Abstract
Purpose
The molecular heterogeneity of glioblastoma has been well recognized and has resulted in the generation of molecularly defined subtypes. These subtypes (classical, neural, mesenchymal, and proneural) are associated with particular signaling pathways and differential patient survival. Less understood is the correlation between these glioblastoma subtypes with immune system effector responses, immune suppression and tumor-associated and tumor-specific antigens. The role of the immune system is becoming increasingly relevant to treatment as new agents are being developed to target mediators of tumor-induced immune suppression which is well documented in glioblastoma.
Experimental Design
To ascertain the association of antigen expression, immune suppression, and effector response genes within glioblastoma subtypes, we analyzed the Cancer Genome Atlas (TCGA) glioblastoma database.
Results
We found an enrichment of genes within the mesenchymal subtype that are reflective of anti-tumor proinflammatory responses, including both adaptive and innate immunity and immune suppression.
Conclusions
These results indicate that distinct glioma antigens and immune genes demonstrate differential expression between glioblastoma subtypes and this may influence responses to immune therapeutic strategies in patients depending on the subtype of glioblastoma they harbor.
Keywords: glioblastoma, immune activation, immune suppression, tumor antigens
Introduction
Glioblastoma remains the most common malignant primary brain tumor in adults. Despite aggressive treatment with surgical resection, radiation, and chemotherapy, the tumor ultimately recurs. A major obstacle to treatment is the subversion of the immune system by the tumor to facilitate proliferation and malignant degeneration of tumor cells. Immune suppression is thought to play a major role in the aggressive nature of gliomas and their resistance to current therapies. The presence of immunosuppressive infiltrates such as FoxP3+ T regulatory cells (Tregs) and M2 macrophages has been documented in gliomas and in some cases, correlates with prognosis (1–3). Additionally, immune responses to glioma can be impaired by the tumor itself through expression of immunosuppressive cytokines such as TGF-β and the up regulation of the signal transducer and activator of transcription 3 (STAT3) (4). Genomic profiling of glioblastomas has shown that up to four genotypic subtypes exist, with two demonstrating marked differences in gene expression and patient survival (proneural and mesenchymal) (5). The STAT3 pathway has been shown to be the key molecular driver of the mesenchymal transformation within glioblastoma (6, 7). Additionally, STAT3 has been implicated in many mechanisms of tumor-mediated immune suppression (8–13) and is a negative prognosticator for survival in mice (14) and in human anaplastic astrocytoma patients (15). These data would suggest that the mesenchymal glioblastoma subtype may be more immune suppressive compared to other glioblastoma subtypes and possibly more refractory to immunotherapy.
Immunotherapy is an appealing treatment for gliomas because it allows for tumor specificity while minimizing collateral damage to normal brain tissue. Clinical trials using dendritic cell or peptide vaccines to target glioma cells have shown promising results (16–19). However, as with other treatments, only subsets of patients respond. This may be due to molecular and genomic factors that affect interactions between the immune system and the tumor. A recent study using glioma lysate-pulsed dendritic cell vaccination showed that glioblastomas of the mesenchymal phenotype had higher levels of CD3+ and CD8+ tumor-infiltrating lymphocytes than glioblastomas of other subtypes (20). Furthermore, patients whose tumors had the mesenchymal gene signature, survived longer after dendritic cell vaccination than controls of the same genetic subtype (20). Although this finding tends to contradict what is thought about the immune suppressive nature of mesenchymal gliomas with respect to aggressiveness and poor patient survival, it suggests that mesenchymal gliomas may be more immunogenic and more responsive to immunotherapy. Interestingly, robust immune suppressive Treg infiltration into the glioblastoma microenvironment is almost always observed with a corresponding influx of T effector cells (2). Thus, one may speculate that the tumor becomes selectively more immune suppressive as a reaction to anti-tumor effector responses. To investigate this paradox, we analyzed mRNA expression levels of immune system genes among the various glioblastoma subtypes using The Cancer Genome Atlas (TCGA) database and found not only a preferential enrichment of immune suppressive genes but also an enrichment of immune effector genes within the mesenchymal subset.
Materials and Methods
TCGA data acquisition
It should be noted that the composition of the analyzed glioblastoma tissue from the TCGA database contains genetic information from both glioma cells and tumor-supportive stroma cells including infiltrating immune cells because these components were not selectively eliminated from the specimen, which was used in the construction of the database. The glioblastoma cancer study set from the TCGA database consists of defined glioblastoma subtypes (21). The TCGA database was analyzed in two distinct ways: 1) using online knowledge bases (Ingenuity Pathway Analysis (IPA) and Uniprot Protein Knowledgebase (www.uniprot.org)) to define genes associated with immune responses; and 2) using a collated list of immune response genes from the literature with an emphasis on those previously documented to have a role in glioblastoma. For the first analysis, mRNA expression levels (available from the TCGA Research Network website: cancergenome.nih.gov, of the proneural (n=107), mesenchymal (n=119), classical (n=115) and neural (n=58) glioblastoma subsets were used as the source data available as of February 2013. This first analysis compares mRNA expression levels using the mesenchymal subset as the reference relative to the other subsets. The second analysis was through the open access cBio Cancer Genomics Portal at www.cbioportal.org (22), in which proneural (n=141), mesenchymal (n=160), classical (n=147) and neural (n=96) were used as the source data. This second analysis compares specific mRNA expression levels between glioblastoma subsets. To analyze mRNA expression based on Agilent microarray of selected genes in the four subtypes of glioma, the z-score threshold for all genes (the number of standard deviations above the mean expression level of the selected gene) was set to > 1 for each of the subtypes (an example of the syntax used for the search is: IL10: EXP>1).
Compilation of immune genes and tumor antigens
The immune effector gene sets were defined by:
-
1
The Ingenuity Pathway Analysis (IPA) tool to identify immune activators with the following characteristics: cytokine, enzyme, G-protein coupled receptor, growth factor, kinase, peptidase, phosphatase, transcription factor, transcription regulator, transmembrane receptor, transporter, and unknown and secondarily the Uniprot knowledge base (www.uniprot.org) for the terms “activation of immune response” and “immune effector process”. Of 814 genes identified by IPA and Uniprot, 734 could be found in the TCGA database and were used for the analysis.
