Abstract
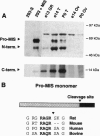
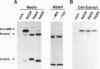
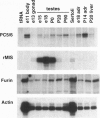
During male gonadal development Müllerian duct regression is mediated by the actions of the hormone Müllerian inhibiting substance (MIS), a member of the transforming growth factor beta superfamily. MIS is considered to be unique among members of this superfamily because bioactivation of MIS via proteolytic processing is hypothesized to occur at its target organ, the Müllerian duct. We find instead that the majority of MIS is processed and secreted from the embryonic testes as a complex in which the mature region remains noncovalently associated with the prodomain. In addition, we have identified two candidate endoproteases that are expressed in the testes and that may be capable of processing MIS in vivo. These kex2/subtilisin-like enzymes, PC5 and furin, are members of the proprotein convertase family that have been implicated in hormone bioactivation via proteolytic processing after dibasic amino acid cleavage recognition sites. Coexpression of PC5 and MIS in transfected mammalian cells results in efficient processing and bioactivation of MIS. Our results suggest that MIS is a natural substrate for PC5, thereby supporting a role for prohormone convertases in the activation of transforming growth factor beta-related hormones during development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J., Mason O. B., Landsberg K. E., Wong P. A., Kiefer M. C., Brake A. J. cDNA and gene structure for a human subtilisin-like protease with cleavage specificity for paired basic amino acid residues. DNA Cell Biol. 1991 Jun;10(5):319–328. doi: 10.1089/dna.1991.10.319. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Finegold M. J., Cate R. L. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994 Nov 4;79(3):415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Bratt T., Cedervall T., Akerström B. Processing and secretion of rat alpha 1-microglobulin-bikunin expressed in eukaryotic cell lines. FEBS Lett. 1994 Oct 31;354(1):57–61. doi: 10.1016/0014-5793(94)01087-0. [DOI] [PubMed] [Google Scholar]
- Dubois C. M., Laprise M. H., Blanchette F., Gentry L. E., Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995 May 5;270(18):10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- Haqq C., Lee M. M., Tizard R., Wysk M., DeMarinis J., Donahoe P. K., Cate R. L. Isolation of the rat gene for Mullerian inhibiting substance. Genomics. 1992 Apr;12(4):665–669. doi: 10.1016/0888-7543(92)90291-y. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K., Hosaka M., Nakagawa T., Nagase M., Shoda A., Murakami K., Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990 Dec 25;265(36):22075–22078. [PubMed] [Google Scholar]
- Horimoto T., Nakayama K., Smeekens S. P., Kawaoka Y. Proprotein-processing endoproteases PC6 and furin both activate hemagglutinin of virulent avian influenza viruses. J Virol. 1994 Sep;68(9):6074–6078. doi: 10.1128/jvi.68.9.6074-6078.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Darlington D. N., Hand T. A., Bloomquist B. T., Mains R. E. PACE4: a subtilisin-like endoprotease prevalent in the anterior pituitary and regulated by thyroid status. Endocrinology. 1994 Sep;135(3):1178–1185. doi: 10.1210/endo.135.3.8070361. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Tucker J. E., Joh R., Landsberg K. E., Saltman D., Barr P. J. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991 Dec;10(10):757–769. doi: 10.1089/dna.1991.10.757. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusson J., Vieau D., Hamelin J., Day R., Chrétien M., Seidah N. G. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6691–6695. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin D. T., Hudson P. L., Graciano A. L., Kenneally M. K., Ragin R. C., Manganaro T. F., Donahoe P. K. Mullerian duct regression and antiproliferative bioactivities of mullerian inhibiting substance reside in its carboxy-terminal domain. Endocrinology. 1992 Jul;131(1):291–296. doi: 10.1210/endo.131.1.1612008. [DOI] [PubMed] [Google Scholar]
