Summary
Humans rely on the fovea, the small region of the retina where receptors are most densely packed, for seeing fine spatial detail. Outside the fovea, it is well established that a variety of visual functions progressively decline with eccentricity [1–5]. In contrast, little is known about how vision varies within the central fovea, as incessant microscopic eye movements prevent isolation of adjacent foveal locations [6–8]. Using a new method for restricting visual stimulation to a selected retinal region, we examined the discrimination of fine patterns at different eccentricities within the foveola. We show that high-acuity judgments are impaired when stimuli are presented just a few arcminutes away from the preferred retinal locus of fixation. Furthermore, we show that this dependence on eccentricity is normally counter-balanced by the occurrence of precisely directed microsaccades, which bring the preferred fixation locus onto the stimulus. Thus, contrary to common assumptions, vision is not uniform within the foveola, but targeted microscopic eye movements compensate for this lack of homogeneity. Our results reveal that microsaccades, like larger saccades, enable examination of the stimulus at a finer level of detail and suggest that a reduced precision in oculomotor control may be responsible for the visual acuity impairments observed in various disorders.
Results and Discussion
Visual functions are believed to be approximately uniform in the foveola, a tiny region covering approximately half the width of a thumb at arm’s length (less than 1° in visual angle). However, mapping foveal vision is an extremely challenging task, primarily because of involuntary fixational eye movements, the microscopic drifts and microsaccades that humans continually perform when attempting to maintain steady gaze [6–8]. These eye movements cause two main methodological problems. First, they create uncertainty at any given time on the exact location of the line of sight—and thus the position of the stimulus on the retina (Figure S1A available online)—a problem that persists even during measurement of oculomotor activity (Figures S1B and S1C). Second, they move the stimulus over many photoreceptors, effectively preventing isolation of closely spaced regions on the retina, especially with the long exposures necessary to achieve high visual acuity [9, 10]. Both effects are likely to homogenize measurements at adjacent retinal locations and may have contributed to conflicting reports on visual acuity [1, 11] and the widespread notion of approximately uniform foveal vision.
In this study, we examined the discrimination of fine spatial patterns at different eccentricities within the fovea. To overcome the problems of previous studies, we relied on a combination of techniques. First, we developed a new procedure for localizing the line of sight, which greatly improved determination of the position of the stimulus on the retina over standard methods (Figures S1D and S1E; see Supplemental Experimental Procedures). Second, we presented stimuli under conditions of retinal stabilization [12–14]; that is, we updated the display according to the subject’s eye movements so as to maintain the stimulus immobile on the retina, thus avoiding contamination from multiple retinal regions. Third, we designed a high-acuity task, which delivered more natural visual input within confined retinal regions than the brief flashes of previous studies. Together, these features enabled selective stimulation of a narrow region within the fovea centered at the desired eccentricity on the horizontal meridian.
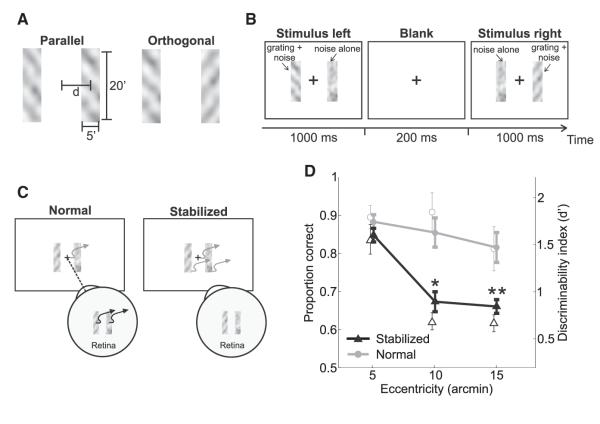
In a forced-choice task, subjects reported whether two sequentially presented gratings were parallel or orthogonal. Gratings were embedded within narrow (5′ wide) rectangular noise fields, which restricted stimulation around the same eccentricity angles in the nasal and temporal hemifields (Figures 1A and 1B). Figure 1D shows results obtained with stimuli at three different eccentricities within the fovea. Even though the stimulated regions were adjacent to each other and their centers only 5′ apart, performance dropped drastically with eccentricity: discrimination percentages measured with stimuli centered at both 10′ and 15′ eccentricity were significantly lower than the values measured at 5′ (Figure 1D, stabilized condition). For comparison, Figure 1D also shows levels of performance measured in the presence of the physiological motion of the retinal image, when fixational eye movements were allowed to normally move the stimuli on the retina (normal condition). In this case, results varied little with eccentricity and discrimination percentages with stimuli at both 10′ and 15′ were only slightly lower than—and statistically undistinguishable from— those measured at 5′ (p > 0.1; two-tailed paired t test). Both levels of performance were significantly higher than the corresponding values measured under retinal stabilization (p < 0.05; two-tailed paired t test). Similar results were also obtained in a control experiment, which disentangled interstimulus distance and eccentricity. Discrimination performance decreased with eccentricity under retinal stabilization but not during normal viewing, even when the two bars were displayed at the same retinal (or monitor) location (Figure S2).
