Abstract
Sickle cell disease is characterized by recurrent episodes of ischemia-reperfusion injury to multiple vital organ systems and a chronic hemolytic anemia, both contributing to progressive organ dysfunction. The introduction of treatments that induce protective fetal hemoglobin and reduce infectious complications has greatly prolonged survival. However, with increased longevity, cardiovascular complications are increasingly evident, with the notable development of a progressive proliferative systemic vasculopathy, pulmonary hypertension (PH) and left ventricular diastolic dysfunction. Pulmonary hypertension is reported in autopsy studies and numerous clinical studies have shown that increased pulmonary pressures are an important risk marker for mortality in these patients. In epidemiological studies, the development of PH is associated with intravascular hemolysis, cutaneous leg ulceration, renal insufficiency, iron overload and liver dysfunction. Chronic anemia in sickle cell disease results in cardiac chamber dilation and a compensatory increase in left ventricular mass. This is often accompanied by left ventricular diastolic dysfunction which has also been a strong independent predictor of mortality patients with sickle cell disease. Both PH and diastolic dysfunction are associated with marked abnormalities in exercise capacity in these patients. Sudden death is an increasingly recognized problem and further cardiac investigations are necessary to recognize and treat high-risk patients.
Keywords: Sickle, Cell, Disease
Background
Sickle cell disease is an autosomal recessive Mendelian disease affecting approximately 1/500 African Americans and 1/1200 Hispanic Americans.(1–4) It is estimated that 72,000 Americans have sickle cell disease. A single point mutation in the beta globin gene results in a substitution of a glutamic acid residue with a valine at position six (beta G6V), with the mutant hemoglobin referred to as hemoglobin S. The hemoglobin functions normally except when deoxygenated. This exposes a hydrophobic area around the valine that produces interactions between beta chains on neighboring hemoglobin tetramers, ultimately resulting in arrangement into a polymer nucleus and then a long polymer bundle. The polymerization process is accelerated by the extent of hemoglobin S deoxygenation and hemoglobin S concentration, and modulated by the presence of fetal hemoglobin, which interferes with polymerization.
Polymerization of deoxygenated hemoglobin S within the erythrocyte ultimately reduces its flexibility and distorts its shape, reducing membrane fluidity and altering it rheological properties in flowing blood. Both physical entrapment and red blood cell-leukocyte-endothelial adhesive interactions driven by secondary inflammation result in obstruction of the microvasculature.(4–8) This produces ischemia-reperfusion injury to vital organs, further amplifying inflammatory and oxidative stress, activation of the innate immune response, and driving infarction of all critical organs such as the spleen, kidneys, liver, muscle, brain, lung and bone.(3,6,9) Infarction of the bone marrow results in edema and necrosis, producing episodes of severe bone pain that characterize the vaso-occlusive painful crisis. This is the most common complication of sickle cell disease, resulting in an average of 2 admissions or emergency room visits a year.(10) However, this is only the tip of the proverbial ice-berg, as most patients experience daily pain but avoid medical evaluation in the hospital setting.(11) In fact, only about a third of patients frequently go to the emergency room for evaluation and therapy.(12)
While in Sub-Saharan Africa babies born to sickle cell disease rarely live beyond two years of age, in the U.S. and Europe patient survival has steadily increased over the last decade.(4) This improved longevity is likely related to reduced infectious co-morbidities related to sanitation and penicillin prophylaxis, improvements in the quality and availability of red cell transfusions, and use of the fetal hemoglobin-inducing therapy hydroxyurea. In the U.S. early mortality from sepsis, severe vaso-occlusive crisis, the acute chest syndrome and childhood stroke, have been significantly reduced with transfusion and hydroxyurea therapy.(13–17) The Cooperative Study of patients with Sickle Cell Disease (CSSCD), a large pediatric and adult registry study performed before the advent of hydroxyurea or prophylactic transfusions to prevent strokes in children at risk, indicated that women survived to a median age of 48 and men to 42,(10) however it is now apparent that many patients have the potential to live well into the 7th decade. This “good news” is tempered by the appreciation that as patients survive to adulthood and old age, they are accumulating end-organ injury and failure, and a progressive systemic and pulmonary vasculopathy.(4,18,19) The development of this vasculopathy is driven primarily by chronic hemolytic anemia but compounded by other comorbidities such as renal and liver failure, systemic hypertension, diastolic left ventricular dysfunction, iron overload, and thrombosis.(3,15,18,20)
Pulmonary Hypertension
Mechanisms of disease and clinical risk factors
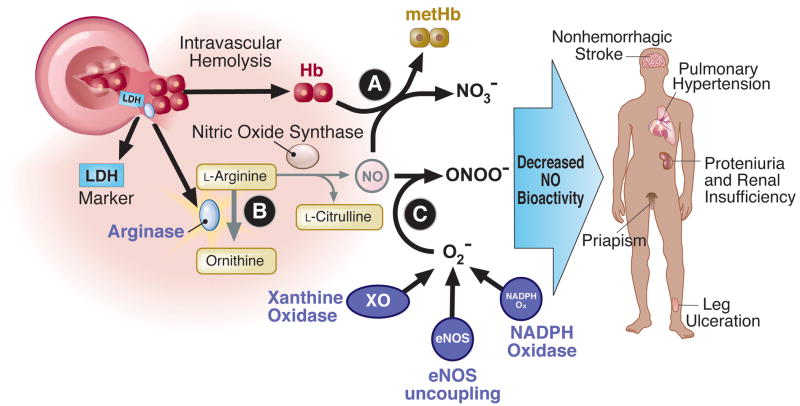
Pulmonary hypertension represents one of the major emerging vasculopathic complications of SCD as this patient population ages. Hemolytic anemia has been proposed as an important mechanism leading to pulmonary vasculopathy. As shown in Figure 1, hemoglobin, when decompartmentalized from the red cell, will react with NO at the near diffusion limit, to oxidize the NO to nitrate.(21,22) This reaction is so fast and irreversible that even levels of cell-free plasma hemoglobin of only 6–10 microM are sufficient to inhibit all NO signaling and produce vasoconstriction.(23,24) In addition to cell free hemoglobin, hemolysis releases other red cell enzymes that have the potential to inhibit NO signaling. Arginase 1 is present in abundance in red cells and metabolizes arginine to ornithine, reducing arginine availability for NO synthesis.(25) In addition, other independent factors that contribute to the development of PH in this population include surgical splenectomy and functional asplenia, thrombo-embolism, lung fibrosis and hypoxemia, increases in other vasoactive mediators such as placental growth factor and endothelin-1, renal insufficiency and genetic factors (summarized in Figure 2).(18,26–28)
Figure 1. Mechanisms of hemolytic anemia in reducing NO bioavailability and association with vasculopathic sub-phenotypes of sickle cell disease.
