Abstract
Head movement during functional magnetic resonance imaging (fMRI) degrades data quality. The effects of small movements can be ameliorated during data postprocessing, but data associated with severe movement is frequently discarded. In discarding these data, it is often assumed that head‐movement is a source of random error, and that data can be discarded from subjects with severe movement without biasing the sample. We tested this assumption by examining whether head movement was related to task difficulty and cognitive status among persons with multiple sclerosis (MS). Thirty‐four persons with MS were scanned while performing a working memory task with three levels of difficulty (the N‐back task). Maximum movement (angle, shift) was estimated for each difficulty level. Cognitive status was assessed by combining performance on a working memory and processing speed task. An interaction was found between task difficulty and cognitive status (high vs. low cognitive ability): there was a linear increase in movement as task difficulty increased that was larger among subjects with lower cognitive ability. Analyses of the signal‐to‐noise ratio (SNR) confirmed that increases in movement degraded data quality. Similar, though far smaller, effects were found in a cohort of healthy control (HC) subjects. Therefore, discarding data with severe movement artifact may bias MS samples such that only those with less‐severe cognitive impairment are included in the analyses. However, even if such data are not discarded outright, subjects who move more (MS and HC) will contribute less to the group‐level results because of degraded SNR. Hum Brain Mapp 35:1–13, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: fMRI, head movement, multiple sclerosis
INTRODUCTION
In the analysis of functional magnetic resonance imaging (fMRI) data, perhaps the single largest factor that degrades data quality is subject motion. This is because when a subject moves his/her head during a scan, one of the fundamental assumptions underlying fMRI data analysis is violated—the assumption that a given voxel corresponds to a given volume of brain tissue across time [see Friston et al., 1996 for a review]. This assumption is critical because in fMRI data analysis, we wish to ascribe variance in the signal from each voxel to our experimental manipulation(s). However, if a given voxel corresponds to one location in the brain at Time 1 and a different location at Time 2, then there are at least two sources of variance in the data: the experimental manipulation and subject motion. To ascribe changes in the blood oxygen level dependent (BOLD) signal to the experimental manipulation(s), it is therefore necessary to ensure that subject motion accounts for little to none of the variance in the data. If this is not done, if data is included in the analyses that have been minimally corrected for motion, the results become unreliable [e.g., Power et al., 2012; Van Dijk et al., 2012]. This is not only because there are two sources of variance, but also because the changes in the BOLD signal associated with movement can be far larger than changes associated with the experimental manipulation. Thus, movement‐related changes can “swamp” changes associated with the experimental paradigm.
In the functional neuroimaging literature, three ways have been proposed and used to minimize the contribution of head motion to variance in the data. One method is to use restraints that make movement difficult [Fitzsimmons et al., 1997; Green et al., 1994]. Nearly all fMRI studies in the literature use restraints such as foam pads that are inserted around the subject's head to help the subject remain still. While these are useful, they do not completely eliminate movement; their value is largely in allowing subjects to feel when they are moving, thereby allowing compliant subjects to remain still. A more invasive method is to use a bite‐bar. This is a device that is anchored to the head‐coil, and that subjects hold in their jaws. While it is very effective in limiting head motion, it is also perceived by some to be aversive and uncomfortable, limiting its utility; this is particularly so for clinical samples.
Another method that is being developed is to measure head motion in real time and to either adjust scanning to account for this motion [Derbyshire et al., 1998; Mathiak et al., 2001; Speck et al., 2006; Thesen et al., 2000; Welch et al., 2002] or to use this information retrospectively to correct for head motion [Tremblay et al., 2005]. Finally, motion can be corrected retrospectively, during image processing [Biswal and Hyde, 1997; Ciulla and Deek, 2002; Friston et al., 1996; Hajnal et al., 1995; Woods et al., 1992]. Several algorithms have been developed for this sort of “motion correction,” but the central approach is largely the same: a canonical image is chosen, and every other image in the time‐series is compared to that canonical image. The extent to which each image differs is quantified in at least six parameters (three angular deviations: roll, pitch and yaw; three translational deviations: shifts in the right/left, posterior/anterior, and superior/inferior dimensions), and corrected by applying a rigid‐body transformation. While this approach has proven very useful for minimizing the effects of small amounts of motion on the BOLD signal, it is less reliable when there are large deviations in the data [Tremblay et al. 2005]. While this problem is difficult in the X and Y directions (i.e., movement that is parallel to the slice acquisition plane), it is nearly impossible in the Z direction (i.e., across slices) because of spin history effects (i.e., it is impossible to know what the data would have been, if it had been acquired at a different time). It is therefore common practice to exclude (discard) data in which movement exceeds ∼1–2 mm, which translates to <1° in angular deviation, and less than one voxel (usually ∼3 × 3 × 3 mm or ∼27 mm3) in translational deviation.
While it is unquestionably good practice to exclude data with excessive motion artifact, there are several potential disadvantages. For example, if there is a systematic relationship between excessive motion and task difficulty (i.e., if subjects tend to move more during more difficult tasks), then the removal of blocks with excessive motion will result in the removal of data from the most difficult conditions, resulting in sampling bias. Moreover, if subjects who tend to move more are systematically different from those who do not (e.g., if they have a lower IQ), then the removal of subjects with excessive motion will result in the removal of subjects with this difference (lower IQ), again introducing sampling bias. Generally, when data is excluded, it is assumed that head movement is random, and not affected by task difficulty or by subjects' cognitive abilities.
Although head motion is common in typical healthy individuals (a recent study on over 1,000 healthy subjects indicated a range of motion from 0.027 to 0.051 mm [Van Dijk et al., 2012], the concern about inadvertently introducing sampling bias when subjects with excessive motion are excluded is stronger when clinical populations are studied. Indeed, motion has been shown to be a problem in fMRI studies of neuropsychiatric populations including multiple sclerosis [Phillips, 2008], traumatic brain injury [for review, see Hillary et al., 2002], stroke [Seto et al., 2001], epilepsy [Lemieux et al., 2007], and schizophrenia [Weinberger et al., 1996]. In addition to clinical samples, studies involving pediatric samples are affected by greater head movement as children are less able to remain still compared to adults [Evans et al., 2010; Yuan et al., 2009]. Despite this, it has not been universally found that head motion is greater in clinical populations. For example, Yoo et al. [Yoo et al., 2005] reported that there was very little head motion in a group of individuals with schizophrenia, and that their head motion was no greater than that seen in a matched group of healthy controls. While this result is reassuring, it is not clear that it is representative of other clinical populations (e.g., multiple sclerosis), nor indeed whether it is generalizable beyond the group studied inasmuch as the sample was very small (n = 11).