Figure 1.
Experimental Procedure and Results
(A) In a forced-choice task, subjects reported whether two gratings (11 cycles/degree tilted by ±45°) were parallel or orthogonal.
(B) Gratings appeared within two rectangular noise bars centered at the desired eccentricity d. They were displayed sequentially first in the left and then in the right bar while subjects maintained fixation at the center of the display (cross).
(C) Stimuli were either displayed at fixed positions on the screen (normal) and normally moved on the retina because of fixational eye movements or at fixed locations on the retina (stabilized) and moved on the display under computer control to compensate for the subject’s eye movements.
(D) Average subject performance (n = 4) as a function of the stimulus eccentricity in the two conditions. Both proportions of correct responses (filled symbols) and d’ values (empty symbols) are shown. In each condition, asterisks mark statistically significant differences with respect to the proportions of correct responses at 5′ (*p < 0.05; **p < 0.005; two-tailed paired t test). Error bars represent SEM.
See also Figures S1 and S2.
The two conditions in Figure 1D only differed for the consequences of oculomotor activity on retinal stimulation: fixational eye movements moved the stimulus on the retina during normal viewing but not under retinal stabilization (Figure 1C).Figure 2 summarizes the effects of eye movements in the normal viewing condition (see also Figure S3). Although minute, gaze shifts were not random; subjects looked preferentially toward the grating: to the left side of the monitor when the grating was displayed in the left bar and to the right side of the monitor when the grating appeared in the right bar (see examples in Figure 2A and Movie S1, Movie S2, and Movie S3). Across subjects, this difference in gaze position was significantly correlated with the eccentricity of the stimuli (r = 0.75; p = 0.01) and was visible even with stimuli at 5′ eccentricity, so that the mean horizontal gaze positions measured during presentation of the left and right gratings differed significantly at all tested eccentricity angles (Figure 2B).
Figure 2.
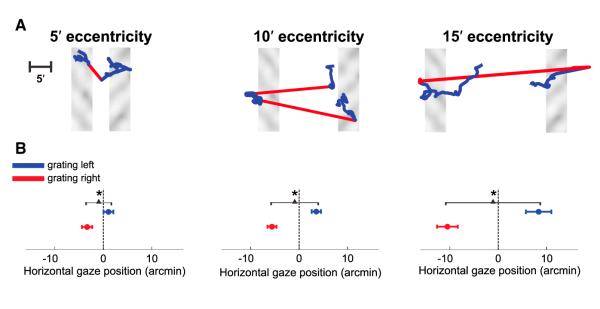
Gaze Location in Normal, Unstabilized Trials
(A) Examples of fixational eye movements. Red and blue segments represent microsaccades and drifts, respectively.
(B) Average horizontal position of the center of gaze during presentation of each of the two gratings.
In both (A) and (B), different panels show data obtained with stimuli at different eccentricities. Error bars represent SEM. Asterisks mark significant differences (p < 0.05; two-tailed paired t test). The center of gaze is defined in this study as the point on the screen projecting onto the center of the preferred retinal locus of fixation. This point was estimated by means of a preliminary calibration procedure, in which the observer maintained prolonged fixation on markers at known positions on the display. See also Figure S3 and Movies S1, S2, and S3.
Gaze shifts were primarily caused by microsaccades rather than fixational drifts. Microsaccades occurred frequently in the experiments and were clearly modulated by the task: they were more likely to occur in the periods immediately following the appearance of each grating (Figure S4C), and both their frequency (Figure S4A) and amplitude (Figure S4B) increased with the eccentricity of visual stimulation [rate: F(2,3) = 7.02, p = 0.03; amplitude: F(2,3) = 14.89, p = 0.005; two-way ANOVA]. Furthermore, regardless of the eccentricity of the stimulus, microsaccades were always more likely to land close to the bar in which the grating was currently displayed than close to the other bar, while the probability of terminating on the background region far from the two bars was always low (Figure 3A). These precise movements were responsible for the average changes in gaze position of Figure 2B: when microsaccades were removed from the eye movement traces and the remaining periods of drifts were concatenated, the line of sight no longer moved away from the center of the screen (Figure 3B). Thus, in the normal condition, stimuli did not remain on the retina around their intended eccentricity angles as under retinal stabilization but moved, because of microsaccades, toward regions at lower eccentricities, where performance in the task was higher (Figure 1D).