Hemolysis releases cell-free plasma hemoglobin and arginase 1 into plasma, which catabolize NO and L-arginine. Activation of vascular oxidases, such as xanthine oxidase, NADPH oxidase and uncoupled eNOS, generate superoxide which scavenges NO. Hemolytic anemia and reduced NO bioavailability are associated with vasculopathic clinical complications in SCD patients.
Figure 2. Mechanisms of pulmonary hypertension in SCD patients.
A pulmonary vessel with severe intimal and smooth muscle proliferation is shown from a 35 year old patient with SCD who died suddenly with known PAH. Mechanisms that contribute to the development of PH identified in animal and human studies are summarized.
In SCD, a number of the chronic complications appear to be related to hemolytic anemia while other complications are related to inflammation and vaso-occlusion (classic “sickling” events).(29) Vaso-occlusive pain crisis and the acute chest syndrome are caused by vaso-occlusion by sickled and adhesive red cells and leukocytes; in epidemiological studies the risk of developing these clinical manifestations are related to high steady state hemoglobin levels (less hemolysis and higher viscocity), high white blood cell count (inflammation), and low levels of fetal hemoglobin (which inhibits hemoglobin S polymerization).(10) In contrast, other complications such as endothelial dysfunction, pulmonary hypertension, cutaneous leg ulceration, proteinuria, renal dysfunction, systolic systemic hypertension, risk of death and possibly stroke may be in part mediated by chronic hemolytic anemia; epidemiological studies show that the risk of developing these complications is related to low steady state hemoglobin with weaker association with rates of painful crisis and acute chest syndrome.(18,20,30–32)
While this hypothesis for subphenotypes has been challenged in editorial forums,(33,34) most studies have supported this hypothesis over the last 7 years using animal models, human vascular studies, and large epidemiological cohort studies. From an epidemiological standpoint, in patients with SCD a number of clinical vasculopathic complications are significantly associated with markers of hemolytic anemia, including PH, priapism, leg ulceration and risk of death.(18,30,35,36) Numerous cohort studies have consistently associated the severity of hemolytic anemia with increasing Doppler-estimated pulmonary artery systolic pressure and high risk of death, including the NIH-PH cohort,(18) the Duke cohort,(37) the UNC cohort,(27) the Muti-Centers Study of Hydroxyurea cohort,(31) the Pediatric Hypoxic Response (PUSH) cohort,(32,38,39) and a recently published Greek study.(40) An analysis of banked plasma samples from the classic cooperative study of sickle cell disease (CSSCD) cohort revealed that an abnormally high NT-proBNP level ≥ 160 pg/ml, a biomarker for pulmonary hypertension in patients with sickle cell disease(31), was present in 27.6 % of adult SCD patients, and high levels were associated with markers of hemolytic anemia such as a low hemoglobin level (P <0.001), high lactate dehydrogenase (P < 0.001), and high total bilirubin levels (P < 0.007).(41) An NT-proBNP level ≥160 pg/ml was a major and independent predictor of mortality (RR 6.24, 95% CI 2.9–13.3, P < 0.0001). The recently published analysis of screening patients in the Walk-PHASST cohort confirms similar strong associations between indices of hemolytic anemia, high NT-proBNP, low walk distance and increased Doppler-echocardiographic estimates of pulmonary artery systolic pressures.(42) Similar associations between markers of hemolytic anemia, pulmonary hypertension, leg ulceration and risk of death have now been confirmed in two PH screening studies based on right heart catheterization (discussed in more detail below).(26,43)
Screening for PH: TRV and NT-proBNP as risk markers in SCD
Echocardiography is a useful non-invasive screening tool for PH, but diagnosis requires right heart catheterization. Using the Bernoulli equation, the tricuspid regurgitation velocity (TRV) provides a calculated estimate of right ventricular and pulmonary artery systolic pressures (PASP ≈ 4*TRV2) after adding an estimate of the central venous or right atrial pressure.(44) In patients with SCD, echocardiographic estimates have been shown to correlate reasonably well with measured pulmonary artery systolic pressures by right heart catheterization (R = 0.77; P<0.001).(18).
Three relatively large screening studies have been performed at the National Institutes of Health (NIH),(18) Duke University, (37) and the University of North Carolina Chapel Hill (UNC).(27) In all of these studies Doppler-echocardiography was performed on sickle cell disease patients in steady state, not during hospitalization with vaso-occlusive pain crisis. A tricuspid regurgitation velocity (TRV) of 2.5 m/s or above was prospectively chosen as a cut-off for high risk patients, and the percentage of patients with a value ≥ 3.0 m/s, a more conventional cut-off for PH screening, was also reported. Approximately 30% of patients had a TRV ≥ 2.5 m/s and 10% had a value ≥ 3.0 m/s. In a recently completed large U.S. and U.K. screening study (called the Walk-PHASST screening study) that included 483 patients with homozygous SS disease, 26% of the subjects had a TRV of 2.7 to <3.0 m/s (mean ± SD 2.8±0.1) and 11% had a TRV value ≥ 3.0 m/s (3.4±0.4).(42)
In all epidemiological studies conducted to date, a mild-to-moderate elevation in Doppler-estimated right ventricular systolic pressure (TRV ≥ 2.5 m/s) was common in adults with SCD and was associated with a 9.24–15.9 risk ratio for early death.(18,27,37) In the multi-center Walk-PHASST cohort, increases in estimated pulmonary artery systolic pressures have been shown to predict high NT-proBNP levels and decreased functional capacity,(42,45) and our preliminary analysis of 2-year mortality data indicates a significantly increased risk of death in the patients with elevated TRV values (P<0.000006).