- Moore C. C., Brentano S. T., Miller W. L. Human P450scc gene transcription is induced by cyclic AMP and repressed by 12-O-tetradecanoylphorbol-13-acetate and A23187 through independent cis elements. Mol Cell Biol. 1990 Nov;10(11):6013–6023. doi: 10.1128/mcb.10.11.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterberg A., Lovell-Badge R. Expression of the mouse anti-müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991 Oct;113(2):613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Hosaka M., Torii S., Watanabe T., Murakami K., Nakayama K. Identification and functional expression of a new member of the mammalian Kex2-like processing endoprotease family: its striking structural similarity to PACE4. J Biochem. 1993 Feb;113(2):132–135. doi: 10.1093/oxfordjournals.jbchem.a124015. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Murakami K., Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS Lett. 1993 Jul 26;327(2):165–171. doi: 10.1016/0014-5793(93)80163-o. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Kim W. S., Torii S., Hosaka M., Nakagawa T., Ikemizu J., Baba T., Murakami K. Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J Biol Chem. 1992 Mar 25;267(9):5897–5900. [PubMed] [Google Scholar]
- Ohagi S., LaMendola J., LeBeau M. M., Espinosa R., 3rd, Takeda J., Smeekens S. P., Chan S. J., Steiner D. F. Identification and analysis of the gene encoding human PC2, a prohormone convertase expressed in neuroendocrine tissues. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4977–4981. doi: 10.1073/pnas.89.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Chow E. P., Mattaliano R. J., Manganaro T. F., Donahoe P. K., Cate R. L. Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. J Biol Chem. 1988 Dec 15;263(35):18961–18964. [PubMed] [Google Scholar]
- Picard J. Y., Josso N. Purification of testicular anti-Müllerian hormone allowing direct visualization of the pure glycoprotein and determination of yield and purification factor. Mol Cell Endocrinol. 1984 Jan;34(1):23–29. doi: 10.1016/0303-7207(84)90155-2. [DOI] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Leunissen J. A., Onnekink C., Bloemers H. P., Van de Ven W. J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986 Sep;5(9):2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M. Pro-protein convertases of subtilisin/kexin family. Methods Enzymol. 1994;244:175–188. doi: 10.1016/0076-6879(94)44015-8. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Hamelin J., Gaspar A., Collard M. W., Chrétien M. Testicular expression of PC4 in the rat: molecular diversity of a novel germ cell-specific Kex2/subtilisin-like proprotein convertase. Mol Endocrinol. 1992 Oct;6(10):1559–1570. doi: 10.1210/mend.6.10.1448111. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Shen W. H., Moore C. C., Ikeda Y., Parker K. L., Ingraham H. A. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994 Jun 3;77(5):651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Takahashi S., Hatsuzawa K., Watanabe T., Murakami K., Nakayama K. Sequence requirements for endoproteolytic processing of precursor proteins by furin: transfection and in vitro experiments. J Biochem. 1994 Jul;116(1):47–52. doi: 10.1093/oxfordjournals.jbchem.a124501. [DOI] [PubMed] [Google Scholar]
- Thacker C., Peters K., Srayko M., Rose A. M. The bli-4 locus of Caenorhabditis elegans encodes structurally distinct kex2/subtilisin-like endoproteases essential for early development and adult morphology. Genes Dev. 1995 Apr 15;9(8):956–971. doi: 10.1101/gad.9.8.956. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Shima H., Yonemura C. Y., Brody J., Donahoe P. K., Cunha G. R. Effect of human recombinant mullerian inhibiting substance on isolated epithelial and mesenchymal cells during mullerian duct regression in the rat. Endocrinology. 1992 Sep;131(3):1481–1488. doi: 10.1210/endo.131.3.1505479. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Wilson C. A., di Clemente N., Ehrenfels C., Pepinsky R. B., Josso N., Vigier B., Cate R. L. Mullerian inhibiting substance requires its N-terminal domain for maintenance of biological activity, a novel finding within the transforming growth factor-beta superfamily. Mol Endocrinol. 1993 Feb;7(2):247–257. doi: 10.1210/mend.7.2.8469238. [DOI] [PubMed] [Google Scholar]
- di Clemente N., Wilson C., Faure E., Boussin L., Carmillo P., Tizard R., Picard J. Y., Vigier B., Josso N., Cate R. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol. 1994 Aug;8(8):1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]