Figure 3.
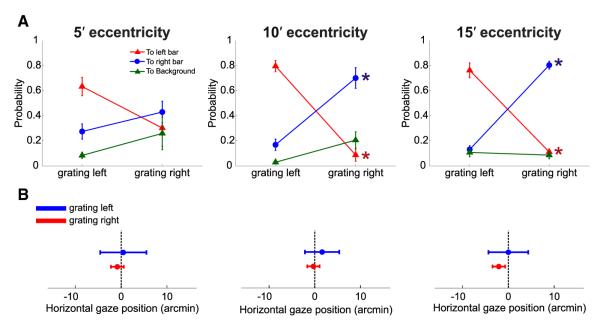
Microsaccades and Gaze Position in the Normal Condition
(A) Proportions of microsaccades landing on one of the two bars and on the background region during the two periods of grating presentation. Microsaccades were more likely to relocate the preferred retinal locus of fixation on the bar currently displaying the grating than anywhere else. Error bars represent SEM. Asterisks mark significant differences between the probabilities of landing in a given region of the image in the two temporal intervals (p < 0.01; two-tailed paired t test).
(B) Average horizontal gaze position during presentation of each of the two gratings (same data as in Figure 2B) after removal of the microsaccades from the recorded eye movement traces. Drift segments for the entire trial duration were concatenated by subtracting all microsaccade displacements, so that the gaze position of the first sample after a microsaccade was made equal to that of the last sample before the microsaccade. Error bars represent SEM.
Together, the results of Figures 1, 2, and 3 suggest that microsaccades normally compensate for nonuniform visual capabilities across the foveola: discrimination performance dropped significantly with eccentricity under retinal stabilization but not during normal viewing, when microsaccades enabled examination of the stimulus with the preferred retinal locus.
To directly assess the importance of microsaccadic gaze shifts, we examined performance in the normal (unstabilized) trials in which microsaccades did not occur. These trials were very rare, particularly during stimulation at larger eccentricities (only 4% of the trials at 15′), which by itself argues in favor of the importance of microsaccades in this task. Figure 4 compares performance with and without microsaccades for two subjects who were tested extensively in order to collect sufficiently large pools of drift-only trials. For both observers, performance dropped in the absence of microsaccades: levels of discrimination at 15′ eccentricity were lower than those measured in the presence of microsaccades, an effect already significant at 10′ eccentricity for one subject. This reduction in performance was similar to that observed under retinal stabilization; for both subjects and at all three eccentricity angles, results in the absence of microsaccades were statistically indistinguishable from those measured with a stabilized retinal image (p > 0.21; paired two-tailed t test).
Figure 4.
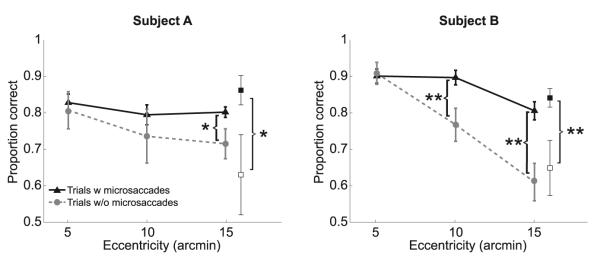
Necessity of Microsaccades
Discrimination performance in the normal (unstabilized) trials with and without microsaccades. The two panels show data for two different observers. The proportions of correct responses in the trials in which microsaccades brought the center of gaze within 5′ from both bars (filled squares) and in the remaining trials, in which microsaccades did occur but were less precise (empty squares), are also shown. Error bars represent SEM; *p < 0.05; **p < 0.01; paired two-tailed t tests.
These impairments were not caused by lack of attention or the absence of the temporal transients of microsaccades. Figure 4 also compares levels of performance in the trials in which microsaccades precisely relocated gaze, enabling the retinal projections of both gratings to fall within 5′ eccentricity and those in which microsaccades occurred but were not as precise. Performance was significantly higher in the trials in which both gratings were accurately fixated. Thus, precise microsaccadic relocations of gaze were necessary for ensuring approximately equal levels of performance during viewing of stimuli at different foveal eccentricities.
