Abstract
Objective(s): Human T-cell lymphotropic virus type-1 is an oncornavirus that causes adult T cell leukemia (ATL) HTLV-I-associated myelopathy⁄tropical spastic paraparesis (HAM/TSP). Golestan province is located in North West of Khorasan province known as an endemic area for HTLV-I in Iran. This study aimed to evaluate seroprevalence of HTLV-I in Golestan province.
Materials and Methods: In this cross-sectional descriptive study in 2007, blood samples were collected from 2034 healthy people residing in different parts of Golestan province. Sera were assessed for HTLV-I/II–specific antibodies by ELISA method and reactive samples were confirmed by Western blot. Demographic and serologic data were entered in SPSS version 11.5 and statistical analysis was performed.
Results: An overall HTLV-I/II prevalence of 0.7% was observed in 15 cases by ELISA. Six out of 15 were confirmed as HTLV-I by western blot. Regional variation in the prevalence of HTLV-I was observed; 0%, 0%, 0.1%, 1.9%, 0.3%, 0%, and 2.6% tested HTLV-I-positive from west to east of Golestan Province regions, respectively. Seropositivity increased with age. No association between HTLV-I infection and sex status was detected.
Conclusion: Highest rate of HTLV-I seroprevalence was shown in east of this region located in neighborhood with Khorasan province, the only confirmed endemic area in Iran. It seems that eastern area of our province is endemic for HTLV-I. Further comprehensive detailed epidemiological and molecular studies are recommended.
Key Words: HTLV-I, Seroprevalence, ELISA, Western Blot, Golestan, Iran
Introduction
Human T-cell lymphotropic virus type-1 (HTLV-I) is a member of Retroviridae family which has been discovered as the first human retrovirus. This oncornavirus causes the T cell malignancy associated with two main diseases; adult T cell leukemia (ATL) and HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (1, 2). Worldwide estimation of HTLV-I infected people is approximately 20 million and it has been suggested more than 90% of them remain asymptomatic carriers during their lives. Geographic distribution of the virus indicates that southwestern Japan, parts of Africa, the Caribbean islands, and Central and South America are the main endemic regions of HTLV-I in the world (3, 4). However, the data should be interpreted based on the population selection criteria and the differences in the diagnostic strategies. Mainly, the data provided from the serological screening of healthy blood donors is the basis for the estimation of the global prevalence of HTLV-I, which tends to underestimate the prevalence of the virus in the population (5, 6). HTLV-I is transmissible through breast milk, semen, and HTLV-I carrier’s lymphocytes and all of transmission routes efficiently localize HTLV-I infection foci within particular family ⁄ethnic groups (7-9). On the other hand, it is considered to study the global prevalence of HTLV-I infection in the context of ethnicity-based, as a new paradigm for cancer research for host factor interaction assay with exogenous carcinogens (10).
Iran has been introduced as an endemic area based on studies reported from Mashhad in Khorasan (A province of Iran recently divided into three provinces) located in the northeast of Iran (11, 12). The latest report from Mashhad showed the overall prevalence of 2.12% HTLV-I infection in the whole population (13). The previous HTLV-I infection prevalence report from Golestan has been limited to the Thalassemia patients with 4.4% (14). Golestan is another province of Iran located in the southeast of Caspian Sea next to Khorasan. Different ethnic groups are living in this province and emigration from the east and northeast of the country to this region is common. In the endemic developed countries and some developing countries, HTLV-I screening of blood donors was already performed. The province of Golestan has not performed HTLV-I screening of blood donors yet. This study aims to evaluate the population-based HTLV-I seroprevalence in the province of Golestan.
Materials and Methods
From all of the seven main cities with an estimated population of 1.5 million, 2034 individuals were selected through multistage cluster sampling in 2007. Demographic information, such as sex, age, and residency status, was collected. The study was approved by Deputy of Research of Golestan University of Medical Sciences regarding scientific and ethical issues. Informed consent was obtained from all participants. Five ml of blood samples were obtained from each individual. Serum was separated through centrifugation and was stored at −20◦C. Serum samples were screened for the presence of anti-HTLV-I antibodies with the HTLV I/II enzyme linked immunosorbent assay (ELISA) (DIA.PRO, Diagnostic Bio probes Srl, Italy) according to the manufacturer’s instructions. All reactive samples on serologic screening were tested further through Western blot (WB) analysis according to the manufacturer’s instructions (HTLV BLOT 2.4, Gene labs Diagnostics). Descriptive data was summarized as the mean, standard deviation, and/or percentages were analyzed by SPSS 11.5 using Chi-square and t-tests. A P-Value of <0.05 was considered statistically significant.
