Abstract
Human Lesch-Nyhan cells, which are coupling and have gap junctions, were fused with mouse cl-lD cells, which are noncoupling and lack gap junctions. The resulting hybrid cells were coupling and had gap junctions while they contained the nearly complete complement of parent chromosomes. As the hybrid cells lost human chromosomes, clones appeared among the segregants, which had reverted to the noncoupling and junction-deficient trait of the mouse parent cell. The human cell appears to contribute a genetic factor to the hybrids that corrects the junctional deficiency of the mouse cell.
Keywords: gap junction, coupling, genetic analysis, cell fusion, electron microscopy
Full text
PDF


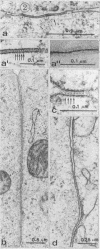
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azarnia R., Loewenstein W. R. Parallel correction of cancerous growth and of a genetic defect of cell-to-cell communication. Nature. 1973 Feb 16;241(5390):455–457. doi: 10.1038/241455a0. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Emmelot P. Hexagonal array of subunits in tight junctions separated from isolated rat liver plasma membranes. J Cell Biol. 1968 Jul;38(1):15–24. doi: 10.1083/jcb.38.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970 Oct;47(1):49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. P., Krauss M. R., Balis M. E., Dancis J. Evidence for transfer of enzyme product as the basis of metabolic cooperation between tissue culture fibroblasts of Lesch-Nyhan disease and normal cells. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1573–1579. doi: 10.1073/pnas.67.3.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. EFFECT OF HALOGENATED PYRIMIDINES AND THYMIDINE ON GROWTH OF L-CELLS AND A SUBLINE LACKING THYMIDINE KINASE. Exp Cell Res. 1964 Jan;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., Potter D. D. Low-resistance junctions between cells in embryos and tissue culture. Curr Top Dev Biol. 1968;3:95–127. doi: 10.1016/s0070-2153(08)60352-x. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Branton D., Satir P. The septate junction: a structural basis for intercellular coupling. Proc Natl Acad Sci U S A. 1970 Sep;67(1):213–220. doi: 10.1073/pnas.67.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerry L. M., Kunkel H. G., Grey H. M. Absence of disulfide bonds linking the heavy and light chains: a property of a genetic variant of gamma-A2 globulins. Proc Natl Acad Sci U S A. 1970 Mar;65(3):557–563. doi: 10.1073/pnas.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. STUDIES ON AN EPITHELIAL (GLAND) CELL JUNCTION. I. MODIFICATIONS OF SURFACE MEMBRANE PERMEABILITY. J Cell Biol. 1964 Sep;22:565–586. doi: 10.1083/jcb.22.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Socolar S. J., Higashino S., Kanno Y., Davidson N. Intercellular Communication: Renal, Urinary Bladder, Sensory, and Salivary Gland Cells. Science. 1965 Jul 16;149(3681):295–298. doi: 10.1126/science.149.3681.295. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. Membrane ultrastructure at mammalian intercellular junctions. Prog Biophys Mol Biol. 1973;26:45–101. doi: 10.1016/0079-6107(73)90017-5. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G. D., Bennett M. V. Specialized junctions involved in electrical transmission between neurons. Ann N Y Acad Sci. 1966 Jul 14;137(2):495–508. doi: 10.1111/j.1749-6632.1966.tb50177.x. [DOI] [PubMed] [Google Scholar]
- Peracchia C. Low resistance junctions in crayfish. I. Two arrays of globules in junctional membranes. J Cell Biol. 1973 Apr;57(1):66–76. doi: 10.1083/jcb.57.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. THE OCCURRENCE OF A SUBUNIT PATTERN IN THE UNIT MEMBRANES OF CLUB ENDINGS IN MAUTHNER CELL SYNAPSES IN GOLDFISH BRAINS. J Cell Biol. 1963 Oct;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Yee A. G., Hudspeth A. J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2924–2927. doi: 10.1073/pnas.68.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P., Gilula N. B. The fine structure of membranes and intercellular communication in insects. Annu Rev Entomol. 1973;18:143–166. doi: 10.1146/annurev.en.18.010173.001043. [DOI] [PubMed] [Google Scholar]
- Slack C., Palmer J. F. The permeability of intercellular junctions in the early embryo of Xenopus laevis, studied with a fluorescent tracer. Exp Cell Res. 1969 Jun;55(3):416–419. doi: 10.1016/0014-4827(69)90577-1. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Three types of gap junctions interconnecting intestinal epithelial cells visualized by freeze-etching. Proc Natl Acad Sci U S A. 1972 May;69(5):1318–1321. doi: 10.1073/pnas.69.5.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Green H. Human-mouse hybrid cell lines containing partial complements of human chromosomes and functioning human genes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1104–1111. doi: 10.1073/pnas.58.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]