Here, we investigated this issue in a cohort of subjects with multiple sclerosis (MS), using a working memory task (the n‐back task), with three levels of difficulty. We hypothesized that movement would be related to task difficulty in MS, based on the idea that the requirement to remain still in the fMRI scanner is similar to adding a second task to the experimental paradigm. It has been shown that when MS subjects must perform a demanding cognitive task while walking, their walking performance declines [Hamilton et al., 2009]. We hypothesized that the same would be true of the ability of MS subjects to remain still in the scanner. Moreover, previous fMRI research investigating differences in brain activation between MS and healthy controls (HCs) has shown that cognitive status moderates group differences. For example, Chiaravalloti et al. 2005 have shown that the activation in high‐functioning MS subjects was similar to HCs, while a lower‐functioning cohort of MS subjects showed a markedly different pattern. Therefore, we also hypothesized that the cognitive status of the MS subjects would moderate the effect of task difficulty on movement in the scanner. We tested these hypotheses by (1) comparing the extent of maximum motion (both in angular and translational deviation) across three levels of task difficulty (0‐, 1‐, 2‐back) in a group of MS subjects, and (2) by comparing the extent of maximum motion across task difficulty in two groups of MS subjects (high vs. low cognitive ability). We also examined the effect of task difficulty on maximum motion in a group of healthy control (HC) subjects.
METHODS
Subjects
There were two groups of subjects: MS and HC. The MS sample was comprised of 34 right‐handed persons (29 women) with MS [McDonald et al., 2001] recruited from local MS clinics and the North Jersey chapter of the National MS Society. Subjects were recruited if they (a) did not have an exacerbation of their MS during the last 4 weeks, (b) were not currently taking corticosteroid medication, (c) were not currently under the care of a physician for any other major medical condition, and (d) had no history of serious psychiatric illness or other neurologic disease other than MS. English was the primary language of all subjects. Mean age was 44.3 (SD = 7.6) years with 15.9 (SD = 2.4) years of education. Mean disease duration was 10.1 (SD = 6.8) years, and MS course included relapsing‐remitting (n = 26), secondary progressive (n = 6) and primary progressive (n = 2).
MS disease severity was mild‐to‐moderate, as indicated by a mean Hauser ambulation index (AI; [Hauser et al., 1983]) score of 2.2 ± 2.3 (range: 0–8). The AI is the ambulation component of the multiple sclerosis functional composite (MSFC) [Cutter et al., 1999], and is highly correlated with other clinical markers of MS disease progression (e.g., correlation of 0.88 with EDSS; [Sumowski et al., 2009]). In terms of the other components of the MSFC, the mean paced auditory serial addition task (PASAT) [Gronwall, 1977] z‐score was −0.52 (1.1) and the mean 9‐Hole Peg Test [Goodkin et al., 1988] z‐score was 0.19 (0.56).
The HC sample was comprised of 20 healthy right‐handed persons (13 women) recruited from the community. These subjects had no history of major medical or psychiatric illness, and English was the primary language of all subjects. Mean age was 30.1 (SD = 6.5) years with 17.2 (SD = 1.2) years of education.
The institutional review boards at UMDNJ and the Kessler Foundation Research Center granted approval for the study. Informed consent was obtained from all subjects prior to participation.
Apparatus and Tasks
During each series, one of three levels of the visual N‐Back working memory task was run: 0‐Back (lowest demand); 1‐Back (intermediate demand); 2‐Back (highest demand). The three tasks were presented in a counterbalanced block design. Each series began with a 28‐s block of rest followed by three repetitions of task (32 s of 0‐Back, 1‐Back, or 2‐Back, in separate series) and rest (32 s). During the 0‐Back task, participants viewed a series of letters, presented one at a time, and pressed a button when a target letter (e.g., “K”) was presented. During the 1‐Back task, participants viewed a different (randomly generated) series of letters, and responded when any letter was the same as the letter immediately preceding it in the series (e.g., “R C K K”). During the 2‐Back task, participants responded when any letter was the same as the letter presented two letters prior in the series (e.g., “R K C K”). Stimuli were presented with the E‐Prime presentation software, which also recorded participants' behavioral performance (accuracy and reaction time [RT]).
Imaging Data and Analyses
The fMRI blood oxygen level dependent (BOLD) signal was acquired in a 3T Siemens Allegra MRI scanner. Three functional acquisition series were collected, each of 115 images (echo time = 30 ms; repetition time = 2,000 ms; field of view = 22 cm; flip angle = 80°; slice thickness = 4 mm, matrix = 64 × 64, in‐plane resolution = 3.438 × 3.438 mm2). The first five images of each series were discarded, allowing magnetization to reach a steady state. A high‐resolution magnetization prepared rapid gradient echo (MPRAGE) image was also acquired (TE = 4.38 ms; TR = 2,000 ms, FOV = 220 mm; flip angle = 8°; slice thickness = 1 mm, NEX = 1, matrix = 256 × 256, in‐plane resolution = 0.859 × 0.859 mm2), and was used to normalize the functional data into standard space.
For each subject, the fMRI data were realigned to correct for subject motion using the 3dvolreg program in the AFNI suite of imaging analysis tools. Fourier interpolation was used, and all images for each subject were realigned to the 10th image in the 0‐Back time‐series. The extent to which each image had to be moved in order for it to be in the same spatial location as the canonical image was recorded. No additional options were used in the realignment. The data were then smoothed (8 mm3 FWHM), scaled, deconvolved, and warped into standard space. The deconvolution used a delayed boxcar function to model the hemodynamic response. The model included regressors for each task (0‐Back, 1‐Back, 2‐Back), as well as nine regressors of no interest: the six movement parameters and three polynomial regressors. The six movement parameters were the shifts in each of the orthogonal directions (right/left, anterior/posterior, superior/inferior) and angular rotations in each of the orthogonal directions (roll, pitch, yaw). The three polynomial regressors accounted for low‐frequency signal drift during the scan. The signal to noise ratio (SNR) was calculated by dividing the estimate of baseline activity for each time‐series (the signal) by the standard deviation of the residual error (the variance in the data not accounted for by the model used in the deconvolution). The voxels in the resulting image were averaged to arrive at an estimate of the SNR for each subject. The steps outlined above were done for all subjects, regardless of whether they had moved an excessive amount or not. Of the 34 MS subjects, 12 moved >1° (movers) while the rest moved less than this (nonmovers).