Results
Out of 2034 people studied in this population, based seroepidemiology, 848 cases were male (41.7%) and 1186 participants were females (58.3%) with the mean age of 38.66±16.54 years. All serum samples were analyzed for anti-HTLV antibodies.
In the primary screening of the samples by ELISA, 15 (0.7%) were positive for HTLV1/2 antibodies. The Western blot results confirmation demonstrated that 6 (0.3%) out of 15 ELISA positive specimens were HTLV-1 positive, but HTLV positivity was not confirmed in 9 cases (0.4%). According to the Western blot results, the overall prevalence of the HTLV-I infection in the population under study was 0.3% (6/2034). We did not find an indeterminate result by Western blot. The HTLV-I infection rate for females was 0.3% (3/1186) and for males was 0.4% (3/848). No significant difference in the seroprevalence was observed between males and females (Table 1).
Table 1.
Demographic factors related to HTLV-I Infection in the general population of Golestan province, Iran
| Variable | No. | Positive (%) | P-Value |
|---|---|---|---|
| Sex | |||
| Male | 848 | 0.4 | |
| Female | 1186 | 0.3 | 0.49 |
| Age (years) | |||
| 1-10 | 44 | 0 | |
| 11-20 | 243 | 0 | |
| 21-30 | 506 | 0.4 | |
| >30 | 1237 | 0.4 | 0.25 |
| Residency area | |||
| Bandar Turkman | 298 | 0 | |
| Kord-Koy | 50 | 0 | |
| Gorgan | 722 | 0.1 | |
| Ali-Abad | 51 | 1.9 | |
| Gonabad | 763 | 0.3 | |
| Minodasht | 69 | 0 | |
| Kalaleh | 75 | 2.6 | 0.034 |
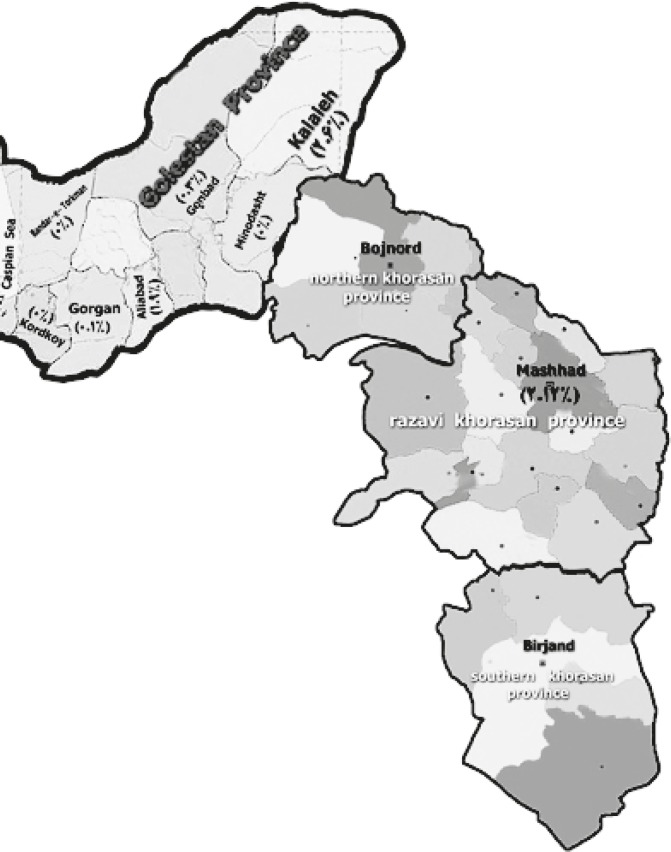
Regional variation in the prevalence of HTLV-I was observed to be 0%, 0% 0.1%, 1.9%, 0.3%, and 2.6% tested HTLV-I-positive from west to east of Golestan regions, respectively (Figure 1). Seroprevalence of the disease increased with age, as observed more among those older than 20 years old(Table 1).
Figure1.
