Abstract
The present study aimed to investigate changes in the mammalian target of rapamycin (mTOR) signaling pathway in the obstructed kidney of rats with unilateral ureteral obstruction (UUO). Male Sprague-Dawley rats were unilaterally obstructed by ligation of the left proximal ureter for 7 days. Control rats were treated in the same way except that no ligature was made. The expression levels of phosphorylated phosphatidylinositol 3-kinase (PI3K), Akt, and mTOR were determined in the kidney by semiquantitative immunoblotting. The protein expression levels of transforming growth factor (TGF)-β1, Bax, and Bcl-2 were also determined in the kidney. The phosphorylation of PI3K, Akt, and mTOR was increased in the kidney of ureteral obstruction rats compared with the control. In the obstructed kidney, the protein expression of TGF-β1 and Bax was also increased, whereas Bcl-2 expression was decreased. In conclusion, the phosphorylation of PI3K/Akt/mTOR was increased in the obstructed kidney of rats with UUO.
Keywords: mTOR protein, rat; Ureteral Obstruction; Fibrosis; Apoptosis
INTRODUCTION
Interstitial fibrosis and tubular atrophy of the kidney is the final common pathogenesis of chronic kidney disease. Unilateral ureteral obstruction (UUO) is a well-known model for progressive renal disease. Prolonged ureteral ligation induces marked hydronephrosis of the obstructed kidney, which exhibits tubulointerstitial fibrosis and apoptosis in association with activation of pro-fibrotic and pro-apoptotic proteins.1
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that plays diverse functions in development, metabolism, aging, cellular proliferation, and cell growth.2-4 Recent data have demonstrated that the mTOR signaling pathway is involved in the pathogenesis of kidney diseases such as acute kidney injury, diabetic nephropathy, and polycystic kidney disease.5-7 Activation of the mTOR pathway in diabetic nephropathy is associated with interstitial fibrosis, tubular atrophy, and progressive decline of the glomerular filtration rate, and blockade of the mTOR pathway slows the progression of diabetic nephropathy.8-10 In addition, we demonstrated that phosphorylation of the mTOR signaling pathway was increased in the kidney of deoxycorticosterone acetate (DOCA)-salt hypertensive rats.11 However, the role of the mTOR signaling pathway in the pathogenesis of kidney injury of the ureteral obstructed kidney has not been fully elucidated.
The present study aimed to investigate changes in the mTOR signaling pathway in the pathogenesis of tubulointerstitial fibrosis and apoptosis in the obstructed kidney of rats with UUO.
MATERIALS AND METHODS
1. Animals
The animal study was approved by the Ethics Committee of Chonnam National University Medical School. The experimental procedure conformed to the institutional guidelines for experimental animal care and use. Male Sprague-Dawley rats weighing 200 to 220 g were used.
2. Induction of UUO
Rats were unilaterally obstructed by ligation of the left proximal ureter for 7 days. Control rats were treated in the same way except that no ligature was made. The rats had free access to standard rat feed and tap water and were sacrificed by decapitation on day 7 after operation. The kidney was rapidly removed, dissected into three zones [cortex and outer stripe of outer medulla (cortex/OSOM), inner stripe of outer medulla (ISOM), and inner medulla (IM)], and processed for semiquantitative immunoblotting as described below.
3. Semiquantitative immunoblotting
The dissected cortex/OSOM, ISOM, and IM were homogenized in ice-cold isolation solution containing 0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride, with pH 7.2. The homogenates were centrifuged at 1,000×g for 15 min at 4℃ to remove whole cells, nuclei, and mitochondria. The total protein concentration was measured (Pierce BCA protein assay reagent kit, Rockford, IL, USA). All samples were adjusted with isolation solution to reach the same final protein concentrations and solubilized at 65℃ for 15 min in SDS-containing sample buffer and then stored at -20℃. To confirm equal loading of protein, an initial gel was stained with Coomassie blue. SDS-PAGE was performed on 9% or 12% polyacrylamide gels. The proteins were transferred by gel electrophoresis (Bio-Rad Mini Protean II, Bio-Rad, Hercules, CA, USA) onto nitrocellulose membranes (Hybond ECL RPN3032D; Amersham Pharmacia Biotech, Little Chalfont, UK). The blots were subsequently blocked with 5% milk in phosphate-buffered saline with Tween 20 (PBST; 80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 hour and incubated overnight at 4℃ with 1:1,000 dilutions of primary antibodies, followed by incubation with 1:1,500 dilutions of secondary anti-rabbit horseradish peroxidase-conjugated antibodies (P448; DAKO, Glostrup, Denmark). The labeling was visualized by use of an enhanced chemiluminescence system.
4. Primary antibodies
The antibodies used were as follows: affinity-purified anti-rabbit antibodies against phosphorylated phosphatidylinositol 3-kinase (p-PI3K; Cell Signaling Technology, Beverly, MA, USA), phosphorylated Akt (p-Akt; Cell Signlaing Technology), phosphorylated mTOR (p-mTOR; Cell Signlaing Technology), transforming growth factor-β1 (TGF-β1; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax (Cell Signaling Technology), and Bcl-2 (Cell Signaling Technology).