RESULTS
Behavior in the MS Group
To assess cognitive functioning, the symbol‐digit modalities test (SDMT) and the paced auditory serial addition task (PASAT) were administered from the minimal assessment of cognitive functioning in MS [MACFIMS; Benedict et al., 2002]. Each subject's score on these tests was converted into a z‐score based on published normative data [Benedict et al., 2006], and the mean of these z‐scores was used as a summary measure of information processing efficiency [for a similar approach, see Sumowski et al., 2009]. For the present MS sample, the z‐score for this summary measure of processing speed was −0.72 (SD = 1.07), which corresponds to the 24th percentile (the z‐score for the SDMT was −1.03 (SD = 1.52), the z‐score for the PASAT was −0.41 (SD = 0.84)). Consistent with previous research [for review, Chiaravalloti and DeLuca, 2008], information processing efficiency in the current sample of persons with MS was below average.
Motion in the MS Group
The motion parameters (angle and shift) were analyzed with repeated measures, one‐way ANOVAs. The factor was task difficulty (0‐Back, 1‐Back, 2‐Back). For angular motion, there was a significant linear effect of task difficulty (F(1,33) = 12.59, P = 0.001, η2 = 0.28). The extent of angular motion increased from 0.65° in the 0‐Back task, to 0.96° in the 1 Back task, to 1.31° in the 2 Back task. Pairwise comparisons showed that all three conditions reliably differed from one another (Ps < 0.05). For translational motion (shift), there was also a significant linear effect of task difficulty (F(1,33) = 20.96, P < 0.0001, η2 = 0.39). As in the case of angular motion, translational motion increased from 0.89 mm in the 0‐Back task, to 1.27 mm in the 1‐Back task, to 1.47 mm in the 2‐Back task. Pairwise comparisons showed that the extent of translational motion in the 0‐Back task was reliably less than in the 1‐Back or 2‐Back tasks (Ps < 0.005), but the difference between 1‐Back and 2‐Back only trended toward significance (P = 0.10).
Motion as a Function of Cognitive Impairment in the MS Group
To assess the effect of cognitive ability on motion in the scanner, the MS group was divided into two groups, based on a median split, using the information processing efficiency z‐score: those with higher information processing efficiency [17 subjects; mean z = 0.16 (SD = 0.60)] and those with lower efficiency [17 subjects; mean z = −1.60 (SD = 0.59)]. We will refer to these groups as those with higher cognitive abilities (cog+) and those with lower cognitive abilities (cog−). The two groups did not differ on age or education, but they were reliably different on their information processing efficiency score (t(32) = −8.72, P < 0.0001; see Table 1). Moreover, the groups did not differ in disease duration, and the distribution of disease type in the two groups was exactly equal, with 13 relapsing‐remitting, 1 primary progressive, and 3 secondary progressive in each group.
Table 1.
The demographics of the MS sample
| Age (years) | Education (years) | Disease duration (years) | Information proc. efficiency (z‐score) | |
|---|---|---|---|---|
| MS Cog+ | 45.3 ± 7.8 | 16.5 ± 2.1 | 9.1 ± 7.5 | 0.2 ± 0.6 |
| MS Cog− | 43.3 ± 7.5 | 15.2 ± 2.6 | 10.2 ± 6.2 | −1.6 ± 0.6 |
The motion parameters (angle and shift) were analyzed with mixed repeated measures ANOVAs. The within‐subjects factor was task difficulty (0‐Back, 1‐Back, 2‐Back) and the between subjects factor was group (cog− vs. cog+). For angular motion, the interaction between task difficulty and group was significant (F(1,32) = 6.90, P = 0.01, η2 = 0.18). This can be seen in Figure 1. The angular motion for the cog− group increased dramatically as task difficulty increased: 0.71°, 1.32°, 1.82° for 0‐Back, 1‐Back, and 2‐Back, respectively. The effect of task difficulty on motion was considerably less for the cog+ group: 0.59°, 0.60°, 0.80° for 0‐Back, 1‐Back, and 2‐Back, respectively. For translational motion (shift), a similar pattern emerged (see Fig. 1): there was a reliable interaction between task difficulty and group (F(1,32) = 3.99, P = 0.05, η2 = 0.11). Both groups moved approximately the same amount in the easiest condition (0‐Back; 0.91 vs. 0.86 mm for the cog− and cog+ groups, respectively), but as the task difficulty increased, the cog− group moved more than the cog+ group: 1.38 vs. 1.16 in the 1‐Back task, and 1.74 vs. 1.20 mm in the 2‐Back task, for the cog− and cog+ groups, respectively.
Figure 1.
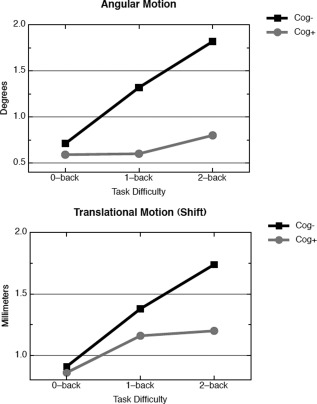
Angular (upper panel) and translational motion (lower panel) in the MS sample, divided into cog− (black squares) and cog+ subjects (gray circles). For angular motion, the cog− group differed between 0‐Back and 1‐Back (d = 0.64, P < 0.05) and between 0‐Back and 2‐Back (d = 0.096, P < 0.01); the cog+ group differed between 0‐Back and 2‐Back only (d = 0.66, P < 0.05). For translational motion, the cog− group differed between 0‐Back and 1‐Back (d = 0.80, P < 0.01), 1‐Back and 2‐Back (d = 0.61, P < 0.05), and between 0‐Back and 2‐Back (d = 1.20, P < 0.001); the cog+ group differed between 0‐Back and 2‐Back only (d = 0.54, P < 0.05).