Map of HTLV-I Infection distribution in Golestan and Khorasan provinces, Iran
Discussion
In the present study, we have reported the seroepidemiology of HTLV-I infection in a representative sample of individuals from different cities of the province of Golestan. The overall prevalence of HTLV-I infection in our study is 0.3% (95% CI: 0.06-0.53%) lower than the previous study with 4.4% in Thalassemia patients in Gorgan (14). This is almost in line of many other studies reported from different investigations in the USA 0.6% (15), Brazil 0.3%, China 0.06% (16), Argentina 0.5% (17), Italy 0.6% (18), and Kuwait 0.02% (19) as well as 0.013 in Boshehr (20) and 0.5% in Sistan-Balutchestan, the provinces of Iran, in donated blood (21). In contrast with the above reports, there are similar data indicating higher prevalence in some regions of Iran specially in Mashhad (11- 13), in the east of our region as well as countries such as Japan (6), Ecuador (22), and Karaeeb region (23). In the last 30 years, a lot of studies on the geographic distribution of the HTLV-I have been conducted. Interpretation of the data from the international prevalence studies should be done based on the population selection criteria and diagnostic strategies performed in the research protocol because of their interference with the final result. Mainly, the serological screening of healthy blood donors was the basis for the estimation of the global prevalence of HTLV-I, but it seems to be an underestimation of the seroprevalence in the population (24).
from different investigations in the USA 0.6% (15), Brazil 0.3%, China 0.06% (16), Argentina 0.5% (17), Italy 0.6% (18), and Kuwait 0.02% (19) as well as 0.013 in Boshehr (20) and 0.5% in Sistan-Balutchestan, the provinces of Iran, in donated blood (21). In contrast with the above reports, there are similar data indicating higher prevalence in some regions of Iran specially in Mashhad (11- 13), in the east of our region as well as countries such as Japan (6), Ecuador (22), and Karaeeb region (23). In the last 30 years, a lot of studies on the geographic distribution of the HTLV-I have been conducted. Interpretation of the data from the international prevalence studies should be done based on the population selection criteria and diagnostic strategies performed in the research protocol because of their interference with the final result. Mainly, the serological screening of healthy blood donors was the basis for the estimation of the global prevalence of HTLV-I, but it seems to be an underestimation of the seroprevalence in the population (24).
Comparison of the regional distribution of the carriers in the present study revealed a significant increase of the HTLV-I carriers in Kalaleh area. The observed changes could be considered mainly due to the migration of people from other areas. This interpretation is supported by the observation of ethno-epidemiology studies on HTLV-I carriers who are either born in the endemic areas or the descendants of migrants from those areas. It has been reported that some ethnically defined factors are likely to be associated with HTLV-I diseases among HTLV-I endemic populations (25).
Previous studies have demonstrated that HTLV-I infection increases with age (26-29). Also, there is a linear trend of an increasing age in the general population of Salvador and Brazil, as the endemic areas of HTLV-I infection (30).
In our study, the age distribution of carriers showed presence of HTLV-I carrier in age groups more than 20 years old and the largest number of carriers was observed in this age group. Recent studies showed the number of carriers in the age groups between 0–9 and 50–59 significantly decreased (13). This decline could be explained by changes in the life styles of people such as smaller number of children per family and shorter period of breast feeding. However, exact reasons remain to be elucidated, especially considering the same tendency observed in the study of Brazilian people (30). Taken together, population-based studies on the HTLV-I infection in all age groups suggest that multidisciplinary factors might be involved in virus transmission (13, 31-33).
In conclusion, serological screening of pregnant women, and the blood donors, along with the prevention of mother-to-child infection transmission by stopping breast feeding will greatly reduce the vertical transmission. In addition, there still remain other modalities of HTLV-I infection, such as sexual transmission and possible transuterine infection (34-37). Further comprehensive and detailed epidemiological and molecular study should be considered to provide valuable information based on the risk factors involved in HTLV-I infection and the cost effective vaccine for viable objective of prophylactic intervention in endemic areas.
Acknowledgment
We would like to thank all the participants for letting us complete this study. Also we should appreciate the personnel of Deputy of Health and their laboratory for their cooperation. This work was supported by Deputy of Research and Technology of Golestan University of Medical Sciences, Golestan, Iran.
