Summary
Mutations of the FOXP2 gene impair speech and language development in humans and shRNA-mediated suppression of the avian orthologue FoxP2 disrupts song learning in juvenile zebra finches. How diminished FoxP2 levels affect vocal control and alter the function of neural circuits important to learned vocalizations remains unclear. Here we show that FoxP2 knockdown in the songbird striatum disrupts developmental and social modulation of song variability. Recordings in anaesthetized birds show that FoxP2 knockdown interferes with D1R-dependent modulation of activity propagation in a corticostriatal pathway important to song variability, an effect that may be partly attributable to reduced D1R and DARPP-32 protein levels. Furthermore, recordings in singing birds reveal that FoxP2 knockdown prevents social modulation of singing-related activity in this pathway. These findings show that reduced FoxP2 levels interfere with the dopaminergic modulation of vocal variability, which may impede song and speech development by disrupting reinforcement learning mechanisms.
Introduction
Individual genes can profoundly affect complex behaviors, including those learned through extensive practice. For example, humans with mutations of FOXP2, a Forkhead box family gene that encodes a transcription factor, exhibit orofacial dyspraxia and fail to develop normal speech and language (Lai et al., 2001; Hurst et al., 1990; Watkins et al., 2002a; Vargha-Khadem et al., 2005; refer to Kaestner et al., 2000 for FOX gene nomenclature). While FOXP2 is expressed in many brain regions, several findings implicate aberrant striatal function in the speech and language deficits that accompany FOXP2 mutations (Lai et al., 2001; Lai et al., 2003). One finding is that imaging studies in humans with FOXP2 mutations reveal a significant reduction in the volume of the head of the caudate nucleus, a region of the striatum involved in controlling head and face movements (Watkins et al., 2002b; Belton et al., 2003). Another clue is that while FOXP2 is expressed in both the striatum and the cerebellum, ‘knock in’ of the humanized FoxP2 in mouse affects synaptic long term depression in the striatum but not in the cerebellum (Reimers-Kipping et al., 2010). Finally, lentiviral shRNA-mediated reduction (i.e., knockdown) of FoxP2 levels in a striatopallidal structure (Area X) of juvenile male zebra finches, which resemble humans in their capacity for imitative vocal learning, prevents them from accurately copying a tutor song (Haesler et al., 2007). While these findings suggest that striatal abnormalities caused by impaired FOXP2 function underlie speech and language deficits, two major issues remain unresolved. First, because FOXP2 is highly expressed in the striatum from embryonic development onwards (Teramitsu et al., 2004), a persistent role of FOXP2 in vocal control cannot be dissociated from a developmentally restricted role in vocal learning. Second, while studies in mice have illuminated structural and functional changes in striatal neurons that result from Foxp2 mutations (Enard et al., 2009; Groszer et al., 2008), how these changes affect neural circuits important to learned vocal control remain unknown.
Songbirds afford a powerful system in which to understand how reduced FoxP2 levels affect corticostriatal circuits important to learned vocalizations and to determine whether FoxP2 plays a persistent role in adult song control in addition to a role in juvenile song learning. Songbirds use auditory information to learn and maintain their songs (Konishi, 1965; Marler, 1970; Doupe and Kuhl, 1999) and the songbird brain contains well-characterized song control circuitry (Figure 1A), including an anterior forebrain pathway (AFP) that plays a critical role in song learning (Nottebohm et al, 1976; Scharff and Nottebohm, 1991). The AFP shares many similarities with mammalian corticostriatal circuitry, including high levels of FoxP2 protein in its striatopallidal component, Area X (Doupe et al., 2005; Fisher and Scharff, 2009). Notably, an adult male zebra finch sings syllables with greater trial-by-trial variability when singing alone (i.e., undirected song) than when singing to a female (i.e., directed song) and this social context-dependent change in variability depends on changes in LMAN activity, the output nucleus of the AFP (Sossinka and Böhner, 1980; Kao and Brainard, 2006; Kao et al., 2005; Leblois et al., 2010). In addition to a demonstrated role for FoxP2 in juvenile song learning, FoxP2 mRNA and protein levels decrease in Area X of both juvenile and adult birds the more they sing (Teramitsu and White 2006; Miller et al., 2008; Teramistu et al., 2010). Further, FoxP2 mRNA levels in adults decrease even more with undirected singing than with directed singing (Teramitsu and White, 2006), although context-dependent differences in FoxP2 protein levels are not evident (Miller et al., 2008). Ultimately, whether changes in FoxP2 mRNA or protein levels are causally linked to song control in adult birds remains unknown. Here we explicitly addressed the issue of causality by reducing FoxP2 protein levels in Area X of adult birds and testing whether this reduction affected their ability to modulate song variability as a function of social context.