Correlations Between Motion and Cognitive Impairment in the MS Group
To assess this relationship without dichotomizing the MS group, we ran partial correlations between cognitive status and the six movement parameters, controlling for brain atrophy. There was no relationship between cognitive status and angular movement during low cognitive demands (0‐Back, r p = −0.10, P > 0.5), but worse cognitive status was associated with more angular movement when cognitive demands increased during the 1‐Back (r p = −0.43, P = 0.01) and 2‐Back (r p = −0.48, P =0.005). That is, as expected, the inverse relationship between cognitive status and angular movement increased as cognitive task demands increased. A similar relationship between cognitive status and shift was not observed (r ps = 0.00, 0.02, −0.20, all Ps > 0.10), perhaps due to lesser variance in shift relative to angular movement.
Correlations Between Motion and Signal‐to‐Noise Ratio in the MS Group
For each level of task difficulty (0‐Back, 1‐Back, 2‐Back), the two motion parameters (angle and shift) were correlated with the signal‐to‐noise ratio (SNR) from the appropriate run (0‐Back, 1‐Back, 2‐Back). As Figure 2 and Table 2 show, there were significant negative correlations between SNR and both angular and translational (shift) movement. This was true for the MS sample as a whole, and also for each group, though the relationship was stronger for the cog+ group. The SNR did not differ between the cog+ and cog− groups at any level of task difficulty, and the stronger relationship in the cog+ group was due to more variance in the SNR in that group at every level of task difficulty (standard deviations for the cog+ and cog− groups at each level of task difficulty were as follows: 0‐Back: 56.56 vs. 45.64; 1‐Back: 56.61 vs. 45.65; 2‐Back: 57.06 vs. 44.85). As expected, the negative correlations show that as subjects moved more, the ratio of signal to noise in their data decreased.
Figure 2.
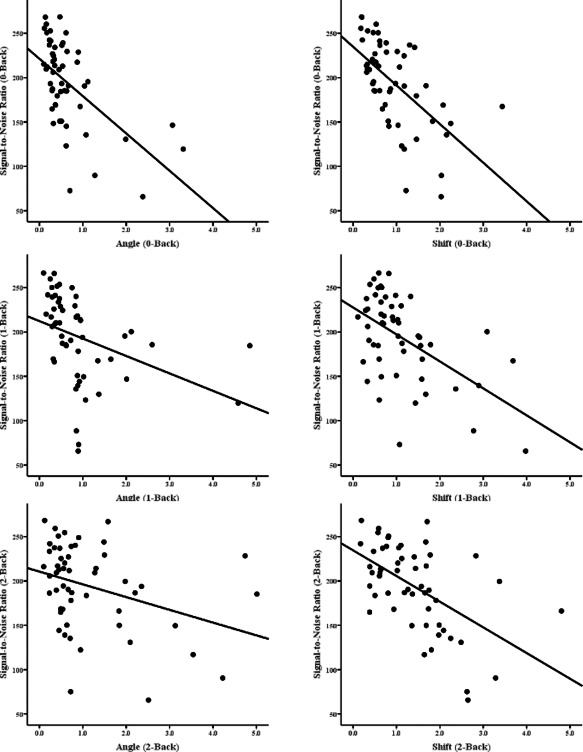
Scatterplots showing signal‐to‐noise ratio (SNR) in the MS groups. The panels on the left show SNR vs. angular motion in the 0‐Back task (upper left), 1‐Back task (middle‐left), and 2‐back task (lower‐left). The panels on the right show SNR vs. translational (shift) motion in each of the levels of the task (0‐, 1‐, 2‐Back in the upper, middle, and lower panels respectively).
Table 2.
The correlation between the signal‐to‐noise ratio and the movement parameters (angle and shift) for each level of task difficulty (0‐Back, 1‐Back, 2‐Back)
| 0‐Back | 1‐Back | 2‐Back | ||||
|---|---|---|---|---|---|---|
| Angle | Shift | Angle | Shift | Angle | Shift | |
| MS | ||||||
| Cog+ | −0.80b | −0.74b | −0.74b | −0.74b | −0.51a | −0.75b |
| Cog− | −0.67b | −0.57a | −0.52a | −0.66b | −0.56a | −0.51a |
| All | −0.70b | −0.64b | −0.40a | −0.70b | −0.42a | −0.55b |
| HC | ||||||
| All | −0.42c | −0.49a | −0.43c | −0.46a | −0.35 | −0.70a |
P < 0.05 (two‐tailed tests in all cases).
**P < 0.01 (two‐tailed tests in all cases).
P < 0.1 (two‐tailed tests in all cases).
fMRI Activation in the MS Group
To better understand the effect of motion on our data, we looked at the functional data, and because the 2‐Back condition was the condition most severely affected, we used this condition to guide our subsequent analyses. A t‐ test was conducted, comparing those who moved 1° in angular motion or more (movers) to those who moved <1° (nonmovers) during the 2‐Back task. The results showed a single area where the movers group showed less activity than the nonmovers group: left middle frontal gyrus, X Y Z = −48 22 34, at P < 0.01 (corrected for multiple comparisons with clustering threshold of 10 contiguous voxels in the original acquisition space).
Random Combinatorial Analysis in the MS Group
The analysis of the beta weights (above) showed there to be a difference between the movers and the nonmovers. However, this difference could be due to several factors (e.g., lower signal to noise in the movers group because of motion, less activity in the movers group because of lower cognitive status, less activity in the movers group simply because of random chance). We therefore conducted a combinatorial analysis in which we first extracted the average beta weight from a sphere (radius = 10 mm), placed over the frontal area that distinguished to two groups. This was done for each subject, in each condition (0‐Back, 1‐Back, 2‐Back). The average signal (beta weight) for 1,000,000 different (random) combinations of 12 subjects was then computed and stored.