While non-invasive estimates of PH require confirmatory right heart catheterization, from a screening standpoint the data suggest that a TRV value less than 2.5 m/s or an NT-BNP < 160 pg/mL are normal screening values, and associated with a low risk of death for a patient with SCD,(18,27,31,37). In contrast, a TRV ≥3 m/s is three standard deviations above the mean, is present in approximately 10% of SCD adults, and is associated with a risk ratio for death of 10.6 (95% CI, 3.3 to 33.6; P<0.001).(3) Based on these data, for patients with TRV ≥3 m/s, standard of care should include clinical evaluation with right heart catheterization and evaluation for PH risk factors, including left ventricular diastolic and/or systolic dysfunction, kidney and liver disease, iron overload, systemic hypertension, thromboembolism, and nocturnal or exercise hypoxemia.
The intermediate group (TRV value of 2.5–2.9 m/s, which is 1–2 SDs above the mean) remains a source of controversy. However, in adults with SCD this group overall also appears to have decreased exercise capacity and increased mortality, with a risk ratio for death of 4.4 (95% CI, 1.6 to 12.2; P<0.001). The label applied to this intermediate risk group of SCD patients with TRV 2.5–2.9 m/s does not change its clinical implications for adults with SCD. Refinement of risk definition in this group in future studies will advance the field.
Diagnosis and characterization of PH using right heart catheterization as gold standard
Three new studies have now been published with PH defined by definitive right heart catheterization. The hemodynamic values reported and samples sizes are summarized in Table 1. The NIH screening study, first published with 195 patients in 2004, has now been extended for 9-years of follow-up in 533 patients, with median follow-up of 4.4 years.(46) In this study, 86 subjects with suspected PH based on elevated TRV underwent right heart catheterization and of these, fifty-six patients (10.5% of the 533 patients evaluated) were diagnosed with PH defined by mean pulmonary artery pressure (mPAP) ≥ 25 mm Hg, which is the consensus definition of PH. Approximately 50% had pulmonary venous hypertension (PVH) and 50% pulmonary arterial hypertension (PAH), based on a pulmonary artery occlusion pressure above or below 15 mm Hg, respectively. This study has confirmed the high risk of death even with more moderate elevations of mPAP (median mPAP of 36 mm Hg). During a median follow-up of 4.4 years, mortality rate was significantly higher in the PH group (20/56 deaths, 36%) than the general sickle cell group with normal Doppler-echocardiographic estimates of pulmonary pressures (50/477 deaths, 13%, P<0.0001). In multivariate analysis, most measures of pulmonary vascular disease were associated with risk of death, including systolic pulmonary arterial pressure, pulse pressure, mean pulmonary artery pressure, transpulmonary gradient, and pulmonary vascular resistance.
Table 1.
Summary of SCD Right Heart Catheterization Studies
Hemodynamic values from right heart catheterization studies in SCD.
| First Author (Ref.#) | Number of Subjects Screened | Exclusions | Criteria for RHC | Number of RHCs | Number of Subjects with PH* | % of RHC with PH | % of Screened Subjects with PH | Mean Age of PH Subjects | mPAP (mmHg) | PVR (dyne* sec/cm5) | CO (Liters/min) | 6MWD (m) | Duration of Follow-up (yrs) | Mortality in PH group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mehari et al.(100) | 533 | none | TRV ≥2.8m/s 6MWD < 500m unexplained dyspnea or desaturation | 86 | 56 | 65 | 11 | 41 | 36 ± 9 | 229 ± 149 | 8 ± 3 | 358 ± 113 | 4.4 | 36% |
| Parent et al. (85) | 445 | renal insufficiency restrictive lung disease liver disease | TRV ≥ 2.5m/s | 96 | 24 | 25 | 5 | 45 | 30 ± 6 | 138 ± 58 | 9 ± 2 | 404 ± 94 | 2.2 | 13% |
| Fonseca et al (84) | 80 | none | TRV ≥ 2.5m/s | 26 | 8 | 31 | 10 | 46 | 33 ± 9 | 179 ± 120§ | 5 ± 2£ | 460 ± 152 | 2.8¤ | 38% |
Pulmonary hypertension defined as mean PAP≥25mmHg
Median (interquartile range)
converted mean value: PVR measured in Woods units as 2.24 ±1.5
Cardiac index (L/min/m2)
Estimated from figure in epub
Parent and colleagues also published a large screening study of pulmonary hypertension in 398 sickle cell disease patients in France.(26) In this study, they performed right heart catheterization in all patients with Doppler-determined TRV ≥ 2.5 m/s. They found that 25% of patients with a TRV ≥ 2.5 m/s had a mPAP ≥ 25 mm Hg diagnosed by right heart catheterization (perhaps not surprising considering values of 2-SD versus 3-SD above the normal mean). In patients with a TRV of ≥ 2.9 meters/sec or a TRV between 2.5 and 2.8 meters/sec per AND either an NT-proBNP level ≥ 164.5 pg/ml or a 6-minute walk distance of < 333 meters, the positive predictive value was 62%, and the false negative rate was reduced to 7%. Similar to other studies, they found an association between PH and high prevalence of cutaneous leg ulcers, low exercise capacity, and increased risk of death (12.5% in PH group versus 0.3% in non-PH group; P=0.002), and lack of association with vaso-occlusive pain crisis and the acute chest syndrome.(26,47)
The Parent study also confirmed risk factors associated with PH from the NIH cohort, including renal insufficiency, markers of hemolysis (lactate dehydrogenase and aspartate aminotransferase released by red cells, but not alanine aminotransferase which is specific for hepatocytes) and markers of cholestatic liver dysfunction (increased alkaline phosphatase and direct bilirubin). These findings support the hypotheses that PH in SCD arises as a consequence of chronic hemolytic anemia and is associated with end-organ dysfunction (renal and liver) but is not a direct consequence of repeated episodes of vaso-occlusion and acute chest syndrome.(3,18,26,47)
A number of differences in study design and analysis may have resulted in small differences in prevalence estimates and could also have biased sensitivity and specificity analysis of echocardiography. In terms of prevalence estimates, the Parent study excluded approximately 9% of patients, those with “severe” renal, liver or lung disease, defined by a creatinine clearance of less than 30 ml/min, an abnormal prothrombin time (international normalized ratio greater than 1.7), and chronic restrictive lung disease defined by a total lung capacity (TLC) of less than 70 percent of predicted.(26) It is not clear why these complications were defined as severe, as hemodialysis was not required in the definition and the threshold of TLC used is classified by the ATS as a moderate reduction. It is also not clear why these patients would be excluded from a prevalence study of SCD related PAH, especially considering the fact that all of these complications represent significant published risk factors for SCD-related PH.(18,42,48) For example, in the Walk-PHASST screening study,(42) 24 of 375 (6.4%) adult Hb SS patients had a creatinine clearance <30 ml/min estimated by the Cockcroft-Gault formula. Of these, 22 (91.7%) had a TRV ≥ 2.5 meters/sec and 13 (54.2%) had a TRV ≥ 3.0 meters/sec, indicating the likely high prevalence of PH in these excluded patients.