References
- 1.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 2.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Thé G, Kazanji M. An HTLV-I/II vaccine: from animal models to clinical trials? J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S191–198. doi: 10.1097/00042560-199600001-00029. [DOI] [PubMed] [Google Scholar]
- 4.Pawson R, Mufti GJ, Pagliuca A. Management of adult T-cell leukaemia/lymphoma. Br J Haematol. 1998;100:453–458. doi: 10.1046/j.1365-2141.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves DU, Proietti FA, Ribas JG, Araujo MG, Pinheiro SR, Guedes AC, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 7.Hino S, Doi H, Yoshikuni H, Sugiyama H, Yamabe T, Tsuji Y, et al. HTLV-I carrier mothers with high-titer antibody are at high risk as a source of infection. Jpn J Cancer Res. 1987;78:1156–1158. [PubMed] [Google Scholar]
- 8.Wiktor SZ, Pate EJ, Rosenberg PS, Barnett M, Palmer P, Medeiros D, et al. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breastfeeding. J Hum Virol. 1997;1:37–44. [PubMed] [Google Scholar]
- 9.Nomura K, Utsunomiya A, Furushou H, Tara M, Hazeki M, Tokunaga M, et al. A family predisposition to adult T-cell leukemia. J Clin Exp Hematop. 2006;46:67–71. doi: 10.3960/jslrt.46.67. [DOI] [PubMed] [Google Scholar]
- 10.Tajima K, Sonoda S. Ethnoepidemiology, a new paradigm, for studying cancer risk factors and prevention strategy. In: Tajima K, Sonoda S, editors. Ethnoepidemiology of Cancer. Gann Monograph No.44. Tokyo: Japanese Scientific Societies Press; 1996. pp. 3–12. [Google Scholar]
- 11.Tavanai Sani A. Serologic prevalence of HTLV-I among blood donors in Mashhad (North eastern of Iran) Arch Iran Med. 2001;4:25–26. [Google Scholar]
- 12.Farid R, Shirdel A. Phylogenetic of HTLV-I in Iranian born in Mashhad. Arch Iran Med. 1999;2:24–25. [Google Scholar]
- 13.Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SKh, Ahmadi S, et al. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: A population-based seroepidemiology survey. J Clin Virol. 2011;52:172–176. doi: 10.1016/j.jcv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Moradi A, Mansurian AR, Ahmadi AR, Ghaemi E, Kalavi KH, Marjani A, et al. Prevalence of HTLV-I among major thalassemic patients in Gorgan (South East of Caspian Sea) J Appl Sci. 2008;8:391–393. [Google Scholar]
- 15.Giuliani M, Rezza G, Lepri AC, Di Carlo A, Maini A, Crescimbeni E, et al. Risk factors for HTLV-I and II in individuals attending a clinic for sexually transmitted diseases. Sex Transm Dis. 2000;27:87–92. doi: 10.1097/00007435-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wang HR, Yan YS, Zhang QW, Zheng J, Liu JM, Feng YY, et al. Sero-epidemiological study on the human T-cell leukemia virus type I/II infection in the east coastal areas of Fujian province. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:428–430. [PubMed] [Google Scholar]
- 17.Biglione MM, Astarloa L, Salomón HE. High prevalence of HTLV-I and HTLV-II among blood donors in Argentina: a South American health concern. AIDS Res Hum Retroviruses. 2005;21:1–4. doi: 10.1089/aid.2005.21.1. [DOI] [PubMed] [Google Scholar]
- 18.Mozzi F, Rebulla P, Lillo F. HIV and HTLV infections in 1305 transfusion-dependent thalassemics in Italy. AIDS. 1992;6:505–508. doi: 10.1097/00002030-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Al-Mufti S, Voevodin A, Ahmed S, Al Hamdan S, Al-Basheer AA. Seroprevalence of human T-Cell leukemia/lymphoma virus type I and type II (HTLV-I/HTLV-II) infection among volunteer blood donors in Kuwait. Med Princ Pract. 1999;1:45–50. [Google Scholar]
- 20.Pour Karim MR, Khamisi Pour GhR, Zandi K, Roustaei MH. Prevalence of anti-HTLV-I & anti-HTLV-II antibodies in blood donors in Bushehr province. Iran South Med J. 2004;2:164–161. [Google Scholar]
- 21.Moradi A, Yaghob Nezhad Z, Mohagheghi AH, Shahraki Sh, Borji A, Firoozkohi MR, et al. Seroepidemiology of HTLV-I antibody in the Thalassemic patients in Zahedan and Zaboul cities in 2001. J Zanjan Univ Med Sci Health Services . 2003;43:47–43. [Google Scholar]