We reasoned that if the difference in the beta weights between the 12 movers and the 22 nonmovers was due simply to chance, then there should be no systematic relationship between the number of movers in the subsample of 12 and the average signal. That is, while it was to be expected that the average signal from the 12 movers would be relatively low, if this were due simply to chance, then one would expect subsamples of 12 of the nonmovers to have equally low average signal. However, if the difference were due to either motion or to cognitive status, then having more movers in the sample would be expected to systematically result in lower average signal. In this case, if the 12 were comprised completely of movers, the signal should be poor; if all 12 were drawn from the remaining 22 subjects (the nonmovers), the signal should be good; and for combinations of subjects that were comprised of a mixture of movers and nonmovers, the signal should be somewhere between these two extremes. While having a propensity to move and having a lower cognitive status are confounded in the 2‐Back condition, they are not in the 0‐Back and 1‐Back conditions: it was only in the 2‐Back that those with lower cognitive status moved an unacceptable amount. We therefore performed the combinatorial analysis on all three levels of the N‐back data. The more movers there were in the sample of 12, the lower we expected the average signal to be because those in the movers group had lower cognitive status. However, we expected the lower signal to noise associated with movement itself to be most evident in the 2‐Back condition.
The results can be seen in Figure 3. For all three conditions, there was a strong relationship between the proportion of movers and the signal: the more movers there were in the sample, the lower the signal. Moreover, this effect appears to be more pronounced in the 2‐Back condition than for either the 0‐Back or the 1‐Back conditions. These observations were subjected to formal analysis with a multiple regression. The factors were mover‐proportion (with 12 levels) and N‐back (0‐back, 1‐back, 2‐back). The main effect of both mover‐proportion and N‐back were reliable, as was the interaction of their slopes (t = −81.81, P < 0.0001). When only 0‐back and 1‐back were included in the analysis, the interaction was no longer significant (t = −1.63, P = 0.10), though both the main effects were highly significant: mover‐proportion (t = −274.88, P < 0.0001), N‐back (t = 298.37, P < 0.0001). When only 1‐Back and 2‐Back were included in the analysis, the interaction was once again highly reliable (t = −75.95, P < 0.0001). This was because the slope of the regression line for the data from the 2‐Back was larger (more negative) than for the 1‐Back.
Figure 3.
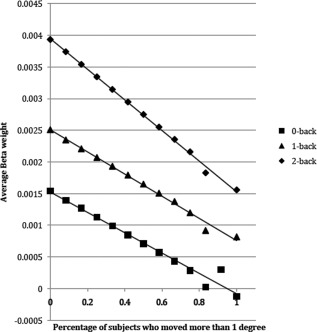
The average activity in an ROI placed in the middle frontal gyrus, as a function of task load (0‐back, 1‐back, 2‐back) and the number of subjects who moved >1° during the 2‐Back condition.
The Effect of Excluding Movers From the Sample
Given the relationship between cognitive status and movement reported above, we predicted that exclusion of MS subjects with greater movement (movers) would bias the sample toward MS subjects with higher cognitive ability. That is, subjects with cognitive impairment would be underrepresented. Investigating this directly, we found that MS subjects with greater movement (movers: subjects with angular movement > 1° on 2‐Back, N = 12) had worse cognitive status than nonmovers (t(32) = 1.913, P = 0.032, one‐tailed). Of note, the effect size of this relationship was medium‐to‐large (d = 0.71). We also investigated this using a Chi‐square analysis in which we tested whether the proportion of patients with and without cognitive impairment would differ after excluding movers from the sample. Our total sample was equally divided between patients with and without cognitive impairment (Ns = 17); however, after excluding patients with excessive movement, there was a significantly greater proportion of cognitively intact (N = 14, 64%) than cognitively impaired (N = 8, 36%) patients in the remaining sample (χ2 = 4.64, P = 0.031). That is, disproportionately more patients with cognitive impairment would be excluded from final fMRI analyses based on excessive movement, thereby biasing the sample away from cognitive impairment.
Analysis of Motion in the HC Group
The motion parameters (angle and shift) were analyzed with repeated measures, one‐way ANOVAs. The factor was task difficulty (0‐Back, 1‐Back, 2‐Back). For angular motion, there was a significant linear effect of task difficulty (F(1,19) = 9.28, P < 0.01, η2 = 0.33). The extent of angular motion increased from 0.62° in the 0‐Back task, to 0.82° in the 1‐Back task, to 0.89° in the 2‐Back task. For translational motion (shift), there was also a linear effect of task difficulty (F(1,19) = 5.06, P < 0.04, η2 = 0.21). The translational motion (shift) was 1.0, 0.80, and 1.29 mm in the 0‐Back, 1‐Back, and 2‐Back tasks, respectively.
Correlations Between Motion and Signal‐to‐Noise Ratio in the HC Group
As for the MS group, correlations were calculated between the two motion parameters (angle and shift) and SNR, for each level of task difficulty (0‐Back, 1‐Back, 2‐Back). The results, shown in Table 2 and Figure 4, were similar to the correlations in the MS group, though weaker. In the HC group, there were reliable negative correlations between translational motion (shift) and SNR for each of the N‐Back conditions. However, for angular motion, the relationship only trended toward conventional levels of significance. As in the MS group, the negative correlations showed that the more subjects moved, the less signal there was in their data, relative to the noise.
Figure 4.
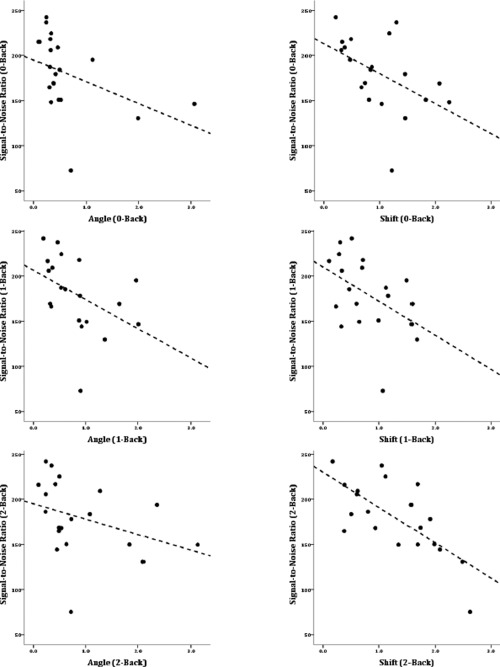
Scatterplots showing signal‐to‐noise ratio (SNR) in the HC group. The panels on the left show SNR vs. angular motion in the 0‐Back task (upper left), 1‐Back task (middle‐left) and 2‐Back task (lower‐left). The panels on the right show SNR vs. translational (shift) motion in each of the levels of the task (0‐, 1‐, 2‐Back in the upper, middle, and lower panels, respectively).