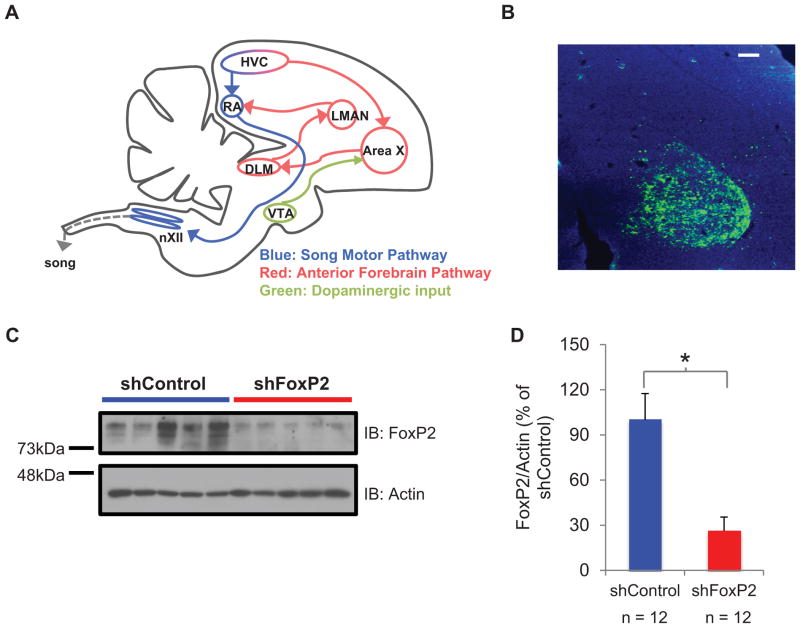
Figure 1. Lentivirus shRNA-mediated knockdown of FoxP2 in Area X of adult male zebra finches.
A) A parasagittal schematic of the song system showing the song premotor pathway (blue) and the anterior forebrain pathway (red). HVC used as a proper name; RA, robust nucleus of the arcopallium; Area X, striatopallidal component of the song system; LMAN, lateral magnocellular nucleus of the anterior nidopallium; DLM, medial nucleus of the dorsolateral thalamus; nXII, nucleus XII. Area X also receives dopaminergic input from VTA; ventral tegmental area (green). Some structures and connections are omitted for simplicity. B) A parasagittal section through Area X, showing expression of ShFoxP2-GFP (immunoreacted with antibodies to GFP, green fluorescence; DAPI staining in blue) 42 days after the injection of lentivirus-ShFoxP2-GFP into the same region. Scale bar, 100 μm. C) A representative immunoblot (IB) showing greater amounts of FoxP2 protein extracted from Area X tissue punches >90 days after the injection of LV-ShC injected birds (left, 5 animals) than after LV-ShFoxp2 injected birds (right, 5 animals). D) Compared to the group of LV-ShC animals, the FoxP2 protein levels in Area X, normalized to actin, were significantly lower in the group injected with LV-ShFoxP2-GFP (73% reduction; mean ± s.e.m.; n = 12 birds in each group, p < 0.05).
Another advantage of using songbirds to study the role of FoxP2 in learned vocal communication is the potential to link synaptic and circuit properties to behavior. During singing, the song premotor nucleus HVC transmits a precise timing signal to the song motor nucleus RA and to Area X (Hahnloser et al., 2002; Kozhevnikov and Fee, 2007; Prather et al., 2008; Long and Fee, 2008; Fujimoto et al., 2011). This timing signal then undergoes context-dependent modulation in the AFP, with the consequence that LMAN neurons display increased trial-by-trial variability, augmented bursting activity and elevated firing rates during undirected singing relative to directed singing (Kao et al., Brainard, 2005; Kao and Brainard, 2006; Stepanek and Doupe, 2010). These changes in LMAN activity drive more variable action potential activity in RA that correlates with greater spectral variability in the bird’s song (Olveczky et al., 2011, Sober et al., 2008). How the AFP modulates signals from HVC is unknown, but in zebra finches dopamine levels in Area X change with social context and in embryonic mice several genes implicated in dopamine signaling are potential targets of Foxp2 regulation (Sasaski et al., 2006; Vernes et al., 2011). These findings suggest that FoxP2 could regulate dopaminergic modulation of signals propagating from HVC through the AFP.