While it is clear that the echocardiogram represents a screening test that must be confirmed using right heart catheterization, the estimates of positive predictive value by Parent and colleagues are greatly influenced by baseline prevalence. By exclusion of 9% of the population at high risk for PH the investigators may have biased their results to lower sensitivity. However, the use of NT-BNP and walk distance to increase the positive predictive value of borderline Doppler-echocardiographic findings (TRV 2.5–2.9 meters/sec) represents a significant contribution by these investigators and an approach that can be advocated in clinical evaluations.
A third right heart catheterization screening study by Fonseca and colleagues in SCD patients screened in Brazil has now been published. This study, with no exclusion criteria applied to screened patients, revealed a 10% prevalence of PH, defined also by mPAP ≥ 25 mm Hg.(43) This study also confirmed associations with hemolytic anemia, renal insufficiency, exercise intolerance and high associated mortality.
Treatment options for PH in SCD
Based on the high associated mortality for PH in SCD, it has been recommended that the underlying sickle cell disease be aggressively controlled.(4) The pulmonary pressures may rise during episodes of vaso-occlusive pain crisis and the acute chest syndrome, and right heart failure may develop at high pulmonary pressures.(49,50) If patients are not on hydroxyurea, this therapy should be started and titrated to a maximally tolerated dose to increase fetal hemoglobin levels (recently reviewed).(51,52) For subjects not responding or tolerating hydroxyurea, as well as those with more significant pulmonary hypertension, a regular simple or exchange transfusion program should be initiated targeting a hemoglobin S level of less than 20% after tranfusions. Patients should be evaluated for iron overload and chelation therapy initiated. Resting, nocturnal and exercise desaturation should be managed with supplemental oxygen. Patients should be evaluated for thrombo-embolic disease, sleep apnea, HIV infection, liver disease, renal insufficiency and other conditions that could contribute to the development or evolution of PH.
In patients with PAH defined by right heart catheterization (mPAP ≥ 25 mm Hg, PCWP < 15 mm Hg and an elevated transpulmonary gradient ≥ 10–12 mm Hg) therapy with PAH specific therapies can be considered. While PAH represents a common complication in patients with sickle cell disease, with a high associated morbidity and mortality, there exist no large randomized placebo-controlled trials to guide therapy decisions. A trial of bosentan, an endothelin A and B receptor blocker, was stopped early based on decision by the sponsor, with only approximately 15 sites activated and 27 subjects enrolled. An underpowered analysis of these subjects showed no evident increase in adverse events in subjects on therapy and a trend towards an increase in cardiac output, but no significant changes in pulmonary pressures.(53) In a case series of 17 SCD patients with PH, open label treatment with either bosentan (ET A and B receptor blocker) or ambrisentan (a selective ET A receptor blocker), resulted in lower NT-proBNP levels, lower TRV measurements and higher six-minute walk distance, suggesting improvement in pulmonary hypertension.(54) There was also a trend towards improved Borg Dyspnea Score and NYHA classification. Based on the lower risk of hepatocellular dysfunction on ambrisentan, and the fact that many patients with SCD have co-morbid iron overload or liver disease, this drug is likely the best selection as a first line agent, with simultaneous diuresis to prevent edema and a rise in filling pressures.
Phosphodiesterase 5 inhibitor (PD5) therapy was considered one of the most promising approaches to SCD associated PH, as these agents appear to be effective in both PAH and PVH related to diastolic dysfunction.(55,56) A small open label study of sildenafil in patients with SCD that were on maximal hydroxyurea therapy or transfusions revealed significant reductions in TRV and NT-proBNP and increases in six minute walk distance. (57) However, a large multi-center trial of sildenafil was discontinued early based on an unexpected increase in hospitalization for pain (45 % of sildenafil, 22% placebo, p=0.022).(42) Consistent with these observations, the use of phosphodiesterase 5 inhibitors in other PAH patient populations, and in large clinical trials of patients with erectile dysfunction, have been associated with an increase in the incidence of myalgias and back pain that could have contributed to the increase in the pain reported in the current study.(58–60) It is increasingly evident that back pain and myalgias represent a class effect of PD5 inhibitors. Based on the results of the Walk-PHASST sildenafil treatment study, therapy with PD5 inhibitors should only be used in SCD patients that are extremely well-controlled on hydroxyurea and transfusion therapy, and should not be considered a first-line therapy.
