Abstract
Selective induction of replication of nucleolar and mitochondrial DNA has been demonstrated in starved-refed cultures of Tetrahymena pyriformis by different techniques.
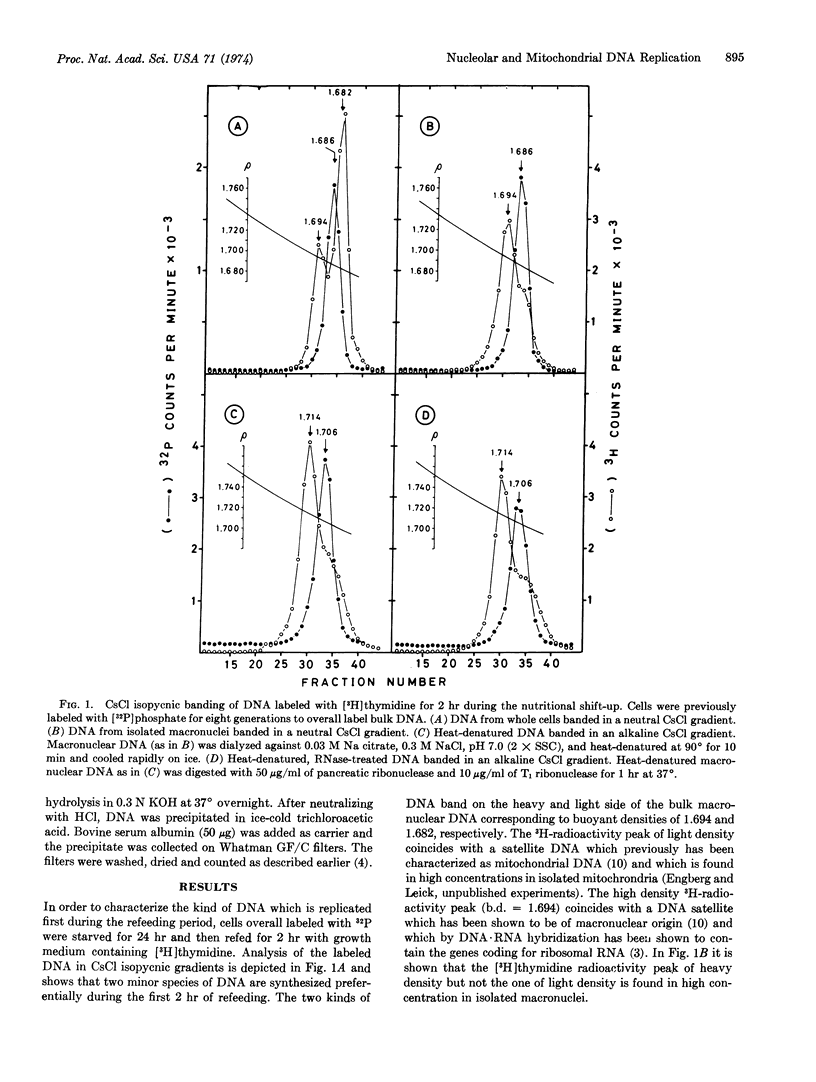
Labeling of starved cells with [3H]thymidine during a nutritional shift-up and analysis of the DNA in isopycnic CsCl gradients shows that the two initially labeled species of DNA are two species banding on the heavy and light side of the bulk macronuclear DNA. In isolated macronuclei the radioactivity is found only in the high density fraction, which has been shown to be of nucleolar origin. In sucrose gradients the newly replicated mitochondrial and nucleolar DNAs sediment considerably slower than the bulk DNA, as one discrete band corresponding to a molecular weight of about 3 to 4 × 107.
Electron microscope autoradiography of cells labeled with [3H]thymidine as above shows that the peripheral nucleoli of the macronucleus as well as the mitochondria are labeled before any radioactivity is found in the chromatin granules of the macronucleus.
The results clearly indicate that nucleolar and mitochondrial DNA replication are under a control independent of that for the replication of bulk DNA.
Keywords: nutritional shift-up, CsCl gradients, EM-autoradiography, sucrose gradients
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk C. F., Hanawalt P. C. Repair of damaged DNA in a eucaryotic cell: Tetrahymena pyriformis. Science. 1967 Nov 3;158(3801):663–664. doi: 10.1126/science.158.3801.663. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Jeter J. R., Jr Synchronization of the cell cycle of Tetrahymena by stravation and refeeding. J Protozool. 1970 Aug;17(3):429–431. doi: 10.1111/j.1550-7408.1970.tb04708.x. [DOI] [PubMed] [Google Scholar]
- Charret R. L'ADN nucléolaire chez Tetrahymena pyriformis: chronoloe de sa réplication. Exp Cell Res. 1969 Mar;54(3):353–361. doi: 10.1016/0014-4827(69)90214-6. [DOI] [PubMed] [Google Scholar]
- Engberg J., Pearlman R. E. The amount of ribosomal RNA genes in Tetrahymena pyriformis in different physiological states. Eur J Biochem. 1972 Apr 11;26(3):393–400. doi: 10.1111/j.1432-1033.1972.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Jones I. G. Mitochondrial deoxyribonucleic acid from Tetrahymena pyriformis and its kinetic complexity. Biochem J. 1970 Mar;116(5):811–817. doi: 10.1042/bj1160811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leick V., Andersen S. B. Polols and turnover rates of nuclear ribosomal RNA in Tetrahymena pyriformis. Eur J Biochem. 1970 Jul;14(3):460–464. doi: 10.1111/j.1432-1033.1970.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Leick V., Engberg J., Emmersen J. Nascent subribosomal particles in Tetrahymena pyriformis. Eur J Biochem. 1970 Apr;13(2):238–246. doi: 10.1111/j.1432-1033.1970.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishi A., Ando T. The DNA of Tetrahymena pyriformis GL strain. A mild method for preparation and its characterization. Biochim Biophys Acta. 1973 Apr 11;299(4):507–515. doi: 10.1016/0005-2787(73)90222-0. [DOI] [PubMed] [Google Scholar]
- Nilsson J. R., Leick V. Nucleolar organization and ribosome formation in Tetrahymena pyriformis GL. Exp Cell Res. 1970 Jun;60(3):361–372. doi: 10.1016/0014-4827(70)90529-x. [DOI] [PubMed] [Google Scholar]
- Parsons J. A., Rustad R. C. The distribution of DNA among dividing mitochondria of Tetrahymena pyriformis. J Cell Biol. 1968 Jun;37(3):683–693. doi: 10.1083/jcb.37.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B. B., Gall J. G. Independent control of ribosomal gene replication in polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1973 May;70(5):1359–1363. doi: 10.1073/pnas.70.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]