Abstract
Patients with glioblastoma typically present when tumors are at an advanced stage. Surgical resection, radiotherapy and adjuvant chemotherapy are currently the standard of care for glioblastoma. However, due to the infiltrative and dispersive nature of the tumor, recurrence rate remains high and typically results in very poor prognosis. Efforts to treat the primary tumor are, therefore, palliative rather than curative. From a practical perspective, controlling growth and dispersal of the recurrence may have a greater impact on disease-free survival, In order for cells to disperse, they must first detach from the mass. Preventing detachment may keep tumors that recur more localized and perhaps more amenable to therapy. Here we introduce a new perspective in which a quantifiable mechanical property, namely tissue surface tension, can provide novel information on tumor behavior. The overall theme of the discussion will attempt to integrate how adhesion molecules can alter a tumor’s mechanical properties and how, in turn, these properties can be modified to prevent tumor cell detachment and dispersal.
Keywords: dispersal velocity, fibronectin matrix assembly, glioblastoma, integrin, matrix stiffness, tissue cohesion, tissue surface tensiometry, wetting
Glioblastoma multiforme (GBM) continues to be one of the greatest challenges in oncology today. Despite extensive molecular and clinical research, median survival of patients with GBM has not significantly increased over the last 20 years. GBMs demonstrate several intriguing properties. These tumors tend to be extremely aggressive within the brain, frequently migrating throughout the brain, yet they very rarely metastasize outside of the CNS. This rapid and extensive dispersal is one of the factors that render GBM refractory to surgical resection, radiation and chemotherapy. It also represents one of the key limiting factors for most of the new molecular therapies for brain tumors, as these new therapies cannot be effectively delivered to the widely dispersed tumor cells. Another important property of GBMs is that histological classification is only an approximation of the way these tumors behave clinically; some patients with GBM may survive 2–3 years, while others may only live 3–4 months. Despite aggressive treatment, GBM almost always recurs. Delaying onset of recurrence or the extent of dispersal would represent a significant advance in managing this disease. Accordingly, understanding the molecular machinery underlying tumor cell detachment and dispersal is key.
In order for cells to disperse, they must first detach from the primary mass. They must then establish an adhesive connection with the structures over which they must move. These processes must involve the actions of adhesion systems that control cell–cell cohesion and cell–substrate adhesion. This article will first present a general overview of GBM. The author will then explore the role of cell–cell cohesion and cell–extracellular matrix (ECM) adhesion in GBM and describe how a shift in the balance between these two opposing forces can significantly reduce detachment and dispersal. The author reviews methods developed to quantify how changes in cell–cell and cell–ECM interaction influence tumor mechanical properties. The author also describes how cell and tissue mechanics can contribute to our understanding of tumor behavior.
General overview
In adults, GBM represents 17% of all primary brain tumors and 54% of all gliomas. GBM in children is more rare, representing approximately 9% of all primary brain tumors [1]. Tumors occur most prevalently in the frontal lobe, temporal lobe and cerebellum, but can also develop in other regions of the brain [2]. In general, GBM behaves similarly in children and adults [1]. A recent study involving an integrative genomic analysis of 200 patient samples of GBM and 1740 genes revealed four distinct tumor subtypes: classical, mesenchymal, proneural and neural. Clinical correlations and treatment efficacy differed between subtypes, suggesting that despite a common morphological diagnosis of GBM, tumors differed markedly in their molecular classification [3]. The histopathology of GBM is extremely variable. GBMs are composed of poorly differentiated, pleomorphic astrocytic cells with marked nuclear atypia and high mitotic activity. Necrosis is an essential diagnostic feature, and prominent microvascular proliferation is common [4]. GBMs can become very large before producing symptoms and can give rise to increased intracerebral pressure by edema and mass effect. Corticosteroids, usually dexamethasone, can reduce edema through rearrangement of the blood–brain barrier, diminishing mass effect and intracerebral pressure [5]. GBM is highly infiltrative and cells can disperse widely throughout the brain. Three anatomical pathways are generally used by dispersing cells: the white matter, the cortex and the perivascular space within these structures [6]. Since white matter and the cortex are densely packed with axons and dendrites, dispersing tumor cells would have to navigate through intercellular spaces as small as 1 µm in size [7]. Accordingly, migrating cells would have to generate sufficient force in order to deform to a shape that would allow them to ‘squeeze’ through these tightly packed brain structures. GTPases, including Rac, RhoA and Cdc42, regulate cell morphology and actin dynamics and have been implicated in this process [8]. By contrast, glioma cells migrating through the perivascular space may not have to deform as much since blood vessels within gliomas have been shown to have an expanded perivascular space [9]. This could facilitate migration and dispersal by providing a path of least resistance to invading cells. In any case, adhesion molecules that mediate cell–ECM interactions must certainly play a role in mediating traction-dependent shape changes or motility during dispersal of GBM cells and have been identified as potential therapeutic targets for GBM [10].
Surgical resection of the tumor is not always possible. However, surgery still represents the best first-line option for patients with operable GBM. The extent of tumor resection is an important consideration given the possible neurologic deficits that may accompany aggressive resection of tumors in critical regions of the brain [11]. Studies have shown that resecting as little as 78% of the tumor provides a survival benefit. However, survival is markedly improved when 95–99% of the tumor is removed [12]. Resection also markedly reduces intracranial pressure and mass effect [13]. Surgical resection is not the only option. Current standard-of-care treatment also includes radiotherapy and adjuvant chemotherapy with alkylating agents, such as temozolomide (TMZ), following what is termed the ‘Stupp protocol’ [14]. Typically, patients who undergo surgical resection followed by TMZ treatment and postoperative radiotherapy survive an average of 3–9 months longer, depending on age, than patients who did not receive the drug [15,16]. To further complicate clinical efficacy, studies have identified stem-like cells in GBM that appear to be resistant to chemotherapy with TMZ [17]. Addition of antiangiogenesis drugs such as bevacisumab (Avastin®; Genentech, CA, USA) or antibodies against VEGFR2 [18]; reduce tumor bulk, but questions have arisen with regard to a potential role in the acceleration of tumor spread [19,20] and cytotoxicity [21]. Inclusion of Gliadel® (Arbor Pharmaceuticals, GA, USA; carmustin wafers implanted preoperatively) was shown to be effective in improving outcome in some studies [22]. However, other studies have shown that addition of Gliadel does not appear to significantly improve clinical outcome and is also associated with increased cytotoxicity [23]. Despite aggressive treatment, long-term survival of patients with GBM remains poor; median survival is less than 30% at 1 year, 5% at 3 years and 3% at 5 years [24]. This is in large part due to a high rate of local recurrence. Tumors typically recur within 2 cm of the operative site, but in some cases can recur more distantly [25]. Reoperation for a recurrence yields little more than a 3-month survival advantage [26]. Since the disease is generally diagnosed at an advanced stage, it is highly likely that tumor cells have already invaded normal brain tissue at the time of diagnosis. Indeed, a study conducted in the 1960s observed that at necropsy, 50% of untreated brain tumors had spread to the contralateral hemisphere [27]. A more recent study using an animal model of GBM showed that intraparenchymal tumor cell invasion is an early event in gliomagenesis and, in fact, precedes tumor mass formation [28]. Thus, tumor cell dispersal beyond the margins of the primary tumor effectively renders surgical efforts to treat the primary tumor palliative rather than potentially curative.