Left Ventricular Dysfunction
LV Dilation
Cardiac complications are a common feature of SCD and are felt to be an important cause of the morbidity and mortality associated with this disease. The chronic anemia of sickle cell disease results in an increase in cardiac output with only a minimal increase in heart rate. Left ventricular stroke volume increases with significant dilation of the left ventricle (61) and the degree of LV dilation is closely linked to the degree of anemia (62). The dilated left ventricle adapts to the increased wall stress by developing eccentric hypertrophy (63) in which wall thickening is increased and myofibers are elongated. Eccentric hypertrophy allows the left ventricle to adapt to chronic volume overload by initially preserving diastolic compliance and maintaining normal filling pressures.
Increased LV Mass and Diastolic Dysfunction
Over time, progressive dilatation leads to increased wall stress and an increase in LV mass. Early studies of sickle cell disease found evidence of increasing LV mass with increasing age (62,64) as well as impaired LV filling (65). The presence of increased mass in both children and adults has been confirmed in the majority of imaging studies. Recent studies using standard Doppler parameters and tissue Doppler have shown that diastolic dysfunction is common in children (66–68), and in adults it was found to be an independent risk factor for mortality with a risk ratio of 4.8 (95% CI 1.9 to 12.1, p<0.001) (69). Importantly, the combination of diastolic dysfunction measures and pulmonary hypertension increases this mortality risk ratio to above 13.
As expected, diastolic abnormalities are associated with older age, increases in blood pressure, increased LV mass and higher creatinine levels. While the association of increased LV mass and diastolic dysfunction is clearly linked to systemic hypertension in the general population, it is unclear whether these findings in sickle cell disease are due to a combination of compensatory hypertrophy secondary to anemia and LV dilation along with a systemic vasculopathy affecting afterload. Direct myocardial damage from microvascular disease and iron deposition have also been postulated as etiologies for the cardiac abnormalities. Systemic blood pressure in SCD is known to be lower than in controls, but the presence of relative systemic hypertension has been linked with renal dysfunction and adverse outcomes (70). In addition, there are significant associations between systolic blood pressure and both increased pulmonary pressures and left ventricular filling pressures(71)
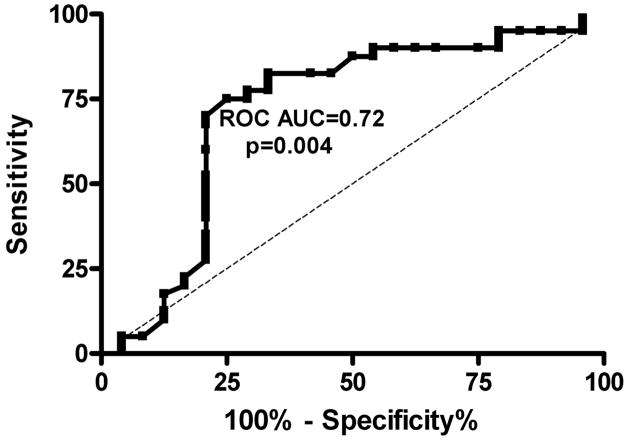
Invasive right heart catheterization measurements of patients with pulmonary hypertension (mean PAP greater than or equal to 25mmHg) show evidence of diastolic dysfunction in approximately half of the patients (48,72). Screening echocardiography studies show a significant variation in the prevalence of diastolic dysfunction due to the well-known difficulty in the non-invasive diagnosis of diastolic dysfunction. Although a tissue Doppler-derived E/e′ ratio>15 is accepted as a predictor of high LV filling pressure in the setting of LV dysfunction, the application of this in patients with preserved systolic function has been difficult (73). Kasner et al (74)studied patients with heart failure and normal ejection fraction and found that an increased E/e′ ratio above 8 was a useful non-invasive predictor of diastolic dysfunction. This ratio has not been prospectively validated in the different hemodynamic circumstances of sickle cell disease. Nonetheless, a retrospective review of the NIH cohort patients undergoing echocardiography and cardiac catheterization within 72 hours suggested that the E/e′ ratio is useful to predict a pulmonary capillary wedge pressure>15mmHg. ROC analysis showed that a cut-off value of 8.2 had a sensitivity of 78%, specificity of 71% (PPV 65%, NPV 82%, area under the curve 0.72) for predicting an elevated wedge pressure (Figure 3)(75). Prospective studies using simultaneous echo and catheterization measurements are needed to further validate this finding in SCD.
Figure 3. Sensitivity and specificity analysis of diastolic dysfunction measures in SCD patients.
Receiver operating characteristic curve for tissue Doppler lateral E/e′ ratio to predict a pulmonary capillary wedge pressure>15mmHg by cardiac catheterization within 72 hours in SCD patients.
LV Systolic Dysfunction
While “heart disease” and “heart failure” have traditionally been considered common in adult SCD patients, recent large screening echocardiographic studies indicate that LV systolic function is preserved in the majority of SCD patients studied in a resting state (69,76,77), and the presence of segmental wall motion abnormalities is rare. When LV dysfunction is present, it has been seen particularly in older patients and those with associated conditions such as hypertension and renal disease(78). Most studies have used parameters such as ejection fraction, velocity of circumferential shortening, shortening fraction, and systolic time intervals to assess LV systolic function. It is important to note that none of these parameters are true indices of contractility since they are known to be affected by heart rate, preload, and afterload. In an early study using a more load-independent measure, the end-systolic stress volume index, Denenberg et al(79) showed that there was significant LV contractile dysfunction in SCD patients compared with controls. More recent evaluations using the end-systolic wall stress to velocity of circumferential shortening relationship have shown mixed results in children. Batra et al (80) found systolic function and contractility to be preserved, while Lamers et al (81) used a slightly different method as well as matched controls and found a significant decrease in contractility in SCD patients.
Right ventricular dysfunction
Imaging studies of SCD patients at steady state without pulmonary hypertension have shown dilated right heart chambers without significant RV dysfunction in most cases (69,76). During acute chest syndrome, pulmonary pressures increase, and in a series of 84 consecutive hospital admissions, cor pulmonale was seen in 13% of patients(50). All of these patients had a TRV≥3m/s during the acute event and they were at particularly high risk for multi-organ failure and sudden death. Our clinical experience suggests that those patients with resting pulmonary hypertension and/or evidence of RV dysfunction are the ones most likely to develop acute right heart failure (Figure 4). Acute pressure overload on top of a chronic pulmonary vasculopathy is felt to be the reason for acute RV decompensation.