The results of this study show that fine spatial vision is not uniform across the central fovea but drops sharply with minute displacements from the preferred retinal locus of fixation. Although idiosyncratic differences exist, cone density is known to decrease rapidly within the fovea outside a very small region of a few squared arcminutes [15]. Thus, the decline in performance with eccentricity observed in our experiments could be the consequence of the variable distribution of cone spacing. However, our stimuli were well within the spatial frequency limits of foveal vision [16] and should have been easily discriminable at all tested eccentricities. Furthermore, subjects used microsaccades to bring the stimulus close to the preferred retinal locus of fixation, a region which not always coincides with the area of highest cone density [17]. These observations suggest that other factors, in addition to cone spacing, contributed to the eccentricity effects shown in Figures 1 and 4. An interesting possibility is that these factors are not restricted to the spatial domain but that changes in temporal sensitivity may also occur within the fovea. Fine spatial vision seems to depend on the temporal modulations resulting from ocular drift, the smooth motion of the eye that continually occurs during the intersaccadic periods [14, 18–22]. Thus, both the local spatial and temporal characteristics of neural processing may be responsible for the emergence of a preferred fixation locus, a region in which neurons optimally extract fine spatial information from temporal modulations.
These findings also provide an answer to the long-investigated and controversial question of the visual function of microsaccades [7, 8]. Microsaccades have been deemed necessary to prevent, during natural viewing, the perceptual fading experienced in the laboratory when retinal image motion is eliminated [23, 24], but the plausibility of this theory has been criticized on multiple grounds [7, 25–28]. In our experiments, image fading did not appear to play any obvious role in the production of microsaccades. Subjects did not notice any fading, not even under retinal stabilization, possibly because stimuli were flashed at high contrast and for brief periods, with an abrupt change caused by the blank interval in between the two gratings, a procedure that minimized neural adaptation. Furthermore, the temporal transients resulting from microsaccades were by themselves not sufficient to improve performance. As shown in Figure 4, discrimination was impaired when microsaccades did occur but were not precisely directed toward the stimuli. Thus, our findings cannot be explained by a mere refreshing of the image following microsaccades.
In contrast, our results show that microsaccades are critical for high-acuity judgments, as they serve, at a microscopic scale, the same explorative function as larger saccades, as long hypothesized [29]: both movements reposition the stimulus on the retina to enable its examination at a finer level of detail. These data provide an explanation for the previous observation of precisely directed microsaccades in high-acuity visuomotor tasks [30] and are consistent with recent findings highlighting the similarities between microsaccades and saccades in terms of the underlying neural substrate [31, 32], their impact on neural responses [33], and their associated perceptual consequences [34–36]. Furthermore, our results imply that fine spatial vision may be compromised when microsaccades are not precisely executed, raising the possibility for a causal link between the abnormal fixational eye movements and the reduced visual acuity that coexist in various disorders [37–44]. Further work is necessary to investigate this hypothesis as well as the factors responsible for the emergence of a preferred fixation locus in the fovea.
Experimental Procedures
Stimuli were rendered by means of a custom-developed system for flexible gaze-contingent display control [45] on a fast-phosphor cathode ray tube monitor (Iyama HM204DT) at a vertical refresh rate of 150 Hz. Stimuli were observed monocularly with the right eye, while the left eye was patched. The movements of the right eye were measured by means of a Generation 6 Dual Purkinje Image eye tracker (Fourward Technologies) and sampled at 1 kHz. A dental-imprint bite bar and a headrest prevented head movements. Informed consent was obtained from all the participants following the procedures approved by the Boston University Charles River Campus Institutional Review Board.
Experiments were conducted in complete darkness with visual stimulation restricted to the two rectangular bars (5′ × 20′) centered at the same absolute eccentricity angle on the horizontal meridian in the nasal and temporal hemifields. In the normal condition, eccentricity angles were measured on the screen relative to the required location of fixation (the center of the display), as it is customary in behavioral measurements of visual functions. Stimuli remained immobile on the screen and normally shifted on the retina with eye movements. In the stabilized condition, eccentricity angles were measured on the retina, relative to the center of gaze (the estimated center of the preferred retinal locus of fixation). Stimuli moved on the screen to compensate for the subject’s eye movements, so that they remained at fixed retinal locations. Subjects did not report any stimulus fading under retinal stabilization and were, in general, unable to tell whether or not a trial was stabilized. To avoid possible stabilization artifacts, performance under retinal stabilization was evaluated over the trials without microsaccades. Results did not change if the stabilized trials with microsaccades were also included in the analysis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant EY18363 and National Science Foundation grant BCS-1127216. We thank Harold E. Bedell, Alice Cronin-Golomb, Austin Roorda, Max Snodderly, and Jonathan D. Victor for their helpful comments on an earlier version of the manuscript.