-
2
By collating a list from the literature of documented proinflammatory effector cytokines (IFN-γ, IL-1, IL-2, IL-4, IL-7, IL-12, IL-15, TNF-α); surface markers reflective of the presence of immune effector cell responses (CD3, CD8) and their associated immune activating markers (CD80, CD86, CD40, HLA); signaling pathways reflective of adaptive immune activation (NF-κB, STAT1, IRAK, STAT4, T-bet, DNAM1, IRF7); and innate immunity and markers reflective of activation (NKp46, NKG2D, NKp30, NKp44, KLRD1, TREM1, TREM2, MIF, TLR2, TLR3, TLR4, TLR9) that participate in anti-tumor effector responses.
The immune suppressive genes were defined by:
-
3
The IPA tool to identify mediators of immune suppression with the following characteristics: cytokine, enzyme, G-protein coupled receptor, growth factor, kinase, peptidase, phosphatase, transcription factor, transcription regulator, transmembrane receptor, transporter, and unknown and secondarily the Uniprot knowledge base for the search terms related to tumor-mediated immunosuppression including: “inhibition of immunity”, “inhibition of immune system process”, “inhibition of effector immunity”, and “inhibition of immune response to tumor cell”. Of 235 genes identified by IPA and Uniprot, 218 could be found in the TCGA database and were used for the analysis.
-
4
By collating a list from the literature of documented immune suppressive cytokines and mechanisms (galectin-3, VEGF, IL-10, IL-23, TGFβ, PD-1, PD-L1, CTLA-4); chemokines (CSF-1, CCL2, CCL22); tumor supportive and immune suppressive myeloid and monocyte-related genes (CD163, CD204, MIC-1, arginase and CD47); immune suppressive signaling pathways (IL-6, gp130, Jak2, STAT3, Pim-1, SOCS3, STAT5A and STAT5B); and markers related to Tregs (CD4, ICOS, IDO, FoxP3) that participate in tumor mediated immune suppression.
The glioma antigen selection was based on the previously analyzed and compiled list by Zhang et al. (23) but was further expanded to include other known potentially overexpressed antigens such as epidermal growth factor receptor (EGFR) (24), and nucleolin (25).
Statistical analysis
A chi square test was used to compare the number of patients between glioblastoma subtypes in which mRNA expression levels of a selected gene were at least one standard deviation above mean expression in a particular subtype. To identify differentially expressed genes between the subtypes, a modified two-sample t test using the limma package was applied. Genes that were differentially expressed (DE) based on the false discovery rate (FDR) of at least <0.01 in the mesenchymal subset relative to the proneural (~7,000), neural (~9,000) and classical (~6,000) subsets were then identified. The beta-uniform mixture (BUM) model, described by Pounds and Morris (26), was used to control FDR. This list of differentially expressed genes was then dichotomized into two subgroups: genes (mRNAs) over-expressed in the mesenchymal subtype (designated DE[m>x]; where m = mesenchymal and x = subtype) and vice-versa (DE[x>m]). Pearson’s chi-square with Yates’ continuity correction and hypergeometric tests were used to determine the enrichment of the immune gene sets within these two groups.
Results
Immune genes have differential expression in glioblastoma subtypes
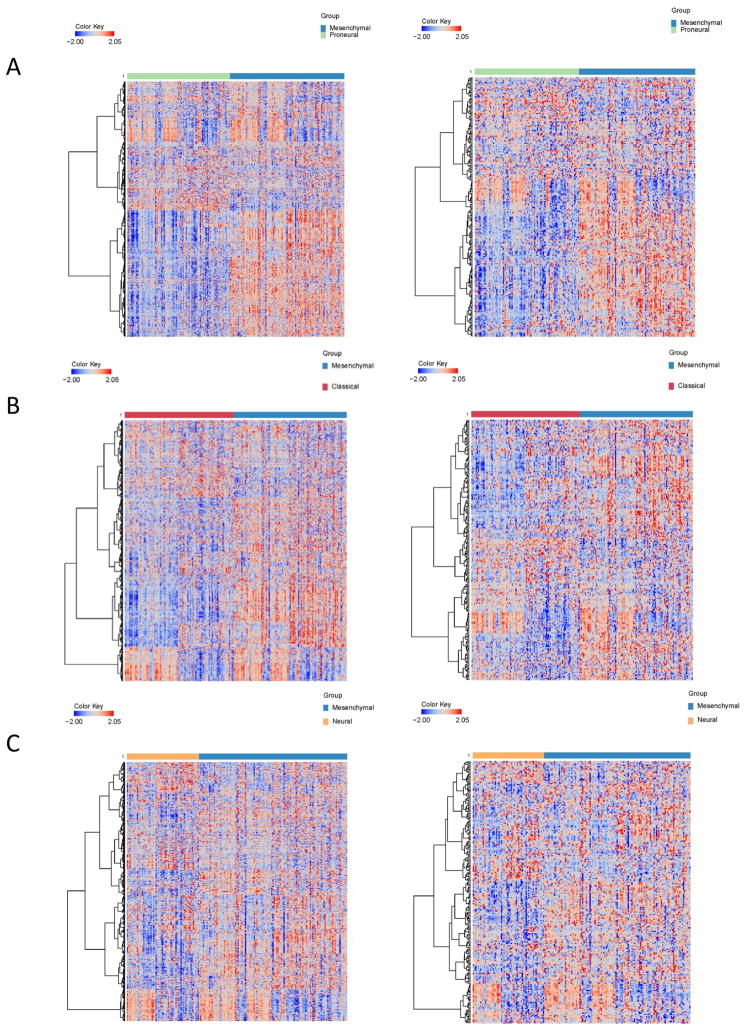
Analysis of the IPA selected immune activators and suppressors revealed that the greatest immunological diversity exists between the proneural and mesenchymal subsets (Figure 1). Only 17% (n=123) of the immune activators and 19% (n=41) of the immune suppressive genes shared differential expression amongst all glioblastoma subtypes (proneural, classical and neuronal) relative to the mesenchymal subset. This diversity of the immune activating and immune suppressive genes can distinguish the various glioblastoma subtypes on heat maps (Figure 2). To ascertain which immune genes were differentially enriched in the mesenchymal subset in comparison to the proneural subset, the immune genes were ranked and then compared to the overall 17,000 genes from the TCGA dataset (Table 1). Using both Chi-square and hypergeometric tests to ascertain if the immune genes were selected by chance, a preferential enrichment of both immune activators (Pearson’s Chi-squared test with Yates’ continuity correction p-value < 2.2×10−16; hypergeometric test p<2.2 ×10−16) and immune suppressors (Pearson’s Chi-squared test with Yates’ continuity correction p-value = 3.5×10−5; hypergeometric test p=2.04×10−5) was found in the mesenchymal subset relative to the proneural subset. Similarly, in the comparison between the classical and mesenchymal subset, immune activators (Pearson’s Chi-squared test with Yates’ continuity correction p-value < 2.2×10−16; hypergeometric p<2.2×10−16) and immune suppressors (Person’s Chi-squared test with Yates’ continuity correction p-value = 2.2×10−08; hypergeometric p=4.88×10−8) were found preferentially enriched in the mesenchymal subset. However, this preferential enrichment was not as evident in the comparison between the mesenchymal and neural subsets [immune activators (Pearson’s Chi-squared test with Yates’ continuity correction p-value = 0.2501; hypergeometric test p=0.8915) and immune suppressors (Pearson’s Chi-squared test with Yates’ continuity correction p-value = 0.5736; hypergeometric test p=0.2847)].