5. Statistical analyses
Results are expressed as means±SEMs. The statistical significance of differences between the groups was determined by using unpaired t-test, and it was defined as a p value less than 0.05.
RESULTS
1. Weight of body and kidney
On day 7 after the ureteral obstruction, the body weight of rats did not differ significantly between the two groups (238±5.8 g in controls vs. 240±4.5 g in UUO, p=0.79). However, the weight of the ureteral obstructed kidney was increased compared with the controls (1.2±0.1 g in controls vs. 2.3±0.2 g in UUO, p<0.05).
2. Expression of mTOR signaling pathway
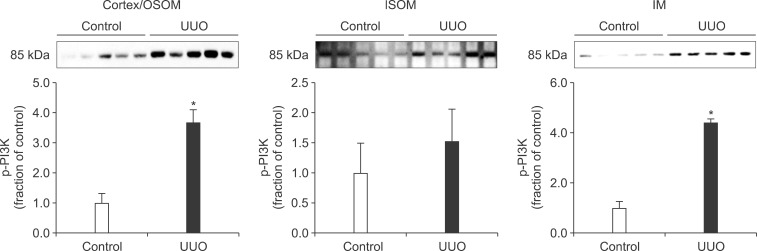
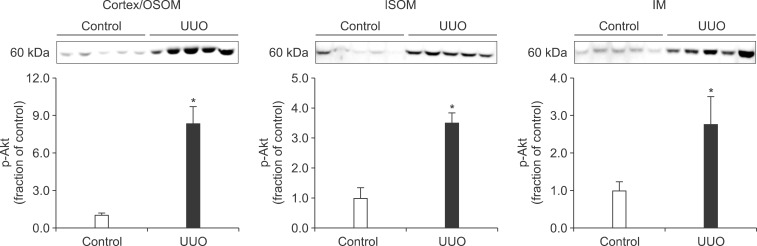
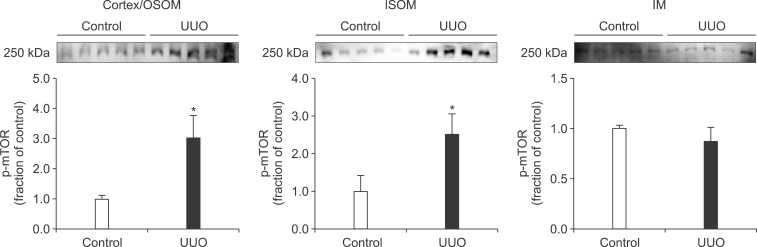
In the ureteral obstructed kidney, the expression of p-PI3K was significantly increased in the cortex/OSOM and IM, whereas it was not significantly changed in the ISOM (Fig. 1). The expression of p-Akt was also increased in the cortex/OSOM, ISOM, and IM (Fig. 2). The expression of p-mTOR was increased in the cortex/OSOM and ISOM (Fig. 3).
FIG. 1.
The protein expression of phosphorylated phosphatidylinositol 3-kinase (p-PI3K) in the kidney. OSOM, outer stripe of the outer medulla; ISOM, inner stripe of the outer medulla; IM, inner medulla; UUO, unilateral ureteral obstruction. Each column represents the mean±SEM of 10 rats. *p<0.05 compared with control kidney.
FIG. 2.
The protein expression of phosphorylated Akt (p-Akt) in the kidney. Legends as in Fig. 1.
FIG. 3.
The protein expression of phosphorylated mammalian target of rapamycin (p-mTOR) in the kidney. Legends as in Fig. 1.
3. Expression of TGF-β1, Bax, and Bcl-2
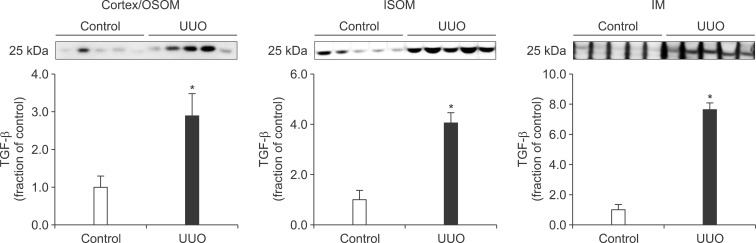
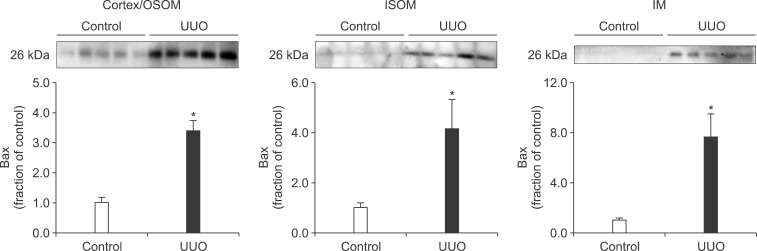
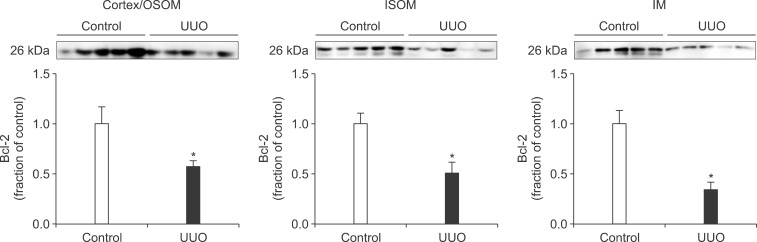
Following the UUO, the protein expression of TGF-β1 was increased in the cortex/OSOM, ISOM, and IM of the obstructed kidney (Fig. 4). The expression of Bax was also increased in the obstructed kidney compared with the control (Fig. 5). The protein expression of anti-apoptotic factor Bcl-2 was decreased in the obstructed kidney (Fig. 6).