Footnotes
Supplemental Information Supplemental Information includes Supplemental Experimental Procedures, four figures, and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.07.007.
References
- 1.Weymouth FW, Hines DC, Acres LH, Raaf JE, Wheeler MC. Visual acuity within the area centralis and its relation to eye movements and fixation. Am. J. Ophthalmol. 1928;11:947–960. [Google Scholar]
- 2.Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Res. 1979;19:1187–1195. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- 3.Legge GE, Kersten D. Contrast discrimination in peripheral vision. J. Opt. Soc. Am. A. 1987;4:1594–1598. doi: 10.1364/josaa.4.001594. [DOI] [PubMed] [Google Scholar]
- 4.Hansen T, Pracejus L, Gegenfurtner KR. Color perception in the intermediate periphery of the visual field. J. Vis. 2009;9:1–12. doi: 10.1167/9.4.26. [DOI] [PubMed] [Google Scholar]
- 5.Nandy AS, Tjan BS. Saccade-confounded image statistics explain visual crowding. Nat. Neurosci. 2012;15:463–469. S1–S2. doi: 10.1038/nn.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- 7.Collewijn H, Kowler E. The significance of microsaccades for vision and oculomotor control. J. Vis. 2008;8:1–21. doi: 10.1167/8.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolfs M. Microsaccades: small steps on a long way. Vision Res. 2009;49:2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Baron WS, Westheimer G. Visual acuity as a function of exposure duration. J. Opt. Soc. Am. 1973;63:212–219. doi: 10.1364/josa.63.000212. [DOI] [PubMed] [Google Scholar]
- 10.Klein SA, Levi DM. Position sense of the peripheral retina. J. Opt. Soc. Am. A. 1987;4:1543–1553. doi: 10.1364/josaa.4.001543. [DOI] [PubMed] [Google Scholar]
- 11.Millodot M. Variation of visual acuity in the central region of the retina. Br. J. Physiol. Opt. 1972;27:24–28. [PubMed] [Google Scholar]
- 12.Keesey UT. Effects of involuntary eye movements on visual acuity. J. Opt. Soc. Am. 1960;50:769–774. doi: 10.1364/josa.50.000769. [DOI] [PubMed] [Google Scholar]
- 13.Millodot M. Foveal and extra-foveal acuity with and without stabilized retinal images. Br. J. Physiol. Opt. 1966;23:75–106. [PubMed] [Google Scholar]
- 14.Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447:851–854. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- 15.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 16.Rossi EA, Roorda A. The relationship between visual resolution and cone spacing in the human fovea. Nat. Neurosci. 2010;13:156–157. doi: 10.1038/nn.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putnam NM, Hofer HJ, Doble N, Chen L, Carroll J, Williams DR. The locus of fixation and the foveal cone mosaic. J. Vis. 2005;5:632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- 18.Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. doi: 10.1016/s0896-6273(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 19.Rucci M. Fixational eye movements, natural image statistics, and fine spatial vision. Network. 2008;19:253–285. doi: 10.1080/09548980802520992. [DOI] [PubMed] [Google Scholar]
- 20.Poletti M, Rucci M. Oculomotor synchronization of visual responses in modeled populations of retinal ganglion cells. J. Vis. 2008;8:1–15. doi: 10.1167/8.14.4. [DOI] [PubMed] [Google Scholar]
- 21.Ahissar E, Arieli A. Seeing via miniature eye movements: A dynamic hypothesis for vision. Front. Comput. Neurosci. 2012;6:89. doi: 10.3389/fncom.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuang X, Poletti M, Victor JD, Rucci M. Temporal encoding of spatial information during active visual fixation. Curr. Biol. 2012;22:510–514. doi: 10.1016/j.cub.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ditchburn RW, Fender DH, Mayne S. Vision with controlled movements of the retinal image. J. Physiol. 1959;145:98–107. doi: 10.1113/jphysiol.1959.sp006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCamy MB, Otero-Millan J, Macknik SL, Yang Y, Troncoso XG, Baer SM, Crook SM, Martinez-Conde S. Microsaccadic efficacy and contribution to foveal and peripheral vision. J. Neurosci. 2012;32:9194–9204. doi: 10.1523/JNEUROSCI.0515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowler E, Steinman RM. Small saccades serve no useful purpose: reply to a letter by R. W. Ditchburn. Vision Res. 1980;20:273–276. doi: 10.1016/0042-6989(80)90113-3. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM. Gaze control under natural conditions. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT Press; Cambridge: 2003. pp. 1339–1356. [Google Scholar]
- 27.Poletti M, Rucci M. Eye movements under various conditions of image fading. J. Vis. 2010;10:1–18. doi: 10.1167/10.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagan I. Microsaccades and image fading during natural vision. 2012 http://www.jneurosci.org/content/32/27/9194/reply.