To disperse, cells must not only detach from the tumor mass, but they must also have the capacity to generate sufficient traction with the structures over which they migrate. Accordingly, forces generated by cell–cell cohesion must be overridden by those forces mediating cell–substratum adhesion. Molecules involved in dispersal probably include cadherins, integrins and various components of the ECM.
Integrins as therapeutic targets in GBM
Integrins perform two basic functions; they mediate cell attachment to the ECM, and in so doing regulate outside-in and inside-out signaling. Integrins mediate cell–ECM adhesion, migration and survival of normal and cancer cells. Several αβ integrin heterodimers are expressed by glioma cells, including αvβ3, αvβ5 [29,30], α6β1/β4 [31], α9β1 [32] and α5β1 [33]. In GBM, αvβ3/β5 expression is upregulated in both tumor cells and endothelial cells, suggesting that it may play a dual role in mediating not only tumor cell adhesion and migration, but also angiogenesis [34]. In GBM, the α6 integrin is coexpressed with stem cell markers and has been used to successfully enrich a stem-like population [31]. This integrin is known to mediate binding to laminin, which is also highly expressed in GBM [35]. The α9β1 integrin binds tenascin, an ECM component in GBM [36]. A recent study proposed a novel mechanism for the α9β1 integrin as a mediator of enhanced cell migration in GBM [32]. The α5β1 integrin is a receptor for fibronectin. Both the α5β1 integrin [37] and fibronectin [38] are upregulated in GBM. Clinically, α51 integrin expression was associated with decreased survival of patients with high-grade glioma [39], and the antagonizing α5αβ1 integrin sensitizes p53 wild-type glioma cells to chemotherapeutic drugs [33]. Knocking down fibronectin expression has also been shown to delay tumor growth in a rodent glioma model [40]. Based on these studies, it is logical to conclude that high levels of α5β1 integrin expression are associated with tumorigenesis and that integrin inhibition may attenuate gliomagenesis. Accordingly, αvβ3 and αvβ5 have emerged as promising targets of anticancer therapy. Cilengitide, a cyclic pentapeptide, is a specific inhibitor of these integrins and has been demonstrated to have antiangiogenic and anti-invasive activity in various glioma models [41]. Cilengitide is currently in clinical trials in patients with recurrent GBM. A recent single-agent study demonstrated potential efficacy with a 6-month progression-free survival rate of 12% [42]. When combined with standard TMZ and radiation therapy, cilengitide improved patient survival as compared with a matched patient sample from the EORTC trial [43]. The emergence of anti-integrin therapy for GBM is promising. Interestingly, other studies have shown that restoring integrin activity or function can also significantly reduce tumor cell dispersal by effectively increasing cohesion between cells in 3D tumor-like tissues.
Integrins as mediators of cell-cell cohesion
The first demonstration of a potential role for integrins as mediators of strong cell–cell cohesion was in human fibrosarcoma HT-1080 cells [44]. Human fibrosarcoma HT-1080 cells express the α5β1 integrin, but are deficient in their ability to assemble fibronectin into a matrix. Treatment of HT-1080 cells with dexamethasone activates the α5 integrin and restores capacity for fibronectin matrix assembly (FNMA) [45]. Restoring this function has been shown to significantly increase tissue cohesion, underscoring a new role for integrins as indirect mediators of cell–cell cohesion of 3D tissues. The concept that α5β1 integrin–fibronectin interaction can act as a mediator of strong tissue cohesion arose from the observation that in tumors (and other tissues) cells are embedded in a 3D meshwork of ECM components. Within this meshwork, integrin–ECM interactions may ‘glue’ adjacent cells together. This concept was demonstrated by showing that transfecting CHO-B2 cells with cDNA for expression of high levels of the α5β1 integrin resulted in the formation of spherical, multicellular aggregates. When such aggregates were subjected to tissue surface tensiometry (TST) [46], a method of measuring intercellular binding energy, they were significantly more cohesive than the parent CHO-B2 line [47]. Moreover, using a series of chimeric α5β1 integrin-transfected cell lines, Robinson et al. showed that only wild-type, fully functional α5β1 integrin could promote FNMA by CHO cells and that only a fully developed fibronectin matrix could give rise to increased intercellular cohesion [48]. The concepts and methods underlying integrin–ECM-based cohesion have now been applied to studies of malignant invasion in various cancer models, including prostate cancer [49] and, more pertinent to this article, GBM [50]. In both models, ECM-based cohesion appears to be correlated with FNMA and could be pharmacologically induced by treatment with dexamethasone. These studies will be described in greater detail below. However, what is important is that restoring FNMA gave rise to increased intercellular cohesion and a decrease in tumor dispersal. In order to more rigorously explore this relationship, it was necessary to develop concepts and methods that could directly quantify intercellular cohesive energy and its relationship to tumor detachment and dispersal.
Methods to quantify tissue-level biomechanics
The measurement of intercellular cohesive energy in tissues is not trivial. Fortunately, several methods have been developed to quantify this property. Most methods involve compression of the tissue and measurement of its response to the compressive force. One such method, TST, has recently been used to measure cohesion and to correlate this property to the dispersal velocity of aggregates composed of brain tumor cells. TST is grounded in the concept of tissue liquidity. The idea that certain tissues behave like ‘living liquids’ was originally developed by Steinberg in the 1960s to explain the ‘rounding-up’ and ‘sorting-out’ behaviors displayed by embryonic tissues [51]. Behaviors that duplicate those of ordinary immiscible liquids include: the rounding up of irregularly shaped tissue fragments toward a spherical shape, which serves to minimize surface area and, consequently, the amount of free energy of the system; the spreading of one tissue mass over another to approach a particular anatomical configuration; and the sorting out of heterotypic cell mixtures to approach the same anatomical configuration adopted by spreading, among others.
This liquid-like behavior serves as the basis of TST, a method employed to measure the cohesivity of spherical aggregates under physiological conditions [46,52]. The method employs a custom-built instrument (Figure 1) to compress spherical cellular aggregates (Figure 2A) between parallel plates to which they cannot adhere. A chart recorder is used to monitor the applied force, and a dissecting microscope is used to capture aggregate geometry. Aggregates are compressed until they reach a shape and force equilibrium, whereupon measurements of aggregate geometry (Figure 2B) and the force of resistance (at equilibrium) are applied to the Young–Laplace equation (Equation 1) [53], producing numerical values of apparent tissue surface tension. Each aggregate is then subjected to a second, greater compressive force and surface tension is again calculated after aggregates have reached a second force and shape equilibrium. Only measurements in which surface tension is independent of the applied force, as would be expected of a liquid system, are used to calculate the average surface tension for any aggregate type. In this context, tissue surface tension represents a physically rigorous assessment of aggregate cohesion. TST has been previously used to measure cohesion of embryonic tissues [52,54–56], genetically engineered cells [57], and spheroids composed of tumor cells from lung [58], prostate [49], muscle [44] and brain [59].