Using in vivo intracellular recordings from LMAN neurons and pharmacological manipulations in Area X of anesthetized adult male zebra finches, we find that FoxP2 knockdown in Area X accelerates signal propagation from HVC to LMAN and renders this propagation insensitive to modulation by dopamine receptor 1 (D1R). Furthermore, FoxP2 knockdown decreases levels of D1R and dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32), a key component in the D1R signal cascade. Finally, we show that FoxP2 knockdown abolishes context-dependent modulation of LMAN activity and context-dependent changes in song variability. These findings advance a model in which FOXP2 mutations disrupt the control of vocal variability by interfering with the dopamine-dependent modulation of premotor signals conveyed by corticostriatal circuits.
Results
FoxP2 knockdown in Area X of adults abolishes context dependent changes in song variability
Lentiviral-mediated expression of short hairpin RNAs (LV-shRNAs) can be used to reduce FoxP2 levels in Area X of juvenile zebra finches, and this treatment renders the juvenile’s copy of a tutor song both inaccurate and imprecise (Haesler et al., 2007). Here we used two LV-shRNA constructs (shFoxP2-h-gfp or the shFoxP2-f-gfp) to explore whether song is affected by reducing FoxP2 levels in Area X of adult male zebra finches (>120 days post hatching (dph)), after song copying is complete in this species (Immelmann, 1969). To determine whether these constructs could infect cells and reduce FoxP2 levels in Area X of adult finches, multiple injections of LV-ShFoxP2 or LV-ShControl (LV-ShC) were made bilaterally in Area X using stereotaxic coordinates (Figure 1A). Two weeks to four months after the injection, visualization of the GFP marker confirmed extensive expression of the LV-ShFoxP2 constructs (Figure 1B, see Methods). To quantify the degree to which these constructs reduced FoxP2 levels, we performed western blots of tissue homogenates obtained from Area X of adult birds previously injected with either LV-ShFoxP2 or LV-ShC (Figure 1C). Optical density measurements indicated that FoxP2 protein levels were reduced by more than two-thirds (~70%) in the LV-ShFoxP2 animals compared to the LV-ShC animals (Figure 1D; n = 12 birds in each group, >90 days post injection; see Methods; p < 0.05; Student’s t-test). This reduction in FoxP2 levels was comparable to that achieved in juvenile birds (Haesler et al., 2007), indicating that the shRNA method is effective at decreasing FoxP2 levels in Area X of adult male zebra finches.
We measured social context-dependent change in syllable variability to probe whether FoxP2 knockdown in Area X of adult finches affected song control independent of learning. Prior to injecting LV-shRNA into Area X of adult male zebra finches, we confirmed that the pitch of syllables in undirected songs varied more from one trial to the next than the pitch of the corresponding syllables in directed songs (Figures 2A–C; blue; Pre-ShFoxP2-undirected versus Pre-ShFoxP2-directed: paired t-test, p = 0.03). We then made bilateral injections of either LV-ShFoxP2 or LV-ShC into Area X of these birds and re-evaluated syllable variability in different social contexts ~ 4 weeks later (ShFoxP2: n = 7 birds, mean age at injection, 127 ± 2 (S.E.M) dph; 26 ± 2 d post injection; ShC: n = 4 birds; mean age at injection, 126 ± 4 dph, 27 ± 2 d post injection). Notably, LV-ShFoxP2 injections in Area X abolished social context-dependent changes in pitch variability (Figure 2B, C; red; Post-ShFoxP2-undirected versus Post-ShFoxP2-directed: paired t-test, p = 0.5; mean pitch in these two states was unaffected, paired t-test, p = 0.7), an effect that was specifically attributable to elevated levels of variability in the directed context (Table S1). In contrast, birds that received LV-ShC injections continued to modulate song variability as a function of social context (Figure 2C; grey; Post-ShC-undirected vs. Post-ShC- directed: paired t-test, p = 0.03). Finally, FoxP2 knockdown did not affect context-dependent modulation of song tempo (Figure S1A), the number of introductory elements per motif (Figure S1B; the motif is a stereotyped syllable sequence produced by zebra finches), or the number of motifs per bout (Figure S1C, ShFoxP2: n = 7 birds, ShC: n = 4 birds, p = 0.3, ANOVA followed by a post-hoc t-test, unless otherwise noted), all of which are song features that usually change with social context but that are unaffected by LMAN lesions and are not regulated by D1R-mediated signaling in Area X (Kao and Brainard, 2006; Leblois and Perkel, 2012). Therefore, manipulating FoxP2 levels in Area X of adult birds can affect context-dependent modulation of song variability, indicating that the role of FoxP2 is not developmentally restricted.
