 8 | 0.93 |
| White-coat hypertension (%) | 38.0 | 26.9 | 19.5 | <0.001 |
| Calcium channel blockers (%) | 31 | 33 | 39 | 0.20 |
| ARB (%) | 0 | 3 | 2 | 0.29 |
| ACE inhibitors (%) | 18 | 19 | 17 | 0.89 |
| Diuretics (%) | 8 | 4 | 5 | 0.28 |
| β-blockers (%) | 0 | 2 | 2 | 0.50 |
| α-blockers (%) | 0 | 1 | 1 | 0.79 |
Data are shown as percentage or mean ± SD.
SBP, systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rates;
ARB, Angiotensin II receptor blockers; ACE, angiotensin-converting enzyme
Acknowledgments
This work was supported by grants-in-aid (1998-1999, 2001-2002, 2004-2005) from the Foundation for the Development of the Community, Tochigi, Japan, and in part by the Banyu Fellowship Program sponsored by Banyu Life Science Foundation International, and NHLBI (R24 HL76857 and P01 HL 47540).
Footnotes
There is no conflict of interest in this paper.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.U. K. Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004;164:1850–1857. doi: 10.1001/archinte.164.17.1850. [DOI] [PubMed] [Google Scholar]
- 4.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Hypertension management in adults with diabetes. Diabetes Care. 2004;27:65S–67S. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Cheung B, Ong K, Man Y, Lam K, Lau C. Prevalence, awareness, treatment, and control of hypertension: United States National Health and Nutrition Examination Survey 2001-2002. J Clin Hypertens (Greenwich) 2006;8:93–98. doi: 10.1111/j.1524-6175.2006.04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 9.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 10.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, for the Systolic Hypertension in Europe Trial I Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 11.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 12.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Hond ED, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 14.Pickering T. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. Am J Hypertens. 1996;9:1–11. doi: 10.1016/0895-7061(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 15.Guidelines Subcommittee World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999;17:151–183. 1999. [PubMed] [Google Scholar]
- 16.Equiluz-Bruck S, Schnack C, Kopp HP, Schernthaner G. Nondipping of nocturnal blood pressure is related to urinary albumin excretion rate in patients with type 2 diabetes mellitus. Am J Hypertens. 1996;9:1139–1143. doi: 10.1016/0895-7061(96)00302-0. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen S, Schmitz A, Poulsen PL, Hansen KW, Mogensen CE. Albuminuria and 24-h ambulatory blood pressure in normoalbuminuric and microalbuminuric NIDDM patients. A longitudinal study. Diabetes Care. 1995;18:1434–1441. doi: 10.2337/diacare.18.11.1434. [DOI] [PubMed] [Google Scholar]
- 18.Torffvit O, Tapia J, Rippe B, Alm P, Willner J, Tencer J. Ambulatory blood pressure in type 2 diabetic patients with albuminuria: Relation to the renal function and structural lesions. J Diabet Complications. 2004;18:328–335. doi: 10.1016/j.jdiacomp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Leitao CB, Canani LH, Bolson PB, Molon MP, Pinotti AF, Gross JL. Urinary albumin excretion rate is associated with increased ambulatory blood pressure in normoalbuminuric type 2 diabetic patients. Diabetes Care. 2005;28:1724–1729. doi: 10.2337/diacare.28.7.1724. [DOI] [PubMed] [Google Scholar]
- 20.Palmas W, Moran A, Pickering T, Eimicke JP, Teresi J, Schwartz JE, Field L, Weinstock RS, Shea S. Ambulatory pulse pressure and progression of urinary albumin excretion in older patients with type 2 diabetes mellitus. Hypertension. 2006;48:301–308. doi: 10.1161/01.HYP.0000232644.98208.65. [DOI] [PubMed] [Google Scholar]
- 21.Moran A, Palmas W, Pickering TG, Schwartz JE, Field L, Weinstock RS, Shea S. Office and ambulatory blood pressure are independently associated with albuminuria in older subjects with type 2 diabetes. Hypertension. 2006;47:955–961. doi: 10.1161/01.HYP.0000216634.73504.7d. [DOI] [PubMed] [Google Scholar]