Fig. 1.
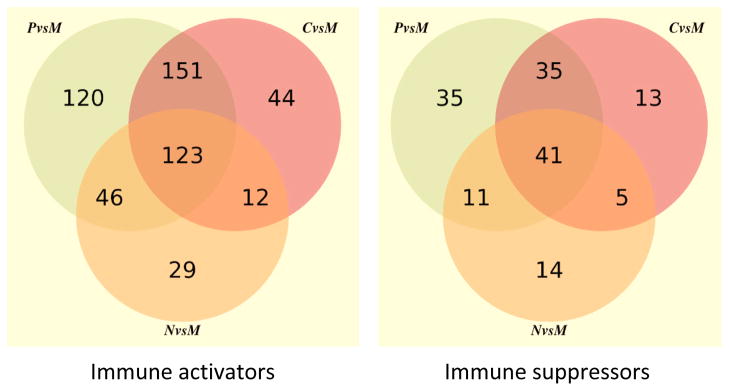
Venn diagrams demonstrating immune activators (left) and immune suppressors (right) that are differentially expressed at a FDR of 0.001.
Fig. 2.
Heatmaps of the 734 immune activator genes (left column) and 218 immune suppressor genes (right column) compared between glioblastoma subtypes. The expression values shown on the heatmap have been standardized and at ±2 standard deviations for display purposes. The scale of the values is indicated in the color key.
Table 1.
Ranking of Differentially Expressed Immune Genes between the Mesenchymal (M) and Proneural (P) Subsets
| Gene | Role | Fold change M relative to P | P value | Rank amongst all genes |
|---|---|---|---|---|
| Immune Activators | ||||
| TIMP1 | Regulates resistance to infection | 4.3 | 4.2 × 10−45 | 12 |
| SERPING1 | Complement activation | 2.3 | 1.1 × 10−34 | 71 |
| TNFRSF14 | Tumor necrosis factor family member | 2.3 | 3.1 × 10−34 | 81 |
| LGALS3 | Galectin-3 that induces immune suppression | 4.8 | 3.0 × 10−33 | 93 |
| TNFAIP3 | Terminates TNF-induced NF-κB responses | 2.0 | 2.6 × 10−30 | 139 |
| CCR2 | Monocyte chemoattractant protein | 2.5 | 7.0 × 10−28 | 213 |
| IL15 | Enhances immunity of CD8+ T cells | 2.3 | 2.2 × 10−27 | 232 |
| LPXN | Substrate for tyrosine kinase | 1.7 | 4.0 × 10−27 | 238 |
| FAS | T cell apoptosis | 2.7 | 8.3 × 10−26 | 294 |
| TNFSF4 | Encodes OX40 ligand | 2.3 | 1.8 ×10−25 | 305 |
| PTGER4 | Prostaglandin E receptor 4 | 2.2 | 2.2 × 10−24 | 346 |
| CCL2 | Monocyte chemotactic protein | 3.7 | 9.9 × 10−24 | 386 |
| SOX11 | Signal transducer molecule | −2.5 | 1.3 × 10−23 | 401 |
| LYN | Inhibitory role in myeloid proliferation | 1.5 | 3.2 × 10−22 | 492 |
| THYBS1 | Thrombospondin 1; assists in tumor death | 1.9 | 8.1 × 10−22 | 532 |
| IRAK3 | Negative regulator of toll-like receptor signaling | 2.0 | 2.3 × 10−21 | 568 |
| GDF15 | TGF-β superfamily member | 2.8 | 7.1 × 10−21 | 611 |
| PRDM1 | Represses IFN-β gene expression | 1.6 | 7.8 × 10−21 | 617 |
| MICB | Activates cytolytic response of NK and CD8 T cells | 1.5 | 5.6 × 10−20 | 696 |
| IL6R | Key immune suppressive pathway | 1.7 | 6.4 × 10−20 | 707 |
| Immune Suppressors | ||||
| TIMP1 | Regulates resistance to infection | 4.3 | 4.2 × 10−45 | 12 |
| ANXA1 | Enhances/inhibits adaptive immunity | 3.3 | 3.4 × 10−41 | 23 |
| TNFRSF1A | Tumor necrosis superfamily protein | 2.2 | 5.4 × 10−40 | 31 |
| MR1 | Antigen presentation function | 2.1 | 2.2 × 10−37 | 45 |
| KLRC2 | Encodes NKG2-C (expressed on NK cells) | −10.9 | 1.3 × 10−36 | 50 |
| SERPING1 | Complement activation | 2.3 | 1.1 × 10−34 | 71 |
| LTBP1 | Activates TGF-β | 3.2 | 1.0 × 10−32 | 100 |
| SWAP70 | Mediates IgE responses/antibody switch | 2.1 | 2.1 × 10−32 | 109 |
| SATB1 | Silencing mimics the effects of IFN-γ treatment | −2.4 | 4.5 × 10−32 | 114 |
| TGFBR2 | TGF-β2 receptor | 1.7 | 6.0 ×10−32 | 120 |
| SHC1 | Inhibits adaptive immune responses | 1.9 | 1.0 × 10−31 | 125 |
| LGALS1 | Galectin 1 – deactivates M1 macrophages | 2.3 | 1.1 × 10−31 | 127 |
| RELB | Interacts with NFκβ2 | 2.0 | 4.1 × 10−31 | 135 |
| DLL1 | T cell activation | −2.0 | 4.4 × 10−30 | 142 |
| BCL3 | Regulates NFκβ | 2.7 | 6.3 × 10−30 | 146 |
| RAB27A | Granulocyte exocytosis | 2.2 | 1.3 × 10−29 | 157 |
| NOD1 | Innate immunity | 1.8 | 1.4 × 10−29 | 160 |
| KLRC1 | Recognition of MHC class I molecules by NK cells | −3.0 | 1.5 × 10−28 | 161 |