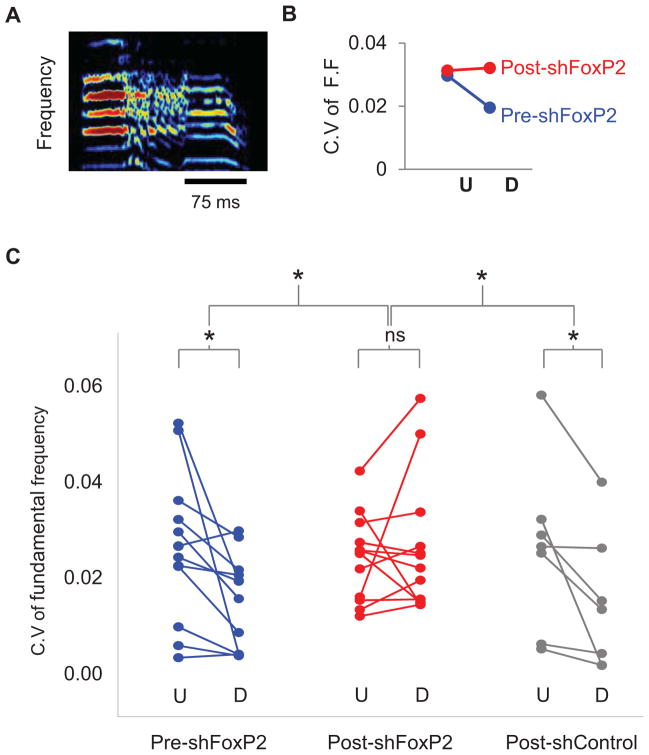
Figure 2. Knockdown of FoxP2 in Area X of adult zebra finches abolishes context-dependent changes in song variability.
A) A sonogram of the type of syllable chosen for analysis of coefficient of variation (C.V) of fundamental frequency (F.F); only the constant frequency portions (i.e., harmonic stacks) were analyzed. Scale bar (black) indicates 75ms, ordinate: 0–9kHz. B) A representative plot of C.V of fundamental frequencies for the syllable shown in A produced during undirected (U) or directed (D) singing pre- (blue) and post-injection (red) of LV-ShFoxP2 into Area X. C) Reducing FoxP2 levels in Area X of the adult abolishes context-dependent differences in syllable variability. In the control conditions (blue), the fundamental frequencies of syllables are more variable in the undirected state and become more stereotyped in the directed state (p = 0.03). Following knockdown of FoxP2 in Area X (red, ShFoxP2 n = 7 birds), syllables from the directed songs are more variable, leading to a loss in context-dependent changes in song variability (p = 0.5). In contrast, the injection of LV-ShC in Area X (grey, ShC n = 4 birds) has no effect on context-dependent changes in song variability (p = 0.03). See also Figure S1.
Juveniles with FoxP2 knockdown in Area X display elevated variability throughout learning
Reinforcement models of song learning posit that trial-by-trial variability enables vocal exploration necessary for the accurate copying of a tutor song (Doya and Sejnowski, 1995). A prior study showed that reducing FoxP2 levels in Area X of the juvenile zebra finch impairs its ability to accurately copy a tutor song (Haesler et al., 2007) and we found here that a similar reduction of FoxP2 levels in the adult finch disrupts its ability to modulate song variability in a context-dependent manner. These observations led us to wonder whether reducing FoxP2 expression in Area X of the juvenile alters levels of song variability during song learning, which in turn could potentially interfere with reinforcement learning mechanisms important to accurate song imitation.