DISCUSSION
This study confirmed that in a clinical sample, such as MS, subjects do indeed move more as task difficulty increases. This shows that subject movement is not a random variable, but that it is related to the experimental manipulation. This is somewhat concerning, particularly if a strict cutoff of 1–2 mm [<1° of angular motion and less than one voxel (∼3 mm)] is used to determine which data to retain and which to discard. As a group, the MS sample moved as much as 1.31° (corresponding to ∼3.43 mm), and 1.47 mm (in the 2‐Back task).
More concerning are the results that emerged when the MS sample was divided into cog− and cog+ groups. In those analyses, it emerged that the cog− group moved far more than the cog+ group. This was true for both angular and translational (shift) motion, but was far more problematic for angular motion. In the 2‐Back task, the cog− group moved nearly 2° (1.82°, or ∼4.76 mm), which is far more than can be reliably corrected for with current image processing software. If these subjects were simply discarded, the sample would be strongly biased toward individuals with MS who have higher cognitive abilities. This would likely result in an underestimation of the effects of MS on brain functions.
One way to avoid the introduction of this sampling bias would be to correct for the motion as much as possible during image‐processing, and then to include the motion parameters in the deconvolution as regressors of no interest. This would minimize the effects of motion on the data (though it would by no means remove them entirely), and might allow some of the subjects who would otherwise be discarded to remain in the sample. However, while this approach works well for event‐related designs, it appears to decrease the sensitivity of the general linear model when block designs are used [Johnstone et al., 2006]. Moreover, the analyses of the SNR in the data presented here show this solution to be flawed as well: the more subjects move, the lower their SNR. This means that the results from subjects who moved very little are stronger than the results from subjects who moved more. This has the unfortunate result that the group‐level statistics will be skewed toward the subjects who moved less: the cog+ subjects. Thus, even if the data from subjects with a large amount of motion are not simply discarded, a bias remains in the group‐level data because of the higher SNR in the data from the subjects who moved less.
The random combinatorial analysis demonstrates the effect of including subjects with excessive motion in the sample. In the 2‐Back condition, 12 subjects moved >1°. As the data from these 12 movers was incrementally added to a subsample of 12 subjects, there was a systematic decrease in the strength of the signal. This was true for 0‐Back and 1‐Back, but was particularly marked for the 2‐Back condition. Because the 12 subjects who moved >1° in the 2‐Back condition (movers) were also all in the cog− group, adding them to the sample would be expected to result in decreased signal for two reasons: the signal from the cog‐ group might be expected to be less than that of the cog+ group, and the SNR would be expected to be less in this group because these 12 subjects moved. However, the difference in SNR should be worst in the 2‐Back condition, since that is where these subjects moved the most.
These data tell an important cautionary tale in relation to fMRI studies of clinical populations such as MS. However, a great many fMRI studies are conducted to better understand brain function in healthy populations. We therefore also assessed whether the motion parameters increase with task difficulty in healthy controls.
As with the MS sample, the HC group showed increasing motion as the task increased in difficulty. However, unlike the MS group, the mean amount of motion in the HC group never exceeded 1° of angular motion or one voxel of translational motion (shift). This is reassuring for those who investigate cognition in healthy samples. However, the fact that SNR was nevertheless correlated with motion (albeit only for translational motion) is concerning. Just as with the MS sample, this means that the results from those who move more will be weaker than the results from those who move less, and that any group‐level statistics will over‐represent those subjects who moved less in the scanner.
The purpose of these experiments was to empirically assess the concern that subject motion (in the scanner) is not a random variable, a concern that is particularly important in clinical samples [e.g., Hillary et al., 2002; Phillips, 2008]. The results suggest that motion is indeed a problem in clinical samples (in this case, MS), particularly in cog− group. If subjects with excessive motion were simply removed from the group‐level analyses, the excluded subjects would overwhelmingly be the cog− subjects. This would introduce sampling bias into the study because the subjects remaining in the group‐level analyses would be biased against cognitive impairment. Thus, any results would not represent MS subjects as a whole, but would rather represent MS subjects who had higher cognitive abilities. This would almost certainly lead to underestimations of the effect of MS on brain activity.
If discarding subjects with excessive motion results in sampling bias, would it be better to leave these subjects in the group‐level analyses (after attempting to mitigate the motion artifact by, for example, including the motion parameters in the deconvolution as regressors of no interest)? Unfortunately, there can be no simple answer to this question. Certainly, including data from subjects with significant motion artifact will not benefit the group‐level analyses: in avoiding sampling bias, spurious activation patterns (associated with motion artifact) would be included in the analyses. Moreover, even if only subjects with no obvious motion artifact are included in the group‐level analyses, the signal‐to‐noise ratio (SNR) will be less from those who moved more (i.e., the group with low cognitive ability). Thus, it is very difficult (though not impossible: see below) to escape from sampling biases in the data, using current techniques.
Relationship to Prior Research
While we found clear evidence of greater motion in our subjects with MS than in our HC subjects, others have reported no such difference in other clinical populations [e.g., Yoo et al., 2005]. While this might have to do with a difference in disease type (MS vs. schizophrenia), it is more likely due to the fact that the cognition of the individuals with schizophrenia used in the Yoo et al. [Yoo et al., 2005] study was relatively intact. Although their performance on working memory tasks was worse than the HCs, their IQ was very high (mean = 111.5), and the estimate of disease severity was very low (brief psychiatric rating scale total score = 25.5). In as much as movement became a larger problem in our sample as cognitive impairment increased, one might not expect the head motion in the sample of individuals with schizophrenia studied by Yoo et al. to be that much more than their healthy counterparts. Moreover, because only 11 subjects were included in the Yoo et al. study, it is possible that the null effect they report is due, at least in part, to a lack of power.