- 29.Cunitz RJ, Steinman RM. Comparison of saccadic eye movements during fixation and reading. Vision Res. 1969;9:683–693. doi: 10.1016/0042-6989(69)90125-4. [DOI] [PubMed] [Google Scholar]
- 30.Ko H-K, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat. Neurosci. 2010;13:1549–1553. doi: 10.1038/nn.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafed ZM, Krauzlis RJ. Similarity of superior colliculus involvement in microsaccade and saccade generation. J. Neurophysiol. 2012;107:1904–1916. doi: 10.1152/jn.01125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engbert R. Computational modeling of collicular integration of perceptual responses and attention in microsaccades. J. Neurosci. 2012;32:8035–8039. doi: 10.1523/JNEUROSCI.0808-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagan I, Gur M, Snodderly DM. Saccades and drifts differentially modulate neuronal activity in V1: effects of retinal image motion, position, and extraretinal influences. J. Vis. 2008;8:1–25. doi: 10.1167/8.14.19. [DOI] [PubMed] [Google Scholar]
- 34.Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- 35.Lavergne L, Vergilino-Perez D, Lappe M, Doré-Mazars K. The spatial pattern of peri-saccadic compression for small saccades. J. Vis. 2010;10:17. doi: 10.1167/10.14.17. [DOI] [PubMed] [Google Scholar]
- 36.Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron. 2013;77:775–786. doi: 10.1016/j.neuron.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Ciuffreda KJ, Kenyon RV, Stark L. Fixational eye movements in amblyopia and strabismus. J. Am. Optom. Assoc. 1979;50:1251–1258. [PubMed] [Google Scholar]
- 38.Bedell HE, White JM, Abplanalp PL. Variability of foveations in congenital nystagmus. Clin. Vis. Sci. 1989;4:247–252. [Google Scholar]
- 39.Abadi RV, Pascal E. Visual resolution limits in human albinism. Vision Res. 1991;31:1445–1447. doi: 10.1016/0042-6989(91)90063-b. [DOI] [PubMed] [Google Scholar]
- 40.Simmers AJ, Gray LS, Winn B. The effect of abnormal fixational eye movements upon visual acuity in congenital nystagmus. Curr. Eye Res. 1999;18:194–202. doi: 10.1076/ceyr.18.3.194.5374. [DOI] [PubMed] [Google Scholar]
- 41.Cesarelli M, Bifulco P, Loffredo L, Bracale M. Relationship between visual acuity and eye position variability during foveations in congenital nystagmus. Doc. Ophthalmol. 2000;101:59–72. doi: 10.1023/a:1002702609387. [DOI] [PubMed] [Google Scholar]
- 42.Parkinson J, Maxner C. Eye movement abnormalities in Alzheimer disease: case presentation and literature review. Am. Orthopt. J. 2005;55:90–96. doi: 10.3368/aoj.55.1.90. [DOI] [PubMed] [Google Scholar]
- 43.Wark HA, Garell PC, Walker AL, Basso MA. A case report on fixation instability in Parkinson’s disease with bilateral deep brain stimulation implants. J. Neurol. Neurosurg. Psychiatry. 2008;79:443–447. doi: 10.1136/jnnp.2007.117192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilikete C, Jasse L, Vukusic S, Durand-Dubief F, Vardanian C, Pélisson D, Vighetto A. Persistent ocular motor manifestations and related visual consequences in multiple sclerosis. Ann. N Y Acad. Sci. 2011;1233:327–334. doi: 10.1111/j.1749-6632.2011.06116.x. [DOI] [PubMed] [Google Scholar]
- 45.Santini F, Redner G, Iovin R, Rucci M. EyeRIS: a general-purpose system for eye-movement-contingent display control. Behav. Res. Methods. 2007;39:350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.