| (Equation 1) |
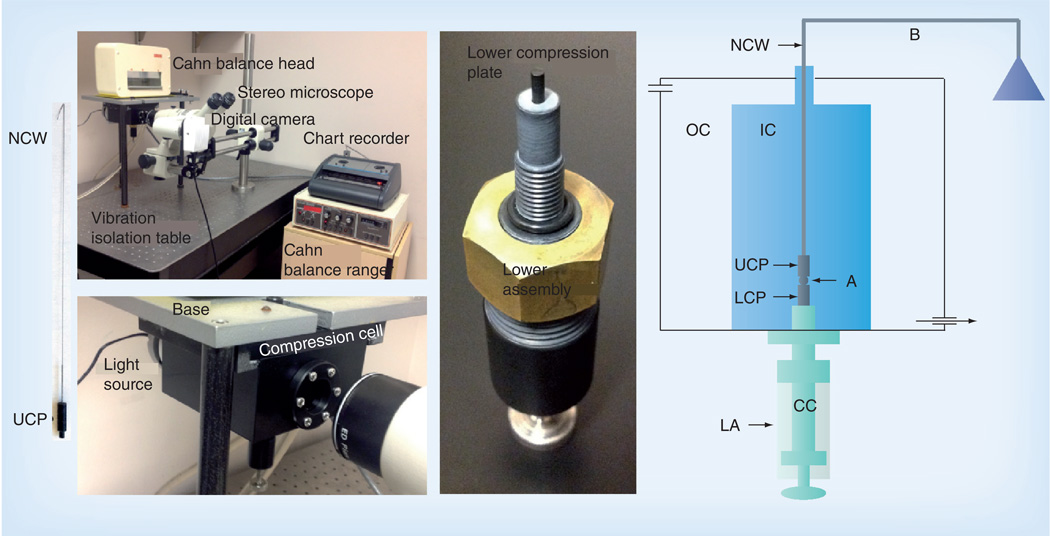
Figure 1. The tensiometer chamber and related equipment.
The instrument is composed of several commercially available systems, including a Cahn C-2000 recording balance ranger and head (Thermo Scientific, NH, USA), a Servogor® 102 chart recorder (Omni Instruments, IN, USA), a Nikon SMZ-10A stereoscopic dissecting microscope (Nikon, Tokyo, Japan) connected to an Infinity 2 black and white digital camera (Lumenera, ON, Canada) and mounted on a digital imaging instruments boom stand. The tensiometer chamber is mounted into the tensiometer base and connected to a Haake® DC10 (Sigma Aldrich, MO, USA) circulating water bath (not shown). The tensiometer sits on a vibration isolation table (TMC Vibration Control, MA, USA). The compression cell is composed of two Delrin® chambers (DuPont, DE, USA). The OC is connected to a 37°C circulating water pump and thus serves to regulate the temperature of the IC. Both the OC and IC have glass windows to facilitate imaging. A light-emitting diode light source illuminates the A. The LA screws into the base of the IC and is used to position the A in the IC, seal the bottom of the IC, elevate the A to initiate compression and control the distance between the parallel plates and hence the compression of the A. The CC of the assembly is adjustable. The tip of the CC is composed of smooth teflon and acts as the LCP. The UCP is a teflon cylinder 15 mm long that hangs from the B by a thin NCW. During the course of an experiment, the cell A is positioned on the lower plate and raised until it contacts the upper plate. The upper plate is connected to the B. Compression of the A causes displacement of the B, which, in turn, is recorded by the chart recorder.
A: Aggregate; B: Balance arm; CC: Central core; IC: Inner chamber; LA: Lower assembly; LCP: Lower compression plate; NCW: Nickel–chromium wire; OC: Outer chamber; UCP: Upper compression plate.
Reproduced with permission from [52].
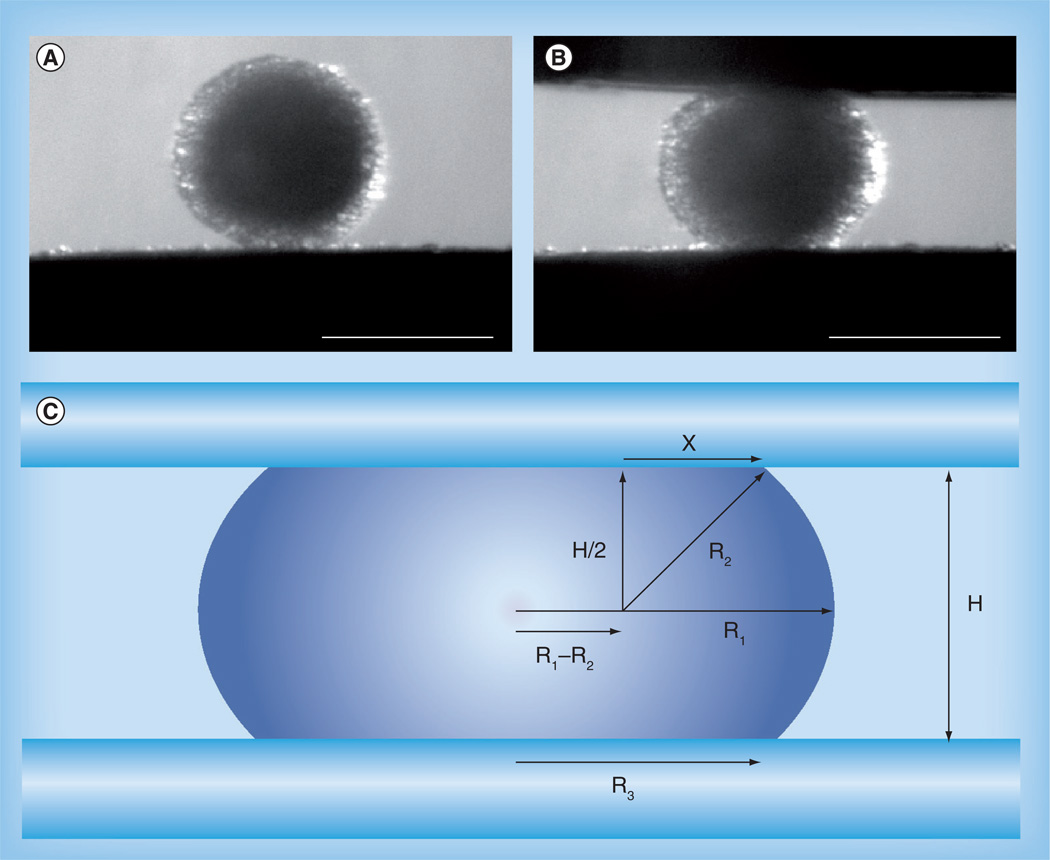
Figure 2. Aggregate geometry in response to compression.
An aggregate of U87-MG glioblastoma cells (A) before and (B) after compression (scale bars: 350 µm). (C) Liquid droplet compressed between two parallel plates to which it poorly adheres at shape equilibrium. R1 and R2 are the two primary radii of curvature, at the droplet’s equator and in a plane through its axis of symmetry, respectively. R3 is the radius of the droplet’s circular area of contact with either compression plate. H is the distance between the upper and lower compression plates. X is one side of a right-angled triangle with hypotenuse R2 extending to a point of contact between the droplet’s surface and either compression plate.