| CHI3L1 | YKL-40 immune suppressor in gliomas | 3.0 | 2.2 × 10−29 | 170 |
| ICAM1 | Transmigration receptor for immune cells | 2.7 | 4.9 × 10−29 | 182 |
Although four subtypes of glioblastoma were identified by Verhaak et al. (21) and three subsets by Phillips et al. (5), the proneural and mesenchymal subsets identified using distinct methodologies and sample sets appear to be the most robust and concordant (27, 28). Since the greatest immunological diversity exists between the proneural and mesenchymal subset, we compared the immune genes of interest between these subsets. Many of the top tier of preferentially expressed immune genes in the mesenchymal glioblastoma subtype relative to the proneural subtype have been shown previously to have a biological role in glioblastoma (Table 1).
Immune suppression predominates in mesenchymal glioblastomas
To determine if the list of genes generated from IPA and Uniprot was artificial or if it contained candidates that might correlate to the growth and maintenance of mesenchymal glioblastoma, we generated a list of known immune suppressive genes associated with glioma biology and then surveyed the TCGA database for over expression within subsets. To ascertain if there was a selective enrichment of the immune suppressive genes among the overexpressed genes in the mesenchymal subset or if this was merely by chance, we compared the immune suppressive gene set with the overall set of differentially expressed genes from the TCGA data set using the chi-square and hypergeometric tests and found selective enrichment of immune suppressive genes within those overexpressed in the mesenchymal subset relative to the other subsets (p<0.05), similar to the findings described above.
We parsed the TCGA dataset using the cBio cancer genomics portal analysis tool to show that the proportion of glioblastoma patients in the mesenchymal subset had significantly higher (relative to the proneural subtype) mRNA expression of previously well-characterized glioma-mediated immune suppressive cytokines such galectin-3 (chi-squared test, p<0.0001), IL-10 (p<0.0001), IL-23 (p=0.0012), TGFβ (p<0.0001), and the immune activation inhibitor PD-L1 (p<0.0001) relative to expression in the proneural subtype (Table 2). Many of these immune suppressive genes are associated with the monocyte and macrophage family including: colony stimulating factor 1 (CSF-1) (p<0.0001), a cytokine that controls the production, differentiation, and function of macrophages; CCL2 (p<0.0001) and CCL-22 (p=0.0008), chemokines that attract monocytes; CD163 (p<0.0001), a marker of immunosuppressive M2 macrophages; CD204 (p<0.0001), a macrophage scavenger receptor; and macrophage inhibitory cytokine-1 (MIC-1)(p<0.0001). CD47, a block used by solid cancers to prevent phagocytosis (29) was not preferentially expressed in the glioblastoma subtype. Arginase, produced by myeloid-derived suppressor cells and an inhibitor of T-cell responses in the glioma microenvironment (30), is also enriched in the mesenchymal subset (p=0.0248). Genes in the IL-6/STAT3 immune suppressive signaling axis was preferentially expressed in the mesenchymal subset; they include: gp130 (p<0.0001), part of the IL-6 receptor family; IL-6 (p<0.0001); STAT3 (p=0.0004); Pim1 (p<0.0001), a STAT3 downstream regulated proto-oncogene; and SOCS (p<0.0001), a negative regulator of cytokine signaling.
Table 2.