- 22.Goubau P, Carton H, Kazadi K, Muya KW, Desmyter J. HTLV seroepidemiology in a central African population with high incidence of tropical spastic paraparesis. Trans R Soc Trop Med Hyg. 1990;84:577–579. doi: 10.1016/0035-9203(90)90046-h. [DOI] [PubMed] [Google Scholar]
- 23.Vrielink H, Reesink HW. HTLV-I/II prevalence in different geographic locations. Transfus Med Rev. 2004;18:46–57. doi: 10.1016/j.tmrv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T. Current status of HTLV-I infection. Int J Hematol. 2011;94:430–434. doi: 10.1007/s12185-011-0934-4. [DOI] [PubMed] [Google Scholar]
- 25.Sonoda Sh, Li HC, Tajima K. Ethnoepidemiology of HTLV-I related diseases: Ethnic determinants of HTLV-I susceptibility and its worldwide dispersal. Cancer Sci. 2011;102 doi: 10.1111/j.1349-7006.2010.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy EL, Figueroa JP, Gibbs WN, Holding-Cobham M, Cranston B, Malley K, et al. Holding-Cobham M, Cranston B, Malley K, et al. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am J Epidemiol . 1991;133:1114–1124. doi: 10.1093/oxfordjournals.aje.a115824. [DOI] [PubMed] [Google Scholar]
- 27.Biggar RJ, Johnson BK, Oster C, Sarin PS, Ocheng D, Tukei P, et al. Regional variation in prevalence of antibody against human T-lymphotropic virus types I and III in Kenya, East Africa. Int J Cancer. 1985;35:763–767. doi: 10.1002/ijc.2910350611. [DOI] [PubMed] [Google Scholar]
- 28.Wiktor SZ, Piot P, Mann JM, Nzilambi N, Francis H, Vercauteren G, et al. Human T cell lymphotropic virus type I (HTLV-I) among female prostitutes in Kinshasa. Zaire. J Infect Dis. 1990;161:1073–1077. doi: 10.1093/infdis/161.6.1073. [DOI] [PubMed] [Google Scholar]
- 29.Kondo T, Kono H, Miyamoto N, Yoshida R, Toki H, Matsumoto I, et al. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. Int J Cancer. 1989;43:1061–1064. doi: 10.1002/ijc.2910430618. [DOI] [PubMed] [Google Scholar]
- 30.Dourado I, Alcantara LC, Barreto ML, da Gloria Teixeira M, Galvão-Castro B. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr. 2003;34:527–531. doi: 10.1097/00126334-200312150-00013. [DOI] [PubMed] [Google Scholar]
- 31.Armah HB, Narter-Olaga EG, Adjei AA, Asomaning K, Gyasi RK, Tettey Y. Seroprevalence of human T-cell lymphotropic virus type I among pregnant women in Accra, Ghana. J Med Microbiol. 2006;55:765–770. doi: 10.1099/jmm.0.46426-0. [DOI] [PubMed] [Google Scholar]
- 32.Kazanji M, Gessain A. Human T-cell lymphotropic virus types I and II (HTLVI/II) in French Guiana: clinical and molecular epidemiology. Cad Saude Publica. 2003;19:1227–1240. doi: 10.1590/s0102-311x2003000500002. [DOI] [PubMed] [Google Scholar]
- 33.Plancoulaine S, Buigues RP, Murphy EL, van Beveren M, Pouliquen JF, Joubert M, et al. Demographic and familial characteristics of HTLV-I infection among an isolated, highly endemic population of African origin in French Guiana. Int J Cancer. 1998;76:331–336. doi: 10.1002/(sici)1097-0215(19980504)76:3<331::aid-ijc8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Astier-Gin T, Portail JP, Londos-Gagliardi D, Moynet D, Blanchard S, Dalibart R, et al. Neutralizing activity and antibody reactivity toward immunogenic regions of the human T cell leukemia virus type I surface glycoprotein in sera of infected patients with different clinical states. J Infect Dis. 1997;175:716–719. doi: 10.1093/infdis/175.3.716. [DOI] [PubMed] [Google Scholar]
- 35.Londos-Gagliardi D, Armengaud MH, Freund F, Dalibart R, Moze E, Huet S, et al. Antibodies directed against a variable and neutralizable region of the HTLV-I envelope surface glycoprotein. Leukemia. 1997;11:38–41. [PubMed] [Google Scholar]
- 36.Hadlock KG, Rowe J, Perkins S, Bradshaw P, Song GY, Cheng C, et al. Neutralizing human monoclonal antibodies to conformational epitopes of human T-cell lymphotropic virus type 1 and 2 gp46. J Virol. 1997;71:5828–5840. doi: 10.1128/jvi.71.8.5828-5840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadlock KG, Rowe J, Foung SK. The humoral immune response to human T-cell lymphotropic virus type 1 envelope glycoprotein gp46 is directed primarily against conformational epitopes. J Virol. 1999;73:1205–1212. doi: 10.1128/jvi.73.2.1205-1212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]