To test this idea, we made bilateral injections of LV-ShFoxP2 or LV-ShC in Area X of juvenile male zebra finches ~ 3 weeks after hatching (ShFoxP2: n = 6; ShC: n = 4; mean age at the time of injection: 21 ± 1 dph). Juveniles were then housed with an adult tutor for a 5–10 day period and their songs were recorded beginning at 45 dph and at regular intervals into adulthood (>90dph). The adult bird’s song was used to retrospectively identify the motif at various times in development (see Methods). As reported previously (Haesler et al., 2007), we observed that birds that received injections of LV-ShFoxP2 in Area X early in juvenile development subsequently sang poorer copies of the tutor song during late juvenile development (65 and 80 dph) and in adulthood than birds that had received LV-ShC (Figures 3A and S2A). We also noted that the LV-ShFoxP2 birds sang poorer copies of the tutor song at the earliest stage that recognizable song motifs are first produced (i.e., 45–55 dph) (Figure 3B). We then measured the pitch variability and entropy of copied syllables at regular intervals throughout development (ShFoxP2: n = 6 birds, 8 syllables; ShC: n = 4 birds, 6 syllables). Notably, juveniles that received injections of LV-ShFoxP2 sang syllables with more variable pitch and higher entropy compared to their LV-ShC injected siblings (Figures 3C, D, and S2B). Moreover, when we analyzed FoxP2 levels in Area X in a subset of adult birds that had received injections of either LV-ShC or LV-ShFoxP2 early in development (~20dph), we found that FoxP2 levels correlated with tutor song similarity (Figure 3E, R2 = 0.65, n = 5 birds in each group). Therefore, reducing FoxP2 levels in Area X of the juvenile affects acute syllable production while also interfering with song learning over a longer time course.
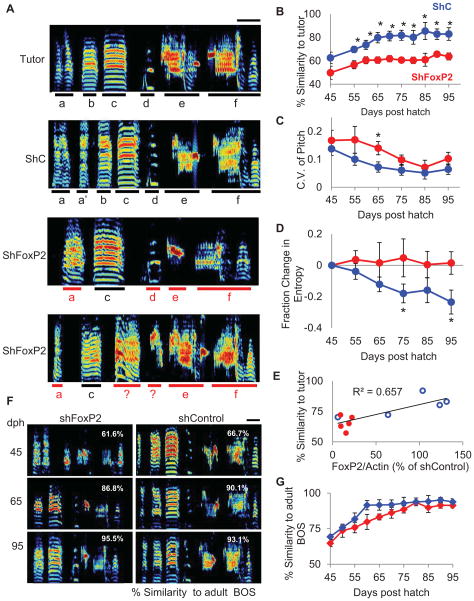
Figure 3. Knockdown of FoxP2 in Area X of juveniles results in elevated song variability throughout song development.
A) Representative sonograms showing a tutor’s song and the adult songs (95 dph) of three of his pupils, including one pupil that received injections of LV-ShC in Area X and two pupils that received injections of LV-ShFoxP2 in Area X early in juvenile life (~20 dph, scale bar indicates 100ms, ordinate: 0–9 kHz). Black bars underline accurately copied individual syllables, red bars underline inaccurately copied syllables. B) Injecting LV-ShFoxP2 (red, ShFoxP2 n = 6 birds) in Area X at ~20 dph disrupts the ability of juvenile birds to copy a tutor’s song, resulting in significantly lower similarity scores to the tutor’s song compared to the LV-ShC injected birds (blue, ShC n = 4 birds; mean ± s.e.m) throughout development (asterisk denotes p < 0.05, ANOVA followed by t-test). C) The coefficient of variation in pitch was higher in the FoxP2 knockdown animals compared to the control animals at 65 dph. D) The entropy of syllables decreased over the course of development in the control animals (blue) but not in the knockdown animals (red). The entropy of each syllable was normalized to its entropy value calculated at 45 dph. E) Levels of FoxP2 in Area X are correlated with the similarity of the adult bird’s song to its tutor’s song. (R2 = 0.65, LV-ShC (blue) or LV-ShFoxP2 (red), n = 5 birds in each group). F) Representative sonograms of songs produced by an LV-ShFoxP2-injected bird (left) and an LV-ShC injected (right) bird recorded at 45, 65, and 95 dph (numbers in white represent average % similarity to the adult BOS for the given day, scale bar indicates 100 ms. G) Comparing each bird’s song recorded at various time points across development to its adult song reveals that both control (blue) and FoxP2 knockdown birds (red) display comparable developmental trajectories. See also Figure S2.