Enrichment of Immune Suppression in the Mesenchymal Glioblastoma Subset
| Immune suppressor/gene | PMID reference | Number of cases; % mRNA over expression | ||||
|---|---|---|---|---|---|---|
| Proneural n=141 | Mesenchymal n=160 | Classical n=147 | Neural n=96 | |||
| Immune suppressive cytokines and checkpoints | Galectin-3/LGALS3 | 22672152 | 2; 1 | 28; 18 | 13; 9 | 6; 6 |
| VEGF/VEGFA | 20549821 | 16; 11 | 26; 16 | 32; 22 | 3; 3 | |
| IL-10/IL10 | 22981868 | 4; 3 | 39; 24 | 5; 3 | 13; 14 | |
| IL-23/IL23A | 20404142 | 4; 3 | 21; 13 | 12; 8 | 5; 5 | |
| TGF-β/TGFB1 | 9597127 | 5; 4 | 50; 31 | 14; 10 | 2; 2 | |
| PD-1/SPATA2 | 11209085 | 28; 20 | 14; 9 | 58; 39 | 27; 28 | |
| PD-L1/PDL1 | 22180678 | 0; 0 | 25; 16 | 14; 10 | 5; 5 | |
| CTLA-4/CTLA-4 | 20578982 | 12; 9 | 30; 19 | 8; 5 | 11; 11 | |
| Tumor-supportive macrophage chemotactic and skewing molecules | CSF-1/CSF | 14709771 | 3; 2 | 30; 19 | 4, 3 | 1, 1 |
| CCL2/CCL2 | 22162712 | 5; 4 | 53; 33 | 9; 6 | 7; 7 | |
| CCL-22/CCL22 | 20518016 | 10; 7 | 33; 21 | 17; 12 | 12; 13 | |
| CD163/CD163 | 15478309 | 8; 6 | 60; 38 | 2; 1 | 11; 11 | |
| CD204/MSR1 | 22083206 | 5; 4 | 53; 33 | 3; 2 | 8; 8 | |
| MIC-1/GDF15 | 20534737 | 7; 5 | 43; 27 | 25; 17 | 14; 15 | |
| Arginase/ARG1 | 20643302 | 9; 6 | 23; 14 | 16; 11 | 22; 23 | |
| CD47/CD47 | 19666525 | 15; 11 | 30; 19 | 10; 7 | 19; 20 | |
| Immune suppressive signaling pathways | IL-6/IL6 | 23248265 | 32; 23 | 83; 52 | 16; 11 | 15; 16 |
| gp130/IL6ST | 20610800 | 0; 0 | 25; 16 | 17; 12 | 8; 8 | |
| Jak2 | 22684105 | 6; 4 | 22; 14 | 9; 6 | 11; 11 | |
| STAT3/STAT3 | 20053772 | 8; 6 | 31; 19 | 26; 18 | 0; 0 | |
| Pim-1/PIM1 | 22384197 | 4; 3 | 44; 28 | 13; 9 | 6; 6 | |
| SOCS3/SOCS3 | 10837055 | 5; 4 | 36; 23 | 10; 7 | 3; 3 | |
| STAT5A/STAT5A | 12835478 | 4; 3 | 48; 30 | 10; 7 | 2; 2 | |
| Markers of Tregs | CD4/CD4 | 20605226 | 5; 4 | 57; 36 | 0; 0 | 9; 9 |
| CD278/ICOS | 23026134 | 8; 6 | 23; 14 | 9; 6 | 9; 9 | |
| IDO/IDO1 | 22932670 | 6; 4 | 25; 16 | 14; 10 | 4; 4 | |
| FoxP3/FOXP3 | 20068105 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |
Red denotes preferential statistically significant enrichment (p<0.05) of mRNA expression in the comparison of the mesenchymal versus proneural subset.
Proinflammatory responses are more frequent in the mesenchymal glioblastoma subtype
We generated a list of key immune genes that reflect immune stimulation/effector responses of both adaptive and innate immunity. Again we found a selective enrichment of the over expressed immune effector genes in the mesenchymal subset relative to all other subsets (p<0.05). Focusing on the differences between immune effector genes expressed between the mesenchymal and proneural subset, genes encoding key receptor interactions for T-cell activation, including CD3 (p<0.0001), CD40 (p<0.0001), CD80 (p=<0.0001), CD86 (p<0.0001), MHC HLA-B (p=0.0001), MHC HLA-DRA (p<0.0001), MHC HLA-DQA1 (p<0.0001), and MHC HLA-DPB1 (p<0.0001) and downstream signaling pathways reflective of T-cell activation, effector function, and immune activating transcription factors such as, STAT4 (p=0.0034), DNAM-1 (p<0.0001), and IRF7 (p=0.0088), were enhanced in the proportion of glioblastoma patients with the mesenchymal phenotype (Table 3). Although there was a trend toward preferential expression of the adaptive proinflammatory cytokines in the proportion of glioblastoma patients with the mesenchymal subtype, only IL-1 (p=0.0007), IL-15 (p<0.0001) and IL-7 (p<0.0001) were significantly expressed at the mRNA level.
Table 3.
Enrichment of Proinflammatory Responses in the Mesenchymal Glioblastoma Subset
| Immune effector/gene | PMID reference | Number of cases; % mRNA over expression | ||||
|---|---|---|---|---|---|---|
| Proneural n=141 | Mesenchymal n=160 | Classical n=147 | Neural n=96 | |||
| Pro-inflammatory cytokines | IFN-γ/IFNG | 21705455 | 17; 12 | 27; 17 | 17; 12 | 12; 13 |
| IL-1/IL1A | 17596594 | 11; 8 | 35; 22 | 6; 4 | 17; 18 | |
| IL-2/IL2 | 20227890 | 14; 10 | 14; 9 | 12; 8 | 11; 11 | |
| IL-4/IL4 | 17389011 | 16; 11 | 29; 18 | 19; 13 | 16: 17 | |
| IL-7/IL7 | 8473051 | 5; 4 | 35; 22 | 20; 14 | 15; 16 | |
| IL-12/IL-12A | 22515181 | 10; 7 | 13; 8 | 35; 24 | 12; 13 | |
| IL-15/IL-15 | 22032984 | 7; 5 | 42; 26 | 4; 3 | 17; 18 | |
| TNF-α/TNF | 22075379 | 19; 13 | 21; 13 | 5; 3 | 13; 14 | |
| T cell effector markers | CD3/CD3D | 23009144 | 8; 6 | 40; 25 | 3; 2 | 9; 9 |
| CD8/CD8A | 18698034 | 12; 9 | 29; 18 | 12; 8 | 9; 9 | |
| CD8/CD8B | 18698034 | 3; 3 | 22; 14 | 9; 6 | 6; 6 | |
| CD80/CD80 | 22798683 | 9; 6 | 36; 23 | 8; 5 | 13; 14 | |
| CD86/CD86 | 20380573 | 4; 3 | 53; 33 | 2; 1 | 19; 20 | |
| CD40/CD40 | 22903346 | 7; 3 | 38; 24 | 6; 4 | 10; 10 | |
| MHC/HLA-A | 23108078 | 12; 9 | 21; 13 | 25; 17 | 10; 10 | |
| MHC/HLA-B | 22426959 | 5; 4 | 28; 18 | 30; 20 | 9; 9 | |
| MHC/HLA-C | 17054674 | 10; 7 | 27; 17 | 25; 17 | 12; 13 | |
| MHC/HLA-DRA | 15688398 | 5; 4 | 42; 26 | 9; 6 | 11; 11 | |
| MHC/HLA-DQA1 | 17016821 | 13; 9 | 46; 29 | 17; 12 | 15; 16 | |
| MHC/HLA-DPB1 | 21716314 | 5; 4 | 45; 28 | 10; 17 | 8; 8 | |