(C) Reprinted permission from [52].
At shape equilibrium, the cohesivity of an aggregate of cells compressed between parallel plates to which it does not adhere can be obtained from the Young–Laplace equation, where σ is cohesivity, F is the force acting to compress the aggregate, πR32 is the area of the surface of the aggregate upon which force is exerted, and R1 and R2 are the radius of the equator of the compressed aggregate and the radius of an arc defining its surface profile normal to the compressing plates and extending between them, respectively.
The concept of tissue liquidity can also be exploited to explore the role of cell–cell cohesion in tumor dispersal velocity. In liquid systems, the degree and rate of spreading are mediated by a balance between cohesive and adhesive forces and is referred to as ‘wetting’. The contact angle between the liquid and substrate is determined by these two opposing forces. As a liquid droplet spreads, the contact angle decreases. A contact angle of 0° indicates perfect wettability, whereas a contact angle of 180° indicates perfect nonwettability [60]. Similarly, if aggregates are deposited on substrates to which they can adhere, they tend to spread to some degree, the extent of which is determined by the relative amount of cell–cell cohesion and cell–ECM adhesion. This was demonstrated using aggregates of genetically engineered cells expressing different types/levels of cadherins, and a library of engineered polymers designed to modulate strength of adhesion to substrate. Either decreasing adhesion to substrate or increasing cell–cell cohesion markedly reduced aggregate spreading rate [61]. More recently, the concept of aggregate wettability was tested by the Brochard-Wyart group. They elegantly demonstrated that tightly cohesive aggregates tend to undergo liquid-like spreading with peripheral cells advancing as a monolayer, whereas weakly cohesive aggregates tend to spread more like a ‘gas’, with cells dispersing away from the advancing mass [62].
Dispersal can be viewed as a tug-of-war between cell–cell cohesion and cell–substratum adhesion. A shift in the balance away from cell–cell cohesion and towards cell–substratum adhesion facilitates dispersal, whereas a shift in the balance towards cell–cell cohesion would probably prevent cell detachment and mitigate dispersal. Shifting the balance between cohesion and adhesion is a reasonable strategy and ideally would be best accomplished pharmacologically. Obvious candidate molecules include cadherins and integrins. Cadherins are classical cell–cell adhesion molecules and their downregulation is often associated with increased invasive potential in tumors of epithelial origins. In GBM, however, the opposite is true. Expression of the most prevalent cadherin in neural tissues, N-cadherin, is typically upregulated in higher-grade tumors [63], and, when expressed in a precursor form, may in fact promote invasion [64]. Moreover, cadherins have been shown to facilitate migration of tumor cells on a cadherin substrate [65]. Regulating cadherin expression is impractical since a genetic approach would probably be necessary. An alternate strategy would be to employ an integrin–ECM-based adhesion system that, when induced pharmacologically, could provide sufficient intercellular binding energy to significantly increase cohesion and discourage detachment of tumor cells from a primary mass. Interestingly, studies have shown that brain injury results in an influx of fibronectin into the brain microenvironment [66]. If the presence of a tumor can stimulate a similar injury response, then it may be possible to conceive of a scenario in which available fibronectin, binding to an activated integrin, could be induced to ‘glue’ cells together to prevent cell detachment. An adhesion mechanism comprising α5β1 integrin–fibronectin interaction, inducible integrin activation and restoration of FNMA has recently been described for GBM [50].
Reactivating the fibronectin matrix increases aggregate cohesion & impedes glioma cell detachment & dispersal
The concepts discussed above have been applied to measure cohesion of aggregates of GBM cells and have provided clues as to how it may be possible to manipulate the ECM in a manner so as to prevent tumor cell dispersal. The study by Winters et al. measured aggregate cohesion of three well-characterized GBM cell lines [59]. All lines formed spherical aggregates when placed in hanging drop culture. Despite the fact that these cell lines were derived from patient tumors that had been pathologically graded as GBM, they had markedly different surface tensions, ranging from 7.0 ± 0.3 dynes/cm for U87-MG to 16.7 ± 0.3 dynes/cm for U118-MG. Surface tension was inversely proportional to in vitro invasive potential, and was markedly increased if cells were treated with dexamethasone. These data raise the possibility that tumor cohesion contains information on tumor behavior that histopathological analysis cannot predict.
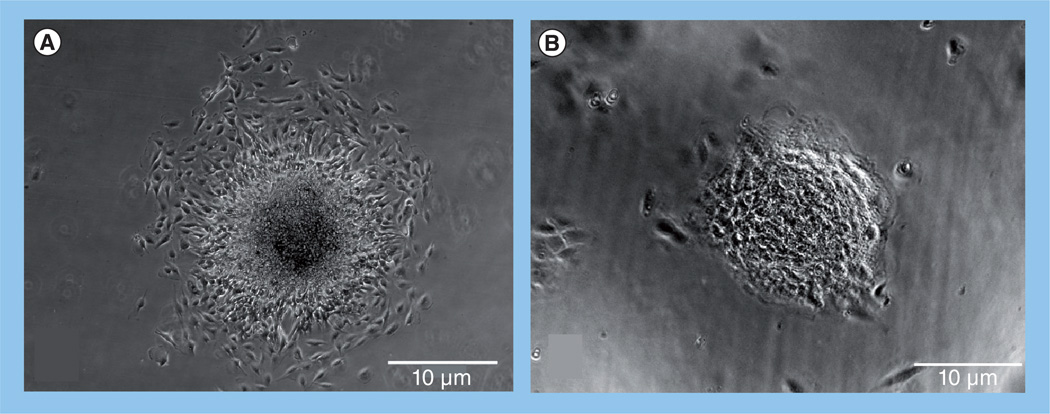
The dexamethasone-mediated increase in surface tension observed by Winters et al. was independent of de novo cadherin expression [59], suggesting activation of some other adhesion-based mechanism. Dexamethasone has been shown to activate FNMA in HT-1080 human fibrosarcoma cells [45]. Sabari et al. asked whether differences in aggregate cohesion could be explained by differences in capacity for FNMA, and whether the dexamethasone-dependent increase in cohesion arose as a result of reactivation of this process [50]. Indeed, the three GBM cell lines varied in their capacity for FNMA. They also differed markedly in their dispersal velocities. That is, when aggregates of cells were plated onto identical substrates, they spread at different rates. U87-MG, the least cohesive of the three lines, spread at a velocity of 21.4 ± 2.9 µ/h, whereas aggregates of the more cohesive LN-229 and U118-MG spread more slowly at 4.9 ± 0.6 and 4.1 ± 0.6 µ/h, respectively. When U87-MG cells were treated with dexamethasone, dispersal velocity was markedly reduced to a level approximating that of the U118-MG line. Of note is that the pattern of dispersal was affected by dexamethasone treatment. Whereas the advancing edge of aggregates of untreated U87-MG dispersed as single cells (Figure 3A), the leading edge of dexamethasone-treated aggregates advanced as a sheet (Figure 3B). Cells at the advancing front were tightly adherent to one another, suggesting that the dexamethasone-mediated decrease in dispersal velocity arose as a consequence of increased cell–cell cohesion. These patterns have been described elsewhere as a shift from a gaseous (low intermolecular forces) to a liquid (high intermolecular forces) state [62]. The involvement of FNMA in this process was confirmed by incubating dexamethasone-treated U87-MG with the 70-kDa fragment of fibronectin [67]. This fragment specifically prevents the incorporation of soluble fibronectin into a dense matrix. This effectively blocked FNMA and rescued dispersal velocity, suggesting that the fibronectin matrix was at least partially responsible for the dexamethasone-mediated increase in cohesion and attendant decrease in dispersal velocity [50].