To dissociate the effects of LV-ShFoxP2 injections on performance and song development independent of the quality of copying, we compared each bird’s song at various stages of development to its adult song. Despite marked differences in their abilities to accurately copy a tutor song, LV-ShFoxP2 and LV-ShC injected animals displayed comparable developmental trajectories to their final adult songs (Figures 3F, G; ShFoxP2: n = 6 birds, ShC: n = 4 birds, p > 0.05). These findings are consistent with the idea that reducing FoxP2 levels in Area X of the juvenile causes it to produce syllables that are more variable in pitch and also noisier, raising the possibility that these acute performance deficits impair the pupil’s ability to accurately and precisely copy a tutor song.
Reducing FoxP2 expression in Area X alters signal propagation through the AFP
How altered FoxP2 expression impacts transmission through corticostriatal circuits to affect learned vocalizations remains unknown. However, the relationship between the modulation of singing-related activity in the AFP and song variability suggests that reduced FoxP2 levels in Area X could affect signal propagation from HVC to LMAN. To test this idea, we made intracellular current clamp recordings from LMAN neurons in anesthetized adult (>90 dph) male zebra finches previously injected in Area X with LV-ShFoxP2 or LV-ShC and applied a brief current pulse through a bipolar stimulating electrode positioned in the ipsilateral HVC (Figure 4A; 40 μA, 400 μs, see Methods; LV injections were made ~20 dph). Electrical stimulation in HVC elicited excitatory synaptic responses in all (72/72) LMAN neurons, regardless of whether they were recorded from LV-ShFoxP2 or LV-ShC animals, indicating that reduced FoxP2 levels in Area X do not prevent signal propagation through the AFP. However, the latency to the onset of the evoked synaptic response was significantly shorter in the LV-ShFoxP2 animals, indicating that signals propagate more quickly from HVC through the AFP (Figures 4B, C; synaptic latency: ShFoxP2 = 12.5 ± 0.3 ms; ShC = 15.5 ± 0.3 ms; ShFoxP2: n = 35 cells, 5 birds; ShC: n = 37 cells, 4 birds; p < 10E-5). In contrast, the mean amplitude and coefficient of variation of latency of the evoked synaptic responses did not differ between LMAN neurons from LV-ShFoxP2 and LV-ShC birds (Figures S3A and S3B). Furthermore, the interspike interval (ISI) distributions and spontaneous firing rates of LMAN neurons did not differ between the two groups (Figures S3C and S3D). Therefore, reducing FoxP2 levels in Area X specifically affects how rapidly signals propagate from HVC through the AFP of anaesthetized birds.
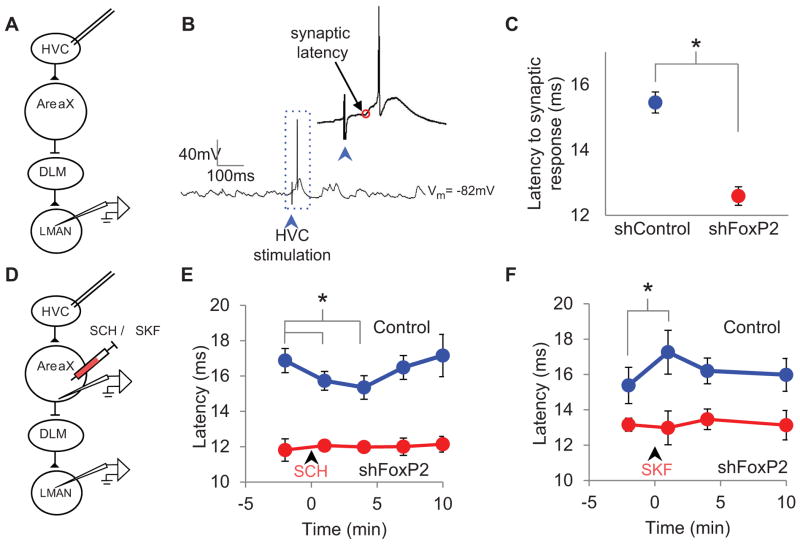
Figure 4. Reducing FoxP2 levels in Area X alters signal propagation times through the AFP.