| beta2- microglobulin/B2M | 22353804 | 8; 6 | 23; 14 | 21; 14 | 8; 8 | |
| Immune effector signaling pathways | NF-κB/RELA | 16724054 | 10; 7 | 17; 11 | 17; 12 | 3; 3 |
| STAT1/STAT1 | 22805310 | 11; 8 | 20; 13 | 30; 20 | 10; 10 | |
| IRAK/IRAK1 | 17265049 | 0 | 0 | 0 | 0 | |
| STAT4/STAT4 | 22121102 | 9; 6 | 28; 18 | 5; 3 | 15; 16 | |
| T-bet/TBX21 | 22186896 | 12; 9 | 20; 13 | 17; 12 | 6; 6 | |
| DNAM-1/CD226 | 16015041 | 8; 6 | 42; 26 | 4; 3 | 11; 11 | |
| IRF7/IRF7 | 22295238 | 8; 6 | 24; 15 | 16; 11 | 17; 18 | |
| Markers of innate immunity | NKp46/NCR1 | 22308311 | 14; 10 | 22; 14 | 22; 15 | 7; 7 |
| NKG2D/KLRK1 | 22530569 | 42; 30 | 12; 8 | 6; 4 | 2; 2 | |
| NKp30/NCR3 | 21877119 | 18; 13 | 24; 15 | 15; 10 | 11; 11 | |
| NKp44/NCR2 | 15728472 | 12; 9 | 16; 10 | 18; 12 | 8; 8 | |
| KLRD1/KLRD1 | 15805295 | 16; 11 | 27; 17 | 11; 7 | 16; 17 | |
| NK/CD244 | 19638467 | 8; 6 | 42; 26 | 4; 3 | 11; 11 | |
| TLR2/TLR2 | 23324344 | 1; 1 | 56; 35 | 3; 2 | 13; 14 | |
| TLR3/TLR3 | 23197495 | 7; 5 | 34; 21 | 16; 11 | 22; 23 | |
| TLR4/TLR4 | 21129170 | 17; 12 | 26; 16 | 8; 5 | 17; 18 | |
| TLR9/TLR9 | 22169598 | 13; 9 | 23; 14 | 23; 16 | 6; 6 | |
| Myeloid markers | TREM1/TREM1 | 22719066 | 12; 9 | 63; 39 | 9; 6 | 2; 2 |
| TREM2/TREM2 | 4; 3 | 27; 17 | 6; 4 | 31; 32 | ||
| MIF/MIF | 21773885 | 13; 9 | 25; 16 | 8; 5 | 14; 15 | |
Red denotes preferential statistically significant enrichment (p<0.05) of mRNA expression in the comparison of the mesenchymal versus proneural subset.
In contrast, genes associated with innate immunity, specifically NK cell markers such as NK cells NKp46, NKG2D, NKp30, NKp44, and KLRD1 were more uniformly distributed across the subtypes, with the exception of the NK activating receptor CD244 (p<0.0001) that was preferentially expressed in the proneural subtype. Other genes involved in the innate immune response, such as Toll-like receptor (TLR) 2 (p<0.0001) and 3 (p<0.0001) were preferentially overexpressed in glioblastoma patients with the mesenchymal subset. The triggering receptors expressed on myeloid cells (TREM) 1 (p<0.0001) and 2 (p<0.0001) that enhance monocyte/macrophage inflammatory responses were also enhanced in the mesenchymal subset. Cumulatively, these data suggest that there is a preferential distribution of both proinflammatory and immune suppressive genes within the mesenchymal subset.
Glioma antigens segregate differentially within glioblastoma subtypes
We postulated that the mesenchymal subset may have greater incidence of tumor-associated and tumor-specific antigens that could contribute to the increased propensity of the immune effector genes present within the mesenchymal subset. Furthermore, there would be a predicted enhancement in immune suppression to counteract the anti-tumor effector responses. Thus, to evaluate if tumor antigens are selectively enriched in the mesenchymal subset, a list of known glioma antigens was compiled, and the frequency of each mRNA overexpression was determined within the various glioblastoma subtypes (Table 4). Although there were predilections of specific tumor antigens for glioblastoma subtypes, there was not a preferential enrichment of antigens found within the mesenchymal subset. Specifically, the EGFR family of antigens including EGFR and ERBB2 were frequently found to be overexpressed at 80% and 24%, respectively, within the classical subtype, consistent with previous reports (21). Within the neural subtype, EGFR (54%) was frequently expressed; whereas within the proneural subtype survivin (32%) and Sart-1 (25%) were more commonly expressed. SART-2 (36%) was the most commonly expressed antigen within the mesenchymal subset. Thus, preferential antigen enrichment in the mesenchymal subset does not appear to be the underlying etiology for enrichment of the immune activator and suppressor genes within this subset.
Table 4.
Antigenic Diversity Amongst Glioblastoma Subset
| Glioma Antigen/gene | Number of cases; % mRNA over expression | |||
|---|---|---|---|---|
| Proneural n=141 | Mesenchymal n=160 | Classical n=147 | Neural n=96 | |
| EGFR/EGFR | 32; 23 | 54; 34 | 118; 80 | 52; 54 |
| Her2/ERBB2 | 4; 3 | 25; 16 | 35; 24 | 7; 7 |
| Survivin/BIRC5 | 43; 32 | 22; 14 | 6; 4 | 19; 20 |
| Nucleolin/NCL | 30; 21 | 22; 14 | 23; 16 | 2; 2 |
| Epha2/EPHA2 | 2; 1 | 27; 17 | 28; 19 | 4; 4 |
| Telomerase/TERT | 6; 4 | 14; 9 | 18; 12 | 5; 4 |
| B-cyclin/CCNB1 | 34; 24 | 20; 13 | 7; 5 | 12; 13 |
| Sart-1/SART1 | 35; 25 | 9; 6 | 18; 12 | 2; 2 |
| Sart-2/DSE | 2; 1 | 57; 36 | 2; 1 | 3; 3 |
| Sart-3/SART3 | 18; 13 | 19; 12 | 20; 14 | 3; 3 |
| Aim-2/AIM2 | 32; 23 | 38; 24 | 5; 3 | 10; 10 |
| Trp-1/TYRP1 | 13; 9 | 23; 14 | 8; 5 | 15; 16 |
| Tyrosinase/TYR | 11; 8 | 15; 9 | 7; 5 | 11; 11 |
| GnT-V/MGAT5 | 5; 4 | 8; 5 | 5; 3 | 1; 1 |
| GP100/PMEL | 7; 5 | 18; 11 | 7; 5 | 6; 6 |
| Mart-1/MLANA | 11; 8 | 24; 15 | 11; 7 | 10; 10 |
| Mage-1/MAGEA1 | 0 | 0 | 0 | 0 |
| Gage-1/GAGE1 | 0 | 0 | 0 | 0 |
Red denotes preferential statistically significant enrichment (p<0.05) of mRNA expression in the comparison of the mesenchymal versus proneural subset.