Figure 4. This echocardiographic image (apical 4-chamber view) from a SCD patient admitted with acute chest syndrome shows a dilated, dysfunctional right ventricle.
A movie image of this patient’s echocardiogram is available on-line.
Myocardial Infarction
Myocardial ischemia and infarction have been reported to occur in SCD, but in almost all cases evaluated in the literature and in our practice at the National Institutes of Health, coronary angiography reveals normal coronary arteries (82–86). Case reports of patients presenting with acute crisis have shown ECG abnormalities (87), nuclear perfusion defects (88,89), MRI abnormalities (84) and troponin elevations (86) suggestive of acute myocardial infarction. These findings have been attributed to acute and chronic microvascular occlusion in the setting of chronic endothelial damage, a procoagulant state, and the systemic vasculopathy described above. Reversal of the cardiac abnormalities has been seen after exchange transfusion and aggressive supportive care for the ischemia (85,90), however, evidence based guidelines for treatment with antiplatelet agents, anticoagulation, and thrombolytics are lacking.
Functional Capacity
Marked abnormalities in exercise capacity have consistently been seen in SCD patients. In addition to possible cardiac filling abnormalities, suggested mechanisms for this limitation in patients studied with cardiopulmonary testing include the anemia itself, pulmonary vascular disease, peripheral vascular disease and/or a myopathy (91). The 6-minute walk test is a useful measure of functional capacity in this population and the walk distance correlates directly with peak oxygen consumption and indirectly with the severity of the pulmonary hypertension.(48) Despite mild increases in pulmonary pressures and pulmonary vascular resistance, studies of SCD patients have shown more severely reduced 6-minute walk distances compared with patients with primary PAH (92). In the Walk-Phasst study, the mean ± SD distance walked in 6-minutes was 458±91 m, 438±98 m and 409±96 m in patients with TRV values of <2.7 meters/sec, 2.7 to <3.0 meters/sec and ≥3.0 meters/sec, respectively (P=0.001)(42). In this study, a reduction in 6-minute walk distance was independently associated with echocardiographic measures of PH (the TRV value) and with measures of diastolic dysfunction, suggesting two major independent determinants of cardiac dysfunction with exercise (71). Although limitations in 6-min walk distance are known to predict morbidity and mortality in heart failure, severe lung disease, and PAH (93), long-term mortality analysis from SCD cohorts is not yet available.
Cardiac Iron Overload
Myocardial iron deposition has been considered as one etiology for the cardiac abnormalities seen in SCD. Although autopsy studies have documented myocardial iron deposition (94), MRI studies using T2* measurements suggest that this finding is rare even in the presence of a significant transfusion history, systemic iron overload, and/or hepatic iron overload (95) (96). The rate of transfusional iron overload in the liver is lower for sickle cell patients than for myelodysplastic syndromes or thalassemia major(97) perhaps due to the intermittent nature of the transfusions or the systemic inflammatory state that limits cellular iron accumulation. In SCD patients, ferritin levels have been found to be a univariate predictor of mortality (31). Myocardial T2* measurements are associated with LV dysfunction and cardiac arrhythmias in thalassemia (98), but this relationship has not been established in SCD suggesting that other etiologies for myocardial dysfunction may be more prevalent.
Dysrhythmia
Electrocardiographic abnormalities, including QT prolongation, are not uncommon in SCD patients at rest (99). Liem et al (100) reported a 38% prevalence of QT prolongation in children and young adults, but they did not find a correlation between the QT measurement and left ventricular hypertrophy in their series. Maisel et al (87) studied 30 SCD patients with 24-hour electrocardiographic monitoring during acute crisis and found significant arrhythmias in 80% of patients with over half the patients having ventricular arrhythmias. A subgroup of these patients underwent gated nuclear studies and there was a trend towards more arrhythmias in patients with ventricular dysfunction. Further evaluation of electrocardiographic findings and arrhythmias is needed in order to identify SCD patients at higher risk for sudden cardiac death.
Sudden Death
Sudden death is an increasingly recognized and reported mechanism of death in the aging sickle cell disease population.(3,18,49,94,101–104) This occurs in the setting of hospitalization with sepsis or multi-organ failure, during recovery from a routine vaso-occlusive event, or at home. While this was historically ascribed to narcotic “overdose” it is now clear that this is a direct complication of pulmonary vascular and cardiac disease.
In a large autopsy series of 306 SCD patients, Manci et al (102) found that death was sudden and unexpected in 40% of patients and was usually associated with acute events. While clinical examination of these patients had reported underlying chronic organ injury in 25% of patients, they found pathologic evidence of chronic organ injury in 75% of patients suggesting that the severity of the underlying disease was often underestimated by clinicians. More recent autopsy series (19,94) have shown that cardiopulmonary causes account for the majority of deaths with sudden death/pulseless electrical activity, heart failure/myocardial infarction, and pulmonary thromboembolism/pulmonary hypertension being the most common findings at the time of death.