Figure 3. Dexamethasone treatment promotes cell–cell contact during aggregate spreading.
Phase-contrast images of (A) control and (B) dexamethasone-treated aggregates of U87-MG glioma cells after 8 h of incubation. Dexamethasone treatment markedly reduces spreading and appears to promote cell–cell cohesion at the advancing cell front.
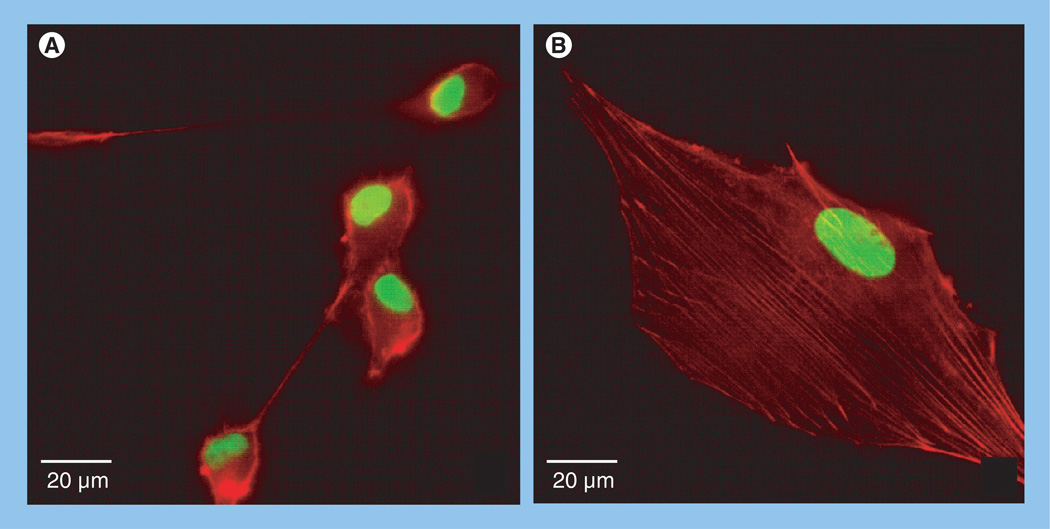
Maximal cell motility is dependent upon an ‘intermediate’ attachment strength of cells to substrate [68]. Too little adhesion would not provide sufficient traction for motility, whereas too much adhesion would prevent motility all together. In order to move, a tumor cell must carry out at least three processes: the leading edge must first detach from the surface and extend, this extension must then re-establish adhesion to the surface and the attached edge must pull the cell forward. Actin is of paramount importance since it can undergo contraction through the action of myosin. Interestingly, dexamethasone treatment resulted in a profound effect on actin organization by U87-MG GBM cells [50]. Actin organization changed from predominantly cortical (Figure 4A) to an arrangement in which actin was arranged into stress fibers (Figure 4B). This was associated with a marked change in cell shape, from round and loosely adherent cells to flatter cells that appeared to adhere more strongly to the substrate. It is possible that dexamethasone treatment not only gives rise to increased cell–cell cohesion, but that it may, by activating integrins, also result in increased adhesion to substrate. Since cells must constantly attach and detach from the substrate in order to move, it is possible that by increasing cell attachment to the substrate, dexamethasone may impede cell movement by making it more difficult for cells to detach from the substrate to which they are adhered. Accordingly, dexamethasone treatment may impart a ‘double hit’, reducing detachment of tumor cells from the mass, while also increasing cell–ECM adhesion to a point that impedes motility.
Figure 4. Dexamethasone treatment promotes actin stress fiber formation.
Immunofluorescence images of (A) untreated and (B) dexamethasone-treated U-87-MG cells. Actin, stained red, is typically cortical in untreated cells, but becomes organized into stress fibers when cells are incubated in dexamethasone. Nuclei are stained green. Note also that dexamethasone treatment results in a marked shape change, from round and poorly adherent, to flatter cells that appear to be more tightly adhered to the surface.
ECM stiffness & glioma cell dispersal
The ECM is fundamentally a mechanical structure whose physical properties, including rigidity, porosity, insolubility and topography, rely strongly on molecular composition and structural organization. Changes in such a milieu would profoundly influence how cells sense and interact with the microenvironment. An important physical property of the ECM is elasticity, which can vary from soft and compliant to stiff and rigid. Many studies have now confirmed that matrix stiffness can profoundly influence cell migration and dispersal [69]. For example, increased ECM stiffness is characteristic of breast tumors, where a five- to 20-fold increase in stiffness is associated with malignant behavior [70]. ECM stiffness has also been shown to influence the migration of glioma cells [71]. In general, tumor cells are more migratory on stiff linearized substrates than on crosslinked fibrous mesh works [72]. More specifically, using a series of glioma cell lines (U373-MG, U87-MG, U251-MG, SNB19 and C6) on fibronectin-coated polymeric ECM substrates of defined mechanical rigidity showed that tumor cells spread extensively and migrated rapidly on rigid ECMs. However, as ECM rigidity was lowered to values comparable to normal brain tissue, tumor cells failed to migrate. Moreover, inhibiting non-muscle myosin II-based contractility blunted this rigidity sensitivity and rescued cell motility on highly compliant substrates [71]. As discussed previously, as GBM cells disperse within the cortex, they encounter a stiff ECM and a ‘meshwork’ of axons and dendrites that limit the effective pore size to the submicron range [7]. To disperse, cells must establish ECM adhesion in order to generate sufficient tractional force for cell movement, but not so much as to become locked in place. Thus, ECM topology must also be considered. Whereas properly aligned ECM fibers can facilitate migration, too dense a meshwork can restrict cell motility by simply introducing a steric barrier [73]. A relationship between ECM stiffness and the spatial confinement experienced by glioma cells can also influence migration [74].
Apart from having to negotiate the ECM, glioma cells must also physically squeeze through pores that are of smaller diameter than that of their nucleus. To do so, they undergo shape change and nuclear deformation – processes that require dramatic changes in cytoskeletal organization and in cellular mechanics [75]. These changes are translated into transitions between membrane- and bulk-dominated elastic responses [76]. To propel the nucleus through the intercellular spaces, glioma cells undergo a non-muscle myosin II-dependent contraction at the rear of the cell and it is this contraction that provides the necessary force to propel the nucleus through the pores. The myosin II inhibitor blebbistatin prevents nuclear protrusion through pores, indicating an absolute requirement for normal myosin II activity. Inhibition of myosin II could, in principle, represent an effective anti-invasive therapy for malignant glioma [77].