- 22.Poulsen PL, Bek T, Ebbehoj E, Hansen KW, Mogensen CE. 24-h ambulatory blood pressure and retinopathy in normoalbuminuric IDDM patients. Diabetologia. 1998;41:105–110. doi: 10.1007/s001250050874. [DOI] [PubMed] [Google Scholar]
- 23.Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578–584. doi: 10.2337/diacare.17.6.578. [DOI] [PubMed] [Google Scholar]
- 24.Nakano S, Ito T, Furuya K, Tsuda S, Konishi K, Nishizawa M, Nakagawa A, Kigoshi T, Uchida K. Ambulatory blood pressure level rather than dipper/nondipper status predicts vascular events in type 2 diabetic subjects. Hypertens Res. 2004;27:647–656. doi: 10.1291/hypres.27.647. [DOI] [PubMed] [Google Scholar]
- 25.Sturrock NDC, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17:360–364. doi: 10.1046/j.1464-5491.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 26.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients : advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 27.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34:2471–2474. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 29.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 30.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 31.Imai Y, Sasaki S, Minami N, Munakata M, Hashimoto J, Sakuma H, Sakuma M, Watanabe N, Imai K, Sekino H, Abe K. The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens. 1992;5:719–726. doi: 10.1093/ajh/5.10.719. [DOI] [PubMed] [Google Scholar]
- 32.White WB, Lund-Johansen P, McCabe EJ. Clinical evaluation of the Colin ABPM 630 at rest and during exercise: an ambulatory blood pressure monitor with gas-powered cuff inflation. J Hypertens. 1989;7:477–483. doi: 10.1097/00004872-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Staessen JA, Asmar R, De Buyzere M, Imai Y, Parati G, Shimada K, Stergiou G, Redon J, Verdecchia P, the Participants of the Consensus Conference on Ambulatory Blood Pressure M Task Force II: Blood pressure measurement and cardiovascular outcome. Blood Press Monit. 2001;6:355–370. doi: 10.1097/00126097-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 35.Strachan MWJ, Gough K, McKnight JA, Padfield PL. Ambulatory blood pressure monitoring: is it necessary for the routine assessment of hypertension in people with diabetes? Diabet Med. 2002;19:787–789. doi: 10.1046/j.1464-5491.2002.00771.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47:1501–1506. doi: 10.2337/diabetes.47.9.1501. [DOI] [PubMed] [Google Scholar]
- 37.Mannucci E, Lambertucci L, Monami M, Fedeli A, Chiasserini V, Marchionni N, Masotti G, Ungar A. Pulse pressure and mortality in hypertensive type 2 diabetic patients. A cohort study. Diabetes Metab Res Rev. 2006;22:172–175. doi: 10.1002/dmrr.598. [DOI] [PubMed] [Google Scholar]
- 38.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 39.White WB. Ambulatory blood-pressure monitoring in clinical practice. N Engl J Med. 2003;348:2377–2378. doi: 10.1056/NEJMp030057. [DOI] [PubMed] [Google Scholar]
- 40.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 41.Working Party of the International Diabetes Federation (European Region) Hypertension in people with Type 2 diabetes: knowledge-based diabetes-specific guidelines. Diabet Med. 2003;20:972–987. doi: 10.1046/j.1464-5491.2003.01021.x. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz J, Monbaron D, Parati G, Perret S, Haesler E, Danzeisen C, Hayoz D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension. 2005;46:162–167. doi: 10.1161/01.HYP.0000169053.14440.7d. [DOI] [PubMed] [Google Scholar]
- 43.Paschalis-Purtak K, Pucilowska B, Kabat M, Sznajderman M. Clinical evaluation of 24 h ambulatory monitoring of blood pressure under various environmental conditions (home and work versus hospital) Blood Press Monit. 1998;3:289–294. [PubMed] [Google Scholar]
- 44.Prisant LM. Blunted nocturnal decline in blood pressure. J Clin Hypertens (Greenwich) 2004;6:594–597. doi: 10.1111/j.1524-6175.2004.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuspidi C, Meani S, Lonati L, Fusi V, Valerio C, Sala C, Magnaghi G, Maisaidi M, Zanchetti A. Short-term reproducibility of a non-dipping pattern in type 2 diabetic hypertensive patients. J Hypertens. 2006;24:647–653. doi: 10.1097/01.hjh.0000217846.65089.19. [DOI] [PubMed] [Google Scholar]