Conclusions
Despite a significant increase in longevity for SCD patients over the last several decades, the mortality rate from cardiovascular and pulmonary complications remains high. Multiple pathologic and clinical studies have shown that patients with pulmonary hypertension and diastolic left ventricular dysfunction represent a particularly high-risk subgroup. These cardiopulmonary complications contribute to a markedly low functional capacity and associated high risk of both sudden death and severe multi-organ dysfunction.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Heart Lung and Blood Institute, National Institutes of Health, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–30. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–65. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 4.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 376:2018–31. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 5.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–9. [PubMed] [Google Scholar]
- 6.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–20. [PubMed] [Google Scholar]
- 8.Platt OS. Sickle cell anemia as an inflammatory disease [comment] J Clin Invest. 2000;106:337–8. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–25. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death [see comments] N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 11.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Aisiku IP, Smith WR, McClish DK, et al. Comparisons of high versus low emergency department utilizers in sickle cell disease. Ann Emerg Med. 2009;53:587–93. doi: 10.1016/j.annemergmed.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Wethers DL. Sickle cell disease in childhood: Part I. Laboratory diagnosis, pathophysiology and health maintenance. Am Fam Physician. 2000;62:1013–20. 1027–8. [PubMed] [Google Scholar]
- 14.Ballas SK. Sickle cell anaemia: progress in pathogenesis and treatment. Drugs. 2002;62:1143–72. doi: 10.2165/00003495-200262080-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: role of nitric oxide and hemolytic anemia. Hematology (Am Soc Hematol Educ Program) 2005:51–7. doi: 10.1182/asheducation-2005.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. Jama. 2003;289:1645–51. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 18.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 19.Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladwin MT, Barst RJ, Castro OL, et al. Pulmonary hypertension and NO in sickle cell. Blood. 116:852–4. doi: 10.1182/blood-2010-04-282095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–6. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 22.Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–48. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 23.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 24.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 27.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 28.Sundaram N, Tailor A, Mendelsohn L, et al. High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood. 116:109–12. doi: 10.1182/blood-2009-09-244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machado RF, Anthi A, Steinberg MH, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. Jama. 2006;296:310–8. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 32.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 86:123–54. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- 34.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 116:687–92. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 35.Nolan VG, Adewoye A, Baldwin C, et al. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133:570–8. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis associated priapism in sickle cell disease. Blood. 2005 doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 38.Naoman SG, Nouraie M, Castro OL, et al. Echocardiographic findings in patients with sickle cell disease. Ann Hematol. 2009 doi: 10.1007/s00277-009-0764-0. [DOI] [PubMed] [Google Scholar]
- 39.Onyekwere OC, Campbell A, Teshome M, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29:309–12. doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 40.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 115:2354–63. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 41.Machado RF, Hildescheim M, Mendelsohn L, Kato GJ, Gladwin MT. NT-Pro Brain Natriuretic Peptide Levels and the Risk of Stroke and Death in the Cooperative Study of Sickle Cell Disease. Blood. 2009;114:1541. doi: 10.1111/j.1365-2141.2011.08777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machado RF, Barst RJ, Yovetich NA, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonseca GH, Souza R, Salemi VC, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterization in sickle cell disease. Eur Respir J. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 44.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev V, Kato GJ, Gibbs JS, et al. Echocardiographic Markers of Elevated Pulmonary Pressure and Left Ventricular Diastolic Dysfunction Are Associated With Exercise Intolerance in Adults and Adolescents With Homozygous Sickle Cell Anemia in the United States and United Kingdom. Circulation. 124:1452–1460. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehari A, Gladwin MT, Tian X, et al. Hemodynamic Determination of Pulmonary Hypertension in Adults with Sickle Cell Disease: Results of 9-year Follow Up of the NIH SCD PH Registry. 2011 manuscript in review. [Google Scholar]
- 47.Maitre B, Mekontso-Dessap A, Habibi A, et al. Pulmonary complications in adult sickle cell disease. Rev Mal Respir. 28:129–37. doi: 10.1016/j.rmr.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–9. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machado RF, Mack AK, Martyr S, et al. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136:319–25. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mekontso Dessap A, Leon R, Habibi A, et al. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177:646–53. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 51.Lanzkron S, Strouse JJ, Wilson R, et al. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–55. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brawley OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148:932–8. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 53.Barst RJ, Mubarak KK, Machado RF, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 149:426–35. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minniti CP, Machado RF, Coles WA, Sachdev V, Gladwin MT, Kato GJ. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147:737–43. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 56.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 57.Machado RF, Martyr S, Kato GJ, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–53. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 59.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–71. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 60.Brock G, Glina S, Moncada I, et al. Likelihood of tadalafil-associated adverse events in integrated multiclinical trial database: classification tree analysis in men with erectile dysfunction. Urology. 2009;73:756–61. doi: 10.1016/j.urology.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 61.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. 1972;83:415–26. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- 62.Lester LA, Sodt PC, Hutcheon N, Arcilla RA. Cardiac abnormalities in children with sickle cell anemia. Chest. 1990;98:1169–74. doi: 10.1378/chest.98.5.1169. [DOI] [PubMed] [Google Scholar]