Discussion
The TCGA database consists of an unselected cellular population that includes glioma-infiltrating immune cells within the genetic composition. The analysis of the mRNA overexpression of immune genes may reflect either gene amplification (increased expression on the immune population) or a relative increase in a designated infiltrating immune population. The interaction between the immune system and molecular subtypes of glioma remains largely unstudied. Although a previous report identified an immune signature that is prognostic for survival in glioblastoma patients, especially within the proneural subtype (31), this is the first report to demonstrate the concordant association of pro-inflammatory and immune suppression in the mesenchymal subset. Specifically, glioblastoma patients that have a pre-existing induced anti-glioma effector response and an actionable immune suppressive target (i.e., the mesenchymal subset) may be “immune reactive” and therefore particularly amenable to immune therapeutic approaches including those targeting immune checkpoints. This is further supported by a retrospective analysis of glioblastoma patients receiving dendritic cell immunotherapy who were more likely to have a mesenchymal subtype glioblastoma (20). These data indicate that the glioblastoma subtype may be a confounding variable in therapeutic response analysis and should be considered in stratification. Additionally, our data indicate that glioblastoma subtype may influence the interpretation of post treatment analysis of immune infiltration. Specifically, because glioblastomas are more likely to transition to the mesenchymal subtype upon recurrence (5), the intratumoral immune analysis after immunotherapy may be more reflective of the biology of the underlying subtype rather than a direct immunotherapy-induced response.
The proinflammatory and immune suppressive gene sets derived from online curated databases identified a set of common genes (e.g. TIMP1). In some cases, the role of these genes is contextual based on the disease state (i.e. autoimmunity versus malignancy) or model system studied. For example, ANXA1 has been shown to both inhibit (32, 33) and enhance inflammation (34, 35). In other instances, that attribution to a particular category simply appeared erroneous (i.e. LTBP1 that activates the immune suppressive cytokine TGF-β or TGFBR2 that encodes the TGF-β2 receptor). Thus, we selected a pro-inflammatory and immunosuppressive list of genes based on the documented roles of these genes in the setting of malignancy with a special emphasis on those operational in glioblastoma. This defined gene list also demonstrated a preferential enrichment of both pro-inflammatory and immunosuppressive genes within the mesenchymal subtype. Although we had concerns regarding the selection of genes by the online curated databases, they did provide novel insights into immune genes that had, thus far, not been appreciated as playing a role in glioblastoma immune biology. Furthermore, these databases revealed the marked diversity of the preferential enrichment of pro-inflammatory or immune suppressive genes in the mesenchymal subtype relative to the others.
In many instances the findings of the immune genes associating with a particular glioblastoma subtype were consistent with and validated previous observations. For example, as STAT3 has been previously demonstrated to be a key molecular hub driving the mesenchymal transformation (6), STAT3 and related down-stream targets, such as Pim-1 and VEGF, were found to be enriched in the mesenchymal subtype relative to the other subsets. Furthermore, multiple monocyte genes were enriched within the mesenchymal subtype as reflected in the expression of CCL2 (a known monocyte chemotactic protein), CD163 (a marker of cells of the monocyte/macrophage lineage), and CD204 (a marker of macrophage scavenger receptors). These data are consistent with our findings in genetically engineered murine models in which the mesenchymal transition was shown to correlate with increased macrophage infiltration (7, 14). Additionally, the proinflammatory T-cell cytokine IL-2 was minimally enriched in any subtype, consistent with our previous reports demonstrating that while T cells may be active in the periphery, upon encountering the local tumor microenvironment, this immune response is markedly down regulated (3). Interestingly, markers of Tregs such as CD4, ICOS, IDO1 and CTLA-4 appeared to be preferentially enriched in the mesenchymal subset. Since robust immune effector response is also present in the mesenchymal subset, these patients may be specifically predisposed to respond to immune therapeutics targeting the Treg population. However, while these markers have been shown to be important for Treg function and are associated with immune inhibition, they do not independently define the Treg population and mechanistic conclusions cannot be drawn from this type of analysis.
Although CCL22 (a chemokine that attracts Tregs) (36), IDO and TGFβ, (inducible Treg factors) (37), and ICOS (critical for the functional stability of Tregs) (38) were present in gliomas, no differential expression of FoxP3 was observed in glioblastoma subtypes. This appears inconsistent with the immunohistochemical data demonstrating a great deal of heterogeneity in the presence of Tregs within glioblastomas (2). However, because the TCGA database is generated by cDNA microarray analysis, which represents gene regulation at the transcriptional level, immune genes that are regulated at the posttranscriptional, translational or posttranslational level may not appear through this screening. A specific example of this is FoxP3, which is regulated either at the mRNA transcription level or at its protein stability level which is degraded through the proteasome rapidly (39). Thus, the discrepancy between the immunohistochemical data and the TCGA data can be explained based on FoxP3 mRNA and protein stability, but also demonstrates a limitation of solely relying on analysis of mRNA data.