A rather different aspect of prior research is that studies investigating functional activity in MS relative to HCs often report “more” activity in the MS group. This increased activity is generally twofold: there is an increase in the intensity of activity in the same brain areas that HCs use to perform a given task, and the extent of the active areas is greater in the MS group [e.g., Chiaravalloti et al., 2005; Sweet et al., 2006]. The results presented here suggest that this frequent finding in the MS literature may represent an underestimate of the increase in activity seen in MS. This is because movement is correlated with decreased SNR, which means that the more people move, the less signal there is to detect (relative to the noise). In as much as individuals with MS move more than HCs, it is more difficult to detect activity in MS. Despite this, we consistently see increased activity in MS cohorts (relative to HCs). Therefore, it seems likely that if individuals with MS moved as little as HCs, the increase in activity seen in MS would be even larger than what is reported in the literature. There is an important caveat to this line of reasoning. In many studies that investigate increasing task difficulty in MS relative to HC, there are large differences when the task is relatively easy, but the differences are less apparent as the task becomes more difficult [for a good example using the N‐back task, see Sweet et al., 2006]. The results of the current paper suggest one possible reason for this perplexing lack of difference at higher levels of task difficulty: increased motion (and therefore decreased SNR) in the MS group as difficulty increases. As the amount of motion in the MS group increases, the concomitant decrease in SNR would eventually begin to make even robust functional activity difficult to detect. Thus, if the results presented here are present in other MS samples (as seems likely) the lack of differences in activity as task difficulty increases may be due, at least in part, to progressive increases in head motion and consequent decreases in SNR in the MS group.
Another frequent observation, when functional activation in MS samples are compared to HCs, is that the MS group shows activation in areas where the HC group shows no reliable activation [e.g., Sweet et al., 2006]. The contribution of motion to this finding is more nuanced. On the one hand, decreased SNR may play a smaller role here: if there is no reliable activation in these regions in the HC group, a smaller increase in the MS group would be detectable (even if this increase was lessened by poorer SNR). On the other hand, motion artifact may result in spurious activation in the MS group, thus producing artifactual “activation.”. Unfortunately, in many studies it is difficult to determine which cause (real activation or motion artifact) produces this type of activation pattern.
Functional MRI research has traditionally considered head motion a source of random error. This would suggest that, at worst, motion reduces the SNR and, therefore, reduces statistical power. In fact, as shown by the current study, head movement may actually be a source of systematic error, which is far more troubling. That is, if clinical samples move more than healthy samples, and impaired patients move more than intact patients, then SNR and statistical power may also vary between groups and within groups as a function of impairment. One current goal of clinical fMRI research is to identify neurophysiologic biomarkers of neurologic disease and behavioral/cognitive impairment. For instance, several studies have demonstrated that functional connectivity within the default network differs between healthy adults and persons with Alzheimer disease [e.g., Greicius et al., 2004; Sorg et al., 2007]. Other studies have correlated continuous measures of behavioral/cognitive impairment with functional connectivity in clinical samples [e.g., Di Martino et al., 2009]. Given that movement impacts SNR and statistical power within functional connectivity analyses in general [Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012] and default network analyses in particular [Van Dijk et al., 2012], it is at least possible that group related differences are due in whole or in part to head movement rather than differences in neurophysiology. However, this conclusion is far from certain: the data presented in the current article show that MS subjects move more as the task becomes increasingly difficult while much of the functional connectivity literature is based on resting state scans which, of course, involve no overt task. Therefore, it may be that clinical samples do not move more during rest than HCs. Nevertheless, the field of neuroimaging must consider head movement within the MR scanner as a possible source of systematic error, and seek ways to ameliorate this confound in the acquisition, analysis, and interpretation of fMRI data.
Why Do Subjects With Cognitive Impairment Move More in the Scanner?
Although it is not clear why task demands and cognitive impairment are associated with greater movement, we have considered one possible explanation. Persons with MS typically require greater cerebral resources (e.g., prefrontal activation) to perform the same cognitive tasks as healthy controls [e.g., Sweet et al., 2006]. This is especially true for MS patients with cognitive impairment [Chiaravalloti et al., 2005]. Experimental fMRI paradigms typically require subjects to perform two tasks simultaneously: (a) perform the cognitive task of interest (e.g., N‐Back), and (b) remain still. As demands of the cognitive task increase (2‐Back), there may be fewer cerebral resources available to maintain the second task (remain still). Healthy persons and MS patients with higher cognitive abilities may process the cognitive task with enough efficiency that cerebral resources remain available for remaining still; however, cerebral inefficiency in MS patients with lower cognitive ability may lead to depleted cerebral resources, resulting in neglect of the second task (remain still).
Another factor which may contribute to increased movement in the MS population is fatigue. Individuals with MS frequently report high levels of both physical and cognitive fatigue [Krupp et al., 2012], and self‐reported fatigue levels often increase during a difficult cognitive task [Johnson et al., 1997]. Although it was not directly studied in the current study, increased fatigue throughout the course of the fMRI paradigm likely leads to increased head movement, which will significantly impact the BOLD signal.
Going Forward
Because we cannot fully correct for subject motion, we are left having to decide between two unpalatable alternatives: (1) exclude subjects with excessive motion and accept the resulting bias in our sample, (2) include as many subjects as possible, and accept the fact that the subjects who moved more will contribute less to the group‐level results. In practice, the latter choice is preferable, but only because the former choice is unacceptable. One insidious problem with the latter choice has to do with the fact that the SNR is almost never reported in fMRI studies. Therefore, when two groups are compared (e.g., individuals with high vs. low cognitive ability), it is almost impossible to tell how much of the difference between the groups is due to differences in SNR. This problem is less concerning in studies involving only HCs, but it would be wise for studies involving clinical samples to include analyses of SNR in their results.
A better solution would be to prospectively coregister all of the images in the fMRI time‐series, adjusting the scanner to track changes in the position of the brain as they occur. Several methods have been devised to do this, ranging the use of three external markers placed on the participant's head [Derbyshire et al., 1998; Speck et al., 2006], to techniques that calculate rigid‐body transformations of the EPI image, similar to algorithms used in retrospective motion correction [Mathiak et al., 2001; Thesen et al., 2000], to techniques that measure differences in k‐space [Welch et al., 2002]. These techniques are very promising and may obviate the need to correct for motion retrospectively by ensuring that the time‐series of EPI images is coregistered at the time of acquisition. This would minimize signal distortions and changes in SNR due to motion, and would thus allow clinical populations to be scanned without the concern that motion artifact will cause differences in signal strength between groups. Indeed, some of these methods have recently become commercially available (e.g., PACE, available on Siemens scanners).
Another solution is to carefully monitor motion parameters from every subject who participates in the study (an approach that should be followed in any case), and to ensure that sufficient numbers of subjects with low cognitive ability are included. From the scatter plots in our analyses, it can be seen there are some subjects with low cognitive ability who were able to remain still. One consequence of this observation is that, despite the fact that many subjects with impaired cognition will move too much to be included, it is possible to sufficiently power a study by continuing to recruit such subjects until a sufficient number who are able to remain still have been found. This is a rather costly option, since it entails the collection of many datasets that will not ultimately be usable, but it is perhaps the best solution for studies of clinical samples.