FIG. 4.
The protein expression of transforming growth factor (TGF)-β1 in the kidney. Legends as in Fig. 1.
FIG. 5.
The protein expression of Bax in the kidney. Legends as in Fig. 1.
FIG. 6.
The protein expression of Bcl-2 in the kidney. Legends as in Fig. 1.
DISCUSSION
Interstitial fibrosis and tubular atrophy are common consequences of chronic ureteral obstruction. TGF-β1 is considered to be a major stimulating factor that regulates extracellular matrix synthesis. It has been suggested that TGF-β1 is increased at the transcriptional level in chronic ureteral obstruction and that it may play a role in initiating fibrogenesis in obstructive nephropathy.12 In addition, it has been established that cellular apoptosis is associated with the upregulation of Bax and down-regulation of Bcl-2. In the present study, the protein expression of TGF-β1 was increased in the obstructed kidney following UUO. The expression of apoptotic factor Bax was increased in the obstructed kidney but that of anti-apoptotic factor Bcl-2 was decreased. These changes may contribute to the kidney injury in UUO. However, diverse mechanisms are involved in the regulation of TGF-β1, Bax, and Bcl-2, and the upstream mechanisms remain elusive.
mTOR is a serine/threonine kinase that is activated through the phosphorylation of PI3K and Akt.13,14 Recently, the mTOR signaling pathway has emerged as an important modulator of several forms of renal disease. The activation of mTOR in the kidney promotes the upregulation of pro-inflammatory and pro-fibrotic factors, which leads to tubulointerstitial fibrosis and atrophy.5-7 The expression of mTOR was increased in the kidney of DOCA-salt hypertensive rats, which was related to the upregulation of TGF-β1, pro-inflammatory proteins, and pro-apoptotic proteins.11 In addition, treatment with mTOR inhibitor ameliorates kidney injury in experimental diabetic nephropathy and a rat model of reduced renal mass through inhibition of TGF-β1 and proinflammatory cytokines.8,9,15,16 In the present study, the expression of p-PI3K, p-Akt, and p-mTOR was significantly increased in the ureteral obstructed kidney. These changes may contribute to the interstitial fibrosis and tubular atrophy in chronic ureteral obstruction.
In conclusion, the phosphorylation of PI3K/Akt/mTOR was increased in the obstructed kidney of rats with UUO, which may be related to the pathogenesis of tubulointerstitial fibrosis and apoptosis.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI10026-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine.
Footnotes
None declared.
References
- 1.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 2.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycinreceptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 4.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 6.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. I. The signaling pathway. Am J Physiol Renal Physiol. 2012;303:F1–F10. doi: 10.1152/ajprenal.00014.2012. [DOI] [PubMed] [Google Scholar]
- 7.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am J Physiol Renal Physiol. 2012;303:F180–F191. doi: 10.1152/ajprenal.00015.2012. [DOI] [PubMed] [Google Scholar]
- 8.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006;17:1395–1404. doi: 10.1681/ASN.2005050549. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, et al. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol. 2007;27:495–502. doi: 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- 10.Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun. 2009;384:471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 11.Ma SK, Choi JS, Joo SY, Kim HY, Kim CS, Bae EH, et al. Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension. Chonnam Med J. 2012;48:150–154. doi: 10.4068/cmj.2012.48.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneto H, Morrissey J, Klahr S. Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993;44:313–321. doi: 10.1038/ki.1993.246. [DOI] [PubMed] [Google Scholar]
- 13.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 14.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann S, Daniel C, Stief A, Vogelbacher R, Amann K, Hugo C. Long-term treatment of sirolimus but not cyclosporine ameliorates diabetic nephropathy in the rat. Transplantation. 2009;87:1290–1299. doi: 10.1097/TP.0b013e3181a192bd. [DOI] [PubMed] [Google Scholar]
- 16.Diekmann F, Rovira J, Carreras J, Arellano EM, Bañón-Maneus E, Ramírez-Bajo MJ, et al. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J Am Soc Nephrol. 2007;18:2653–2660. doi: 10.1681/ASN.2007010087. [DOI] [PubMed] [Google Scholar]