Conclusion
Integrins, expressed at physiological levels and in an appropriate ECM microenvironment, play a critical role in maintaining normal tissue homeostasis. Integrin dysfunction gives rise to a change in normal cell behavior that, in the context of GBM, can result in a decrease in tumor cohesion and increased tumor cell dispersal. Restoring normal integrin function, particularly in a way that increases tumor cohesion, can potentially interfere with the dispersal of tumor cells when the tumor recurs. That dexamethasone is able to restore integrin function is an advantage since dexamethasone is currently routinely used to treat brain tumor-related cerebral edema [78]. Its role in mediating tumor cohesion in vivo is as yet unexplored. As discussed earlier, GBM almost always recurs. How quickly this happens depends on several factors, including propensity for dispersal. By administering agents that effectively increase cell–cell cohesion and impede cell motility at the appropriate time after initial surgery, it may be possible to effectively decrease dispersal (Figure 5). While not a cure, preventing tumor cell dispersal could significantly improve patient outcome by increasing time-to-recurrence. This, in turn, may delay the time course of reoperation and/or other therapeutic interventions for recurrent disease.
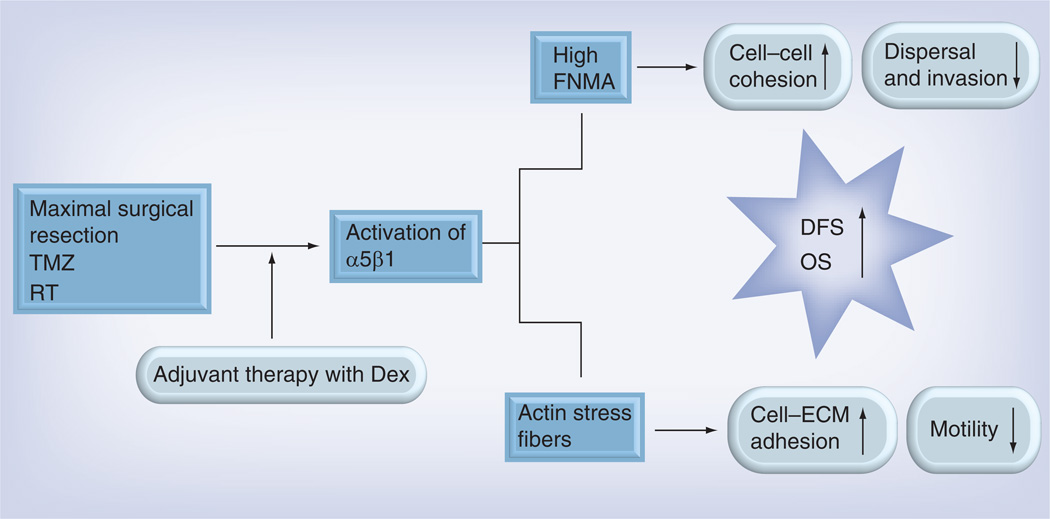
Figure 5. How dexamethasone-mediated activation of the α5β1 integrin can reduce tumor cell detachment and dispersal.
Activation of α5β1 integrin by dexamethasone gives rise to the assembly of fibronectin into a dense matrix. This increases tumor cohesion by effectively ‘gluing’ cells together. This increase in cohesion prevents cells from detaching from the mass, resulting in a decrease in dispersal and invasion into surrounding normal tissue. Activation of α5β1 significantly alters cell shape from round, loosely adherent cells, to flatter cells that appear to adhere more strongly to substrate. This apparent increase in cell–ECM adhesion could, in principle, impede cell motility by rendering cells too adherent to the substrate. Collectively, the increase in cell–cell cohesion and cell–ECM adhesion would prevent dispersal. Keeping the tumors that recur more contained would render them more amenable to surgical re-excision or stereotactic radiotherapy. This, in turn, may increase the length of DFS and OS.
Dex: Dexamethasone; DFS: Disease-free survival; ECM: Extracellular matrix; FNMA: Fibronectin matrix assembly; OS: Overall survival; RT: Radiotherapy; TMZ: Temozolomide.
Future perspective
Current treatment strategies for GBM only yield a modest improvement in disease-free and overall survival. This is mainly due to the fact that tumors invariably recur. Recurrence is mainly due to the dispersive nature of the tumor cells since it is not possible to completely resect the tumor and achieve disease-free margins. One possible strategy to improve clinical outcome is to delay the onset of recurrence. This can be achieved by containing the spread of the recurrent tumor. This article discussed how an increase in cell–cell cohesion and cell–ECM adhesion could potentially prevent detachment and impede motility of tumor cells. The author proposed that cohesion/adhesion impart to tumors measurable mechanical properties that are highly predictive of tumor behavior. In the next 5–10 years, tumor biomechanics could be exploited as a means of providing information that is more predictive of dispersive or invasive behavior. Methods used to measure tissue mechanical properties and the physical concepts underlying them will provide a framework for developing new approaches to understand and, ultimately, to control tumor cell dispersal. One such method, TST, measures both intercellular cohesion and actin-based cortical tension [79], both of which can be markedly altered by various drugs, including dexamethasone, a steroid currently in use to treat tumor-related edema. That dexamethasone may also reduce dispersal, at least in vitro, suggests a potentially new indication for use after initial resection to contain spread of the recurrent tumor. For reoperable GBM, controlled release delivery of carmustine (Gliadel) from biodegradable polymer wafers implanted into the surgical resection cavity maximizes drug delivery to the local tumor microenvironment while minimizing systemic toxicity [80]. In the future, an alternative/complimentary approach may require implantation of wafers composed of engineered biomaterials designed to attract tumor cells back towards the surgical margin, perhaps by releasing a chemoattractant. Promoting cell–ECM adhesion to the material would keep tumor cells better confined and, therefore, amenable to ablative therapy, such as surgical re-excision or stereotactic radiotherapy. Incorporating contrast-enhancing agents into the bio-material could further facilitate specific targeting of these cells. Alternatively, it may be possible to engineer the biomaterial to degrade upon cell contact and in so doing release agents that drive tumor cells toward senescence or apoptosis. Incorporation of ECM-coated biomaterials with stiffness designed to modulate tumor cell behavior may also represent a promising approach. By tuning the stiffness of the biomaterial, it may be possible to initiate cell signaling to regulate cell fate towards a less aggressive phenotype [81].
GBM is a devastating disease. Despite decades of intense research, it remains intractable to therapy. Any improvement in the length of disease-free or overall survival would be of clinical benefit. Reducing tumor cell detachment and dispersal could contain the recurrent tumor and render it more amenable to targeted therapy and, as such, delay progression of this disease.
Executive summary.
Tissue-level biomechanics & glioma cell dispersal
-
▪
Tissue biomechanics can provide information about tumor behavior.
-
▪
A shift in the balance between forces of cell–cell cohesion and cell–extracellular matrix adhesion determines whether cells can detach from a primary mass.
-
▪
Changes in integrin–extracellular matrix-based adhesion can influence this shift in forces.
Methods to quantify tissue-level biomechanics
-
▪
Tissue surface tensiometry can be used to measure intercellular binding energy in 3D tissues.