Another limitation of the current study is that the analysis does not directly reflect the systemic immune status or functional status of these immune responses. Some of the immune suppressive targets may be markedly enhanced within the tumor microenvironment, with marginal elevations systemically, and agents without significant glioma penetration may fail to show a correlation of treatment response with tumor expression levels. Furthermore, the analysis of the frequency of overexpression is relative to the mean expression levels of a marker across all gliomas, and thus more clinical responders may be identified within any given phenotype because the threshold of minimal expression necessary to result in a therapeutic response has not yet been characterized. Additionally, the mRNA expression levels analyzed in our study may not correspond to the protein expression levels especially in the case of B7-H1 which has been shown to be post-transcriptionally regulated by cytokines (40). Finally, although there is an association of immune effector and suppressor genes within the mesenchymal subtype, this has not yet been immunologically functionally validated. Not previously reported, but as part of a previous analysis, we found that 26% of newly diagnosed glioblastoma tumors have very little immune infiltration; whereas approximately 50% have both effector (CD8+ T cells) and suppressor (FoxP3+) immune responses in the glioblastoma (2). The correlation of these immunohistochemical findings with glioblastoma subtype is an area of future investigation along with the association of observed immune gene signatures and patient outcome.
Although the mesenchymal glioblastoma subtype may be “immunologically reactive,” as defined by the preexistence of immune effector responses and possessing the therapeutic targets of immune suppressive modulators, it is unlikely that this is the sole subtype that can potentially benefit from immunotherapeutic strategies. We have previously demonstrated that a peptide vaccine targeting the EGFR variant III (EGFRvIII) in newly diagnosed glioblastoma patients significantly increased median survival time to more than 26 months (17, 18). Based on the TCGA data, it is likely that most of these patients have glioblastomas within the classical subtype because EGFRvIII expression almost always coincides with EGFR amplification (24, 41). This indicates that with specific immune therapeutic strategies, patients who have distinct glioblastoma subtypes may preferentially benefit.
In conclusion, the analysis of these selected immune effector and suppressor genes may provide a way to programmatically prioritize different types of potentially competing immune therapeutic strategies, and to identify subsets of patients that may respond to a particular strategy and thus selectively enrich for potential responders during early or small-scale clinical trials.
Acknowledgments
We thank Audria Patrick and David M. Wildrick, Ph.D., for editorial assistance..
Grant Support
Grant support was provided by the National Institutes of Health RO1-CA1208113 (ABH), 5P50 CA127001 (TD, GR, KA, ABH), Dr. Marnie Rose Foundation (ABH), the Cynthia and George Mitchell Foundation (ABH) and the Vaughn Foundation (ABH).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
Authors Contributions
Conception and design: Mark Gilbert, Amy B. Heimberger
Development of methodology: Tiffany Doucette, Ganesh Rao, Kenneth Aldape, Arvind Rao, Li Shen
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computation analysis): Tiffany Doucette, Ganesh Rao, Kenneth Aldape, Arvind Rao, Li Shen, Jun Wei, Kristine Dziurzynski, Amy B. Heimberger
Writing, review and/or revision of the manuscript: Tiffany Doucette, Ganesh Rao, Amy B. Heimberger
Administrative, technical or material support: Kenneth Aldape, Amy B. Heimberger
Study supervision: Amy B. Heimberger
References
- 1.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucette TA, Kong LY, Yang Y, Ferguson SD, Yang J, Wei J, et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 2012 doi: 10.1093/neuonc/nos139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: Evidence for Stat3-dependent and independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 14.Kong LY, Wu AS, Doucette T, Wei J, Priebe W, Fuller GN, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res. 2010;16:5722–33. doi: 10.1158/1078-0432.CCR-10-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–35. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 17.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Research. 2001;61:842–7. [PubMed] [Google Scholar]
- 20.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13:566–75. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 25.Galzio R, Rosati F, Benedetti E, Cristiano L, Aldi S, Mei S, et al. Glycosilated nucleolin as marker for human gliomas. J Cell Biochem. 2012;113:571–9. doi: 10.1002/jcb.23381. [DOI] [PubMed] [Google Scholar]
- 26.Pounds S, Morris SW. Estimating the occurrence of false positives and false negatives in microarray studies by approximating and partitioning the empirical distribution of p-values. Bioinformatics. 2003;19:1236–42. doi: 10.1093/bioinformatics/btg148. [DOI] [PubMed] [Google Scholar]
- 27.Huse JT, Phillips HS, Brennan CW. Molecular subclassification of diffuse gliomas: seeing order in the chaos. Glia. 2011;59:1190–9. doi: 10.1002/glia.21165. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JX, Zhang J, Yan W, Wang YY, Han L, Yue X, et al. Unique genome-wide map of TCF4 and STAT3 targets using ChIP-seq reveals their association with new molecular subtypes of glioblastoma. Neuro Oncol. 2013;15:279–89. doi: 10.1093/neuonc/nos306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vauleon E, Tony A, Hamlat A, Etcheverry A, Chiforeanu DC, Menei P, et al. Immune genes are associated with human glioblastoma pathology and patient survival. BMC Med Genomics. 2012;5:41. doi: 10.1186/1755-8794-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold R, Pepinsky RB, Zettl UK, Toyka KV, Hartung HP. Lipocortin-1 (annexin-1) suppresses activation of autoimmune T cell lines in the Lewis rat. J Neuroimmunol. 1996;69:157–64. doi: 10.1016/0165-5728(96)00086-0. [DOI] [PubMed] [Google Scholar]
- 33.Yang YH, Morand EF, Getting SJ, Paul-Clark M, Liu DL, Yona S, et al. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheum. 2004;50:976–84. doi: 10.1002/art.20201. [DOI] [PubMed] [Google Scholar]
- 34.D’Acquisto F, Merghani A, Lecona E, Rosignoli G, Raza K, Buckley CD, et al. Annexin-1 modulates T-cell activation and differentiation. Blood. 2007;109:1095–102. doi: 10.1182/blood-2006-05-022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang YH, Song W, Deane JA, Kao W, Ooi JD, Ngo D, et al. Deficiency of annexin A1 in CD4+ T cells exacerbates T cell-dependent inflammation. J Immunol. 2013;190:997–1007. doi: 10.4049/jimmunol.1202236. [DOI] [PubMed] [Google Scholar]
- 36.Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J, Ye ZJ. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin Cancer Res. 2009;15:2231–7. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–61. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188:1064–74. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- 39.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–74. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 40.Holets LM, Carletti MZ, Kshirsagar SK, Christenson LK, Petroff MG. Differentiation-induced post-transcriptional control of B7-H1 in human trophoblast cells. Placenta. 2009;30:48–55. doi: 10.1016/j.placenta.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu J-L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12:3935–41. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]