REFERENCES
- Benedict RH, Fischer JS, Archibald CJ, Arnett PA, Beatty WW, Bobholz J, Chelune GJ, Fisk JD, Langdon DW, Caruso L, Foley F, LaRocca NG, Vowels L, Weinstein A, DeLuca J, Rao SM, Munschauer F (2002): Minimal neuropsychological assessment of MS patients: A consensus approach. Clin Neuropsychol 16:381–397. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, Weinstock‐Guttman B (2006): Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 12:549–558. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hyde JS (1997): Contour‐based registration technique to differentiate between task‐activated and head motion‐induced signal variations in fMRI. Magn Reson Med 38:470–476. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J (2008): Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N, Hillary F, Ricker J, Christodoulou C, Kalnin A, Liu WC, Steffener J, DeLuca J (2005): Cerebral activation patterns during working memory performance in multiple sclerosis using FMRI. J Clin Exp Neuropsychol 27:33–54. [DOI] [PubMed] [Google Scholar]
- Ciulla C, Deek FP (2002): Performance assessment of an algorithm for the alignment of fMRI time series. Brain Topogr 14:313–332. [DOI] [PubMed] [Google Scholar]
- Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker BG, Antel JP, Confavreux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E (1999): Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122(Part 5):871–882. [DOI] [PubMed] [Google Scholar]
- Derbyshire JA, Wright GA, Henkelman RM, Hinks RS (1998): Dynamic scan‐plane tracking using MR position monitoring. J Magn Reson Imaging 8:924–932. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP (2009): Relationship between cingulo‐insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry 166:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW, Todd RM, Taylor MJ, Strother SC (2010): Group specific optimization of fMRI processing steps for child and adult data. Neuroimage 50:479–490. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons JR, Scott JD, Peterson DM, Wolverton BL, Webster CS, Lang PJ (1997): Integrated RF coil with stabilization for fMRI human cortex. Magn Reson Med 38:15–18. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Goodkin DE, Hertsgaard D, Seminary J (1988): Upper extremity function in multiple sclerosis: Improving assessment sensitivity with box‐and‐block and nine‐hole peg tests. Arch Phys Med Rehabil 69:850–854. [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MV, Seidel J, Stein SD, Tedder TE, Kempner KM, Kertzman C, Zeffiro TA (1994): Head movement in normal subjects during simulated PET brain imaging with and without head restraint. J Nucl Med 35:1538–1546. [PubMed] [Google Scholar]
- Gronwall DM (1977): Paced auditory serial‐addition task: A measure of recovery from concussion. Percept Mot Skills 44:367–373. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Saeed N, Soar EJ, Oatridge A, Young IR, Bydder GM (1995): A registration and interpolation procedure for subvoxel matching of serially acquired MR images. J Comput Assist Tomogr 19:289–296. [DOI] [PubMed] [Google Scholar]
- Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ (2009): Walking and talking: An investigation of cognitive‐motor dual tasking in multiple sclerosis. Mult Scler 15:1215–1227. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, Mills JA, Weiner HL (1983): Intensive immunosuppression in progressive multiple sclerosis. A randomized, three‐arm study of high‐dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med 308:173–180. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Steffener J, Biswal BB, Lange G, DeLuca J, Ashburner J (2002): Functional magnetic resonance imaging technology and traumatic brain injury rehabilitation: Guidelines for methodological and conceptual pitfalls. J Head Trauma Rehabil 17:411–430. [DOI] [PubMed] [Google Scholar]
- Johnson SK, Lange G, DeLuca J, Korn LR, Natelson B (1997): The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol 4:145–153. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR (2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, Serafin DJ, Christodoulou C (2010): Multiple sclerosis‐associated fatigue. Expert Rev Neurother 10:1437–1447. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek‐Haddadi A, Lund TE, Laufs H, Carmichael D (2007): Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging 25:894–901. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Posse S (2001): Evaluation of motion and realignment for functional magnetic resonance imaging in real time. Magn Reson Med 45:167–171. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg‐Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001): Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127. [DOI] [PubMed] [Google Scholar]
- Phillips MD (2008): Functional faults: fMRI in MS. Neurology 70:248–249. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E, Sela G, McIlroy WE, Black SE, Staines WR, Bronskill MJ, McIntosh AR, Graham SJ (2001): Quantifying head motion associated with motor tasks used in fMRI. Neuroimage 14:284–297. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM (2007): Selective changes of resting‐state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA 104:18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Hennig J, Zaitsev M (2006): Prospective real‐time slice‐by‐slice motion correction for fMRI in freely moving subjects. MAGMA 19:55–61. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Deluca J (2009): Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol 31:913–926. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravalloti N, Deluca J (2009): Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc 15:606–612. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA (2006): Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 27:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR (2000): Prospective acquisition correction for head motion with image‐based tracking for real‐time fMRI. Magn Reson Med 44:457–465. [DOI] [PubMed] [Google Scholar]
- Tremblay M, Tam F, Graham SJ (2005): Retrospective coregistration of functional magnetic resonance imaging data using external monitoring. Magn Reson Med 53:141–149. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Mattay V, Callicott J, Kotrla K, Santha A, van Gelderen P, Duyn J, Moonen C, Frank J (1996): fMRI applications in schizophrenia research. Neuroimage 4(3, Part 3):S118–S126. [DOI] [PubMed] [Google Scholar]
- Welch EB, Manduca A, Grimm RC, Ward HA, Jack CR Jr, (2002): Spherical navigator echoes for full 3D rigid body motion measurement in MRI. Magn Reson Med 47:32–41. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC (1992): Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16:620–633. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Choi BG, Juh R, Pae CU, Lee CU (2005): Head motion analysis during cognitive fMRI examination: Application in patients with schizophrenia. Neurosci Res 53:84–90. [DOI] [PubMed] [Google Scholar]
- Yuan W, Altaye M, Ret J, Schmithorst V, Byars AW, Plante E, Holland SK (2009): Quantification of head motion in children during various fMRI language tasks. Hum Brain Mapp 30:1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]