-
▪
Tissue surface tensiometry is rooted in the concept of tissue liquidity, which can also be used to model spreading or dispersal behavior as a liquid-like phenomenon.
Reactivating fibronectin matrix assembly impedes dispersal
-
▪
Glioblastoma cell lines differ in their capacity for fibronectin matrix assembly.
-
▪
Cell lines that can assemble a matrix disperse more slowly than those lacking the capacity for fibronectin matrix assembly.
-
▪
Dispersal velocity is inversely proportional to tissue surface tension.
-
▪
Reactivating matrix assembly by treatment with dexamethasone increases cohesion and decreases dispersal velocity.
-
▪
Reactivating α5β1 integrin promotes actin stress fiber formation, resulting in a marked change in cell shape. Treated cells also appear to adhere more strongly to substrate. This could, in principle, impede cell motility.
Acknowledgments
This work was supported by the NIH grant number R01CA118755.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Dohrmann GJ, Farwell JR, Flannery JT. Glioblastoma multiforme in children. J. Neurosurg. 1976;44(4):442–448. doi: 10.3171/jns.1976.44.4.0442. [DOI] [PubMed] [Google Scholar]
- 2.Zada G, Bond AE, Wang YP, Giannotta SL, Deapen D. Incidence trends in the anatomic location of primary malignant brain tumors in the United States: 1992-2006. World Neurosurg. 2012;77(3–4):518–524. doi: 10.1016/j.wneu.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 3.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1 . Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988;62(10):2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.French LA, Galicich JH. The use of steroids for control of cerebral edema. Clin. Neurosurg. 1964;10:212–223. doi: 10.1093/neurosurgery/10.cn_suppl_1.212. [DOI] [PubMed] [Google Scholar]
- 6.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39(2):235–250. doi: 10.1097/00006123-199608000-00001. discussion 250-232. [DOI] [PubMed] [Google Scholar]
- 7.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl Acad. Sci. USA. 2006;103(14):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowska A, Symons M. Signaling determinants of glioma cell invasion. Adv. Exp. Med. Biol. 2013;986:121–141. doi: 10.1007/978-94-007-4719-7_7. [DOI] [PubMed] [Google Scholar]
- 9.Arismendi-Morillo G, Castellano A. Tumoral micro-blood vessels and vascular microenvironment in human astrocytic tumors. A transmission electron microscopy study. J. Neurooncol. 2005;73(3):211–217. doi: 10.1007/s11060-004-5674-3. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain MC, Cloughsey T, Reardon DA, Wen PY. A novel treatment for glioblastoma: integrin inhibition. Expert Rev. Neurother. 2012;12(4):421–435. doi: 10.1586/ern.11.188. [DOI] [PubMed] [Google Scholar]
- 11.Yong RL, Lonser RR. Surgery for glioblastoma multiforme: striking a balance. World Neurosurg. 2011;76(6):528–530. doi: 10.1016/j.wneu.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanai N, Polley MY, Mcdermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 13.Markert J, Devita V, Hellman S, Rosenberg S. Glioblastoma Multiforme. MA, USA: Jones and Bartlett Publishers; 2005. [Google Scholar]
- 14.Stupp R, Newlands E. New approaches for temozolomide therapy: use in newly diagnosed glioma. Semin. Oncol. 2001;28(4 Suppl. 13):S19–S23. doi: 10.1016/s0093-7754(01)90067-3. [DOI] [PubMed] [Google Scholar]
- 15.Barker CA, Chang M, Chou JF, et al. Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J. Neurooncol. 2012;109(2):391–397. doi: 10.1007/s11060-012-0906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 17.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008;26(18):3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- 19.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J. Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 20.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desjardins A, Sampson JH. Avastin: more questions than answers. J. Neurosurg. 2012;116(2):336–340. doi: 10.3171/2011.8.JNS111107. discussion 340. [DOI] [PubMed] [Google Scholar]
- 22.Mcgirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J. Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bonis P, Anile C, Pompucci A, et al. Safety and efficacy of gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir. 2012;154(8):1371–1378. doi: 10.1007/s00701-012-1413-2. [DOI] [PubMed] [Google Scholar]
- 24.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit. Rev. Oncol. Hematol. 2008;67(2):139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Rhys. 1989;16(6):1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 26.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch. Neurol. 2010;67(3):279–283. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 27.Matsukado Y, Maccarty CS, Kernohan JW. The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J. Neurosurg. 1961;18:636–644. doi: 10.3171/jns.1961.18.5.0636. [DOI] [PubMed] [Google Scholar]
- 28.Sampetrean O, Saga I, Nakanishi M, et al. Invasion precedes tumor mass formation in a malignant brain tumor model of genetically modified neural stem cells. Neoplasia. 2011;13(9):784–791. doi: 10.1593/neo.11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell O, Krebs B, Wagner E, et al. Expression of integrin αvβ3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18(3):378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60(3):502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 31.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin α6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veeravalli KK, Ponnala S, Chetty C, Tsung AJ, Gujrati M, Rao JS. Integrin α9β1-mediated cell migration in glioblastoma via SSAT and Kir4.2 potassium channel pathway. Cell Signal. 2012;24(1):272–281. doi: 10.1016/j.cellsig.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinkova E, Maglott A, Leger DY, et al. α5β1 integrin antagonists reduce chemotherapy-induced premature senescence and facilitate apoptosis in human glioblastoma cells. Int. J. Cancer. 2010;127(5):1240–1248. doi: 10.1002/ijc.25187. [DOI] [PubMed] [Google Scholar]
- 34.Tabatabai G, Weller M, Nabors B, et al. Targeting integrins in malignant glioma. Targeted Oncol. 2010;5(3):175–181. doi: 10.1007/s11523-010-0156-3. [DOI] [PubMed] [Google Scholar]
- 35.Chintala SK, Sawaya R, Gokaslan ZL, Fuller G, Rao JS. Immunohistochemical localization of extracellular matrix proteins in human glioma, both in vivo and in vitro. Cancer Lett. 1996;101(1):107–114. doi: 10.1016/0304-3835(96)04124-9. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi M, Ohnishi T, Arita N, Hiraga S, Hayakawa T. Expression of tenascin in human gliomas: its relation to histological malignancy, tumor dedifferentiation and angiogenesis. Acta Neuropathol. 1993;85(5):481–487. doi: 10.1007/BF00230486. [DOI] [PubMed] [Google Scholar]
- 37.Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am. J. Pathol. 2005;167(5):1379–1387. doi: 10.1016/S0002-9440(10)61225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei KC, Huang CY, Chen PY, et al. Evaluation of the prognostic value of CD44 in glioblastoma multiforme. Anticancer Res. 2010;30(1):253–259. [PubMed] [Google Scholar]