- 63.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerry JL, Baird MG, Fortuin NJ. Evaluation of left ventricular function in patients with sickle cell anemia. Am J Med. 1976;60:968–72. doi: 10.1016/0002-9343(76)90568-4. [DOI] [PubMed] [Google Scholar]
- 65.Balfour IC, Covitz W, Arensman FW, Eubig C, Garrido M, Jones C. Left ventricular filling in sickle cell anemia. Am J Cardiol. 1988;61:395–9. doi: 10.1016/0002-9149(88)90952-6. [DOI] [PubMed] [Google Scholar]
- 66.Hankins JS, McCarville MB, Hillenbrand CM, et al. Ventricular diastolic dysfunction in sickle cell anemia is common but not associated with myocardial iron deposition. Pediatr Blood Cancer. 55:495–500. doi: 10.1002/pbc.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson MC, Kirkham FJ, Redline S, et al. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood. 116:16–21. doi: 10.1182/blood-2009-06-227447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caldas MC, Meira ZA, Barbosa MM. Evaluation of 107 patients with sickle cell anemia through tissue Doppler and myocardial performance index. J Am Soc Echocardiogr. 2008;21:1163–7. doi: 10.1016/j.echo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2008;83:15–8. doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sachdev V, Kato GJ, Gibbs SR, et al. Echocardiographic Markers of Elevated Pulmonary Pressure and Left Ventricular Diastolic Dysfunction Are Associated With Exercise Intolerance in Adults and Adolescents wtih Homozygous Sickle Cell Anemia in the United States and United Kingdom [manuscript in press] Circulation. 2011:124. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–61. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 73.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 74.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–47. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 75.Hannoush H, Kato GJ, Machado RF, et al. A Lower Tissue Doppler E/Ea Threshold Predicts Elevated Pulmonary Capillary Wedge Pressure in Sickle Cell Patients [abstract] Journal of the American Society of Echocardiography. 2011;24:B18. [Google Scholar]
- 76.Covitz W, Espeland M, Gallagher D, Hellenbrand W, Leff S, Talner N. The heart in sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD) Chest. 1995;108:1214–9. doi: 10.1378/chest.108.5.1214. [DOI] [PubMed] [Google Scholar]
- 77.Dham N, Ensing G, Minniti C, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104:713–20. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willens HJ, Lawrence C, Frishman WH, Strom JA. A noninvasive comparison of left ventricular performance in sickle cell anemia and chronic aortic regurgitation. Clin Cardiol. 1983;6:542–8. doi: 10.1002/clc.4960061105. [DOI] [PubMed] [Google Scholar]
- 79.Denenberg BS, Criner G, Jones R, Spann JF. Cardiac function in sickle cell anemia. Am J Cardiol. 1983;51:1674–8. doi: 10.1016/0002-9149(83)90208-4. [DOI] [PubMed] [Google Scholar]
- 80.Batra AS, Acherman RJ, Wong WY, et al. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol. 2002;70:306–12. doi: 10.1002/ajh.10154. [DOI] [PubMed] [Google Scholar]
- 81.Lamers L, Ensing G, Pignatelli R, et al. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J Am Coll Cardiol. 2006;47:2283–8. doi: 10.1016/j.jacc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Bode-Thomas F, Hyacinth HI, Ogunkunle O, Omotoso A. Myocardial ischaemia in sickle cell anaemia: evaluation using a new scoring system. Ann Trop Paediatr. 31:67–74. doi: 10.1179/1465328110Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pannu R, Zhang J, Andraws R, Armani A, Patel P, Mancusi-Ungaro P. Acute myocardial infarction in sickle cell disease: a systematic review. Crit Pathw Cardiol. 2008;7:133–8. doi: 10.1097/HPC.0b013e3181668ac3. [DOI] [PubMed] [Google Scholar]
- 84.Pavlu J, Ahmed RE, O’Regan DP, Partridge J, Lefroy DC, Layton DM. Myocardial infarction in sickle-cell disease. Lancet. 2007;369:246. doi: 10.1016/S0140-6736(07)60114-7. [DOI] [PubMed] [Google Scholar]
- 85.Deymann AJ, Goertz KK. Myocardial infarction and transient ventricular dysfunction in an adolescent with sickle cell disease. Pediatrics. 2003;111:E183–7. doi: 10.1542/peds.111.2.e183. [DOI] [PubMed] [Google Scholar]
- 86.Dang NC, Johnson C, Eslami-Farsani M, Haywood LJ. Myocardial injury or infarction associated with fat embolism in sickle cell disease: a report of three cases with survival. Am J Hematol. 2005;80:133–6. doi: 10.1002/ajh.20385. [DOI] [PubMed] [Google Scholar]
- 87.Maisel A, Friedman H, Flint L, Koshy M, Prabhu R. Continuous electrocardiographic monitoring in patients with sickle-cell anemia during pain crisis. Clin Cardiol. 1983;6:339–44. doi: 10.1002/clc.4960060707. [DOI] [PubMed] [Google Scholar]
- 88.Norris S, Johnson CS, Haywood LJ. Sickle cell anemia: does myocardial ischemia occur during crisis? J Natl Med Assoc. 1991;83:209–13. [PMC free article] [PubMed] [Google Scholar]
- 89.de Montalembert M, Maunoury C, Acar P, Brousse V, Sidi D, Lenoir G. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004;89:359–62. doi: 10.1136/adc.2003.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson WH, Jr, McCrary RB, Mankad VN. Transient left ventricular dysfunction in childhood sickle cell disease. Pediatr Cardiol. 1999;20:221–3. doi: 10.1007/s002469900447. [DOI] [PubMed] [Google Scholar]
- 91.Callahan LA, Woods KF, Mensah GA, Ramsey LT, Barbeau P, Gutin B. Cardiopulmonary responses to exercise in women with sickle cell anemia. Am J Respir Crit Care Med. 2002;165:1309–16. doi: 10.1164/rccm.2002036. [DOI] [PubMed] [Google Scholar]
- 92.Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 93.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 94.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–63. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 95.Inati A, Musallam KM, Wood JC, Sheikh-Taha M, Daou L, Taher AT. Absence of cardiac siderosis by MRI T2* despite transfusion burden, hepatic and serum iron overload in Lebanese patients with sickle cell disease. Eur J Haematol. 2009;83:565–71. doi: 10.1111/j.1600-0609.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 96.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–6. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 97.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 364:146–56. doi: 10.1056/NEJMct1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–8. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mueller BU, Martin KJ, Dreyer W, Bezold LI, Mahoney DH. Prolonged QT interval in pediatric sickle cell disease. Pediatr Blood Cancer. 2006;47:831–3. doi: 10.1002/pbc.20539. [DOI] [PubMed] [Google Scholar]
- 100.Liem RI, Young LT, Thompson AA. Prolonged QTc interval in children and young adults with sickle cell disease at steady state. Pediatr Blood Cancer. 2009;52:842–6. doi: 10.1002/pbc.21973. [DOI] [PubMed] [Google Scholar]
- 101.Graham JK, Mosunjac M, Hanzlick RL. Sickle cell lung disease and sudden death: a retrospective/prospective study of 21 autopsy cases and literature review. Am J Forensic Med Pathol. 2007;28:168–72. doi: 10.1097/01.paf.0000257397.92466.50. [DOI] [PubMed] [Google Scholar]
- 102.Manci EA, Culberson DE, Yang YM, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–65. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 103.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–43. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 104.Liesner RJ, Vandenberghe EA. Sudden death in sickle cell disease. J R Soc Med. 1993;86:484–5. doi: 10.1177/014107689308600822. [DOI] [PMC free article] [PubMed] [Google Scholar]