- 39.Janouskova H, Maglott A, Leger DY, et al. Integrin α5βl plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012;72(14):3463–3470. doi: 10.1158/0008-5472.CAN-11-4199. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta S, Nandi S, Hindi ES, Wainwright DA, Han Y, Lesniak MS. Short hairpin RNA-mediated fibronectin knockdown delays tumor growth in a mouse glioma model. Neoplasia. 2010;12(10):837–847. doi: 10.1593/neo.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada S, Bu XY, Khankaldyyan V, Gonzales-Gomez I, McComb JG, Laug WE. Effect of the angiogenesis inhibitor cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59(6):1304–1312. doi: 10.1227/01.NEU.0000245622.70344.BE. discussion 1312. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert MR, Kuhn J, Lamborn KR, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a Phase II trial with measures of treatment delivery. J. Neurooncol. 2012;106(1):147–153. doi: 10.1007/s11060-011-0650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabors LB, Mikkelsen T, Hegi ME, et al. A safety run-in and randomized Phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306) Cancer. 2012;118(22):5601–5607. doi: 10.1002/cncr.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foty RA, Corbett SA, Schwarzbauer JE, Steinberg MS. Dexamethasone up-regulates cadherin expression and cohesion of HT-1080 human fibrosarcoma cells. Cancer Res. 1998;58(16):3586–3589. [PubMed] [Google Scholar]
- 45.Brenner KA, Corbett SA, Schwarzbauer JE. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene. 2000;19(28):3156–3163. doi: 10.1038/sj.onc.1203626. [DOI] [PubMed] [Google Scholar]
- 46.Foty RA, Forgacs G, Pfleger CM, Steinberg MS. Liquid properties of embryonic tissues: measurement of interfacial tensions. Phys. Rev. Lett. 1994;72(14):2298–2301. doi: 10.1103/PhysRevLett.72.2298. [DOI] [PubMed] [Google Scholar]
- 47.Robinson EE, Zazzali KM, Corbett SA, Foty RA. α5β1 integrin mediates strong tissue cohesion. J. Cell Sci. 2003;116(Pt 2):377–386. doi: 10.1242/jcs.00231. [DOI] [PubMed] [Google Scholar]
- 48.Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates α5β1-mediated cell cohesion. Mol. Biol. Cell. 2004;15(3):973–981. doi: 10.1091/mbc.E03-07-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia D, Entersz I, Butler C, Foty RA. Fibronectin matrix-mediated cohesion suppresses invasion of prostate cancer cells. BMC Cancer. 2012;12:94. doi: 10.1186/1471-2407-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabari J, Lax D, Connors D, et al. Fibronectin matrix assembly suppresses dispersal of glioblastoma cells. PLoS One. 2011;6(9):e24810. doi: 10.1371/journal.pone.0024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg MS. Adhesion in development: an historical overview. Dev. Biol. 1996;180(2):377–388. doi: 10.1006/dbio.1996.0312. [DOI] [PubMed] [Google Scholar]
- 52.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122(5):1611–1620. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 53.Davies JT, Rideal EK. Interfacial Phenomena. NY, USA: Academic Press; 1963. [Google Scholar]
- 54.Davis GS, Phillips HM, Steinberg MS. Germ-layer surface tensions and “tissue affinities” in Rana pipiens gasttulae: quantitative measurements. Dev. Biol. 1997;192(2):630–644. doi: 10.1006/dbio.1997.8741. [DOI] [PubMed] [Google Scholar]
- 55.Schotz EM, Burdine RD, Julicher F, Steinberg MS, Heisenberg CP, Foty RA. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J. 2008;2(1):42–56. doi: 10.2976/1.2834817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz MA, Zheng H, Legan S, Foty RA. Lung self-assembly is modulated by tissue surface tensions. Am. J. Respir. Cell Mol. Biol. 2011;44(5):682–691. doi: 10.1165/rcmb.2009-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 2005;278(1):255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Foty RA, Steinberg MS. Measurement of tumor cell cohesion and suppression of invasion by E- or P-cadherin. Cancer Res. 1997;57(22):5033–5036. [PubMed] [Google Scholar]
- 59.Winters BS, Shepard SR, Foty RA. Biophysical measurement of brain tumor cohesion. Int. J. Cancer. 2005;114(3):371–379. doi: 10.1002/ijc.20722. [DOI] [PubMed] [Google Scholar]
- 60.Shafrin EG, Zisman WA. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J. Phys. Chem. 1960;64(5):519–524. [Google Scholar]
- 61.Ryan PL, Foty RA, Kohn J, Steinberg MS. Tissue spreading on implantable substrates is a competitive outcome of cell-cell vs cell-substratum adhesivity. Proc. Natl Acad. Sci. USA. 2001;98(8):4323–4327. doi: 10.1073/pnas.071615398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Douezan S, Guevorkian K, Naouar R, Dufour S, Cuvelier D, Brochard-Wyart F. Spreading dynamics and wetting transition of cellular aggregates. Proc. Natl Acad. Sci. USA. 2011;108(18):7315–7320. doi: 10.1073/pnas.1018057108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Utsuki S, Sato Y, Oka H, Tsuchiya B, Suzuki S, Fujii K. Relationship between the expression of E-, N-cadherins and β-catenin and tumor grade in astrocytomas. J. Neurooncol. 2002;57(3):187–192. doi: 10.1023/a:1015720220602. [DOI] [PubMed] [Google Scholar]
- 64.Maret D, Gruzglin E, Sadr MS, et al. Surface expression of precursor N-cadherin promotes tumor cell invasion. Neoplasia. 2010;12(12):1066–1080. doi: 10.1593/neo.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cifarelli CP, Titus B, Yeoh HK. Cadherin-dependent adhesion of human U373MG glioblastoma cells promotes neurite outgrowth and increases migratory capacity. Laboratory investigation. J. Neurosurg. 2011;114(3):663–669. doi: 10.3171/2010.3.JNS091451. [DOI] [PubMed] [Google Scholar]
- 66.Tate CC, Garcia AJ, Laplaca MC. Plasma fibronectin is neuroprotective following traumatic brain injury. Exp. Neurol. 2007;207(1):13–22. doi: 10.1016/j.expneurol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Mckeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 1985;100(2):364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 1993;122(3):729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Ulrich TA, De Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010;22(5):697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integr. Biol. 2011;3(4):267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 74.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA. 2012;109(26):10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf K, Wu YI, Liu Y, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9(8):893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 76.Colbert MJ, Brochard-Wyart F, Fradin C, Dalnoki-Veress K. Squeezing and detachment of living cells. Biophys. J. 2010;99(11):3555–3562. doi: 10.1016/j.bpj.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P. The role of myosin II in glioma invasion of the brain. Mol. Biol. Cell. 2008;19(8):3357–3368. doi: 10.1091/mbc.E08-03-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr. Opin. Oncol. 2004;16(6):593–600. doi: 10.1097/01.cco.0000142076.52721.b3. [DOI] [PubMed] [Google Scholar]
- 79.Manning ML, Foty RA, Steinberg MS, Schoetz EM. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl Acad. Sci. USA. 2010;107(28):12517–12522. doi: 10.1073/pnas.1003743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin SH, Kleinberg LR. Carmustine wafers: localized delivery of chemotherapeutic agents in CNS malignancies. Expert Rev. Anticancer Ther. 2008;8(3):343–359. doi: 10.1586/14737140.8.3.343. [DOI] [PubMed] [Google Scholar]
- 81.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal Interact. 2007;7(4):335. [PubMed] [Google Scholar]