A) A schematic showing the placement of the stimulation and recording electrodes. B) In vivo intracellular membrane potential recording from an LMAN neuron showing the synaptic response evoked by brief electrical stimulation in HVC (40 μA, 400 μs, bipolar electrodes). C) Signal propagations times (synaptic latency) from HVC to LMAN are shorter in LV-ShFoxP2- than in LV-ShC-injected birds (p < 0.001, ShFoxP2 n = 37 cells, ShC n = 35 cells, mean ± s.e.m). D) A schematic depicting the placement of the stimulation and recording electrodes and the puffer pipette. E) In adult male zebra finch controls, the AFP signal propagation time transiently decreases following the injection of D1R antagonist (SCH) into Area X (1 and 4 min post drug delivery, p < 0.05, blue, n = 8 cells, 4 birds). In contrast, the AFP signal propagation time in adult finches previously injected with LV-ShFoxP2 (red, n = 5 cells, 3 birds) is unaffected by the SCH injection. F) Conversely, the AFP signal propagation times measured in control finches transiently increases following the injection of D1R agonist (SKF) into Area X (blue, 1 min post drug delivery, p < 0.05, n = 5 cells, 5 birds). In contrast, AFP signal propagation times in the FoxP2 knockdown animals (red, n = 5 cells, 3 birds) are unaffected by SKF injection. See also Figure S3.
A prior study reported that infusing a D1R antagonist into Area X of adult male zebra finches abolishes the reduced syllable variability characteristic of directed singing (Leblois and Perkel, 2010), similar to the effects of injecting LV-ShFoxP2 into Area X of adult birds observed here. These similar behavioral effects, along with the theorized role for dopamine receptor-dependent signaling in modulating singing- related neural activity in the AFP (Doya and Sejnowksi, 1995; Fee and Goldberg, 2011), led us to wonder whether altered AFP signal propagation times in LV-ShFoxP2 birds reflect impaired striatopallidal sensitivity to dopamine signaling. To test this idea, we measured synaptic responses in LMAN neurons evoked by periodic (0.17 Hz) electrical stimulation in HVC before and after we injected a D1R antagonist (SCH) into Area X through a puffer pipette (Figures 4D and S3E, see Methods). Applying SCH to Area X of adult male zebra finches significantly and reversibly decreased the mean latency of synaptic responses evoked in LMAN by HVC stimulation, indicating that dopaminergic signaling in Area X influences how rapidly signals propagate through the AFP (Figures 4E and S3F; synaptic latency: baseline = 16.9 ± 0.6 ms, SCH: 15.2 ± 0.6 ms; control: n = 8 cells in 4 adult finches not injected with any LV-constructs; p = 0.03 and 0.02 for 1 and 5 min post drug injection, respectively). In contrast, the latency of HVC-evoked synaptic responses recorded in LMAN neurons of LV-ShFoxP2- injected adult finches was unchanged by SCH application in Area X (Figures 4E and S3G; synaptic latency ShFoxP2, baseline: 11.8 ± 0.6 ms, SCH: 12.0 ± 0.3 ms, n = 5 cells in 3 birds; p = 0.7). Similarly, infusing a D1R agonist (SKF) into Area X significantly and reversibly increased AFP propagation times in control but not in LV-ShFoxP2 birds (Figures 4F and S3H, I; synaptic latency in controls: baseline = 15.4 ± 1 ms, SKF = 17.3 ± 1.2 ms, n = 5 cells in 5 birds, p = 0.01 for 1 min post drug injection; ShFoxP2 baseline = 13.1 ± 0.4 ms, SKF = 13 ± 1 ms, n = 5 cells in 3 birds, p = 0.9). In addition, applying SKF or SCH in Area X did not alter the resting membrane potentials or spontaneous firing rates of LMAN neurons in either control or LV-ShFoxP2 birds (Table S2). Thus, D1R-mediated signaling in Area X modulates how rapidly activity propagates from HVC to LMAN and reducing FoxP2 levels in Area X renders the AFP signal propagation time insensitive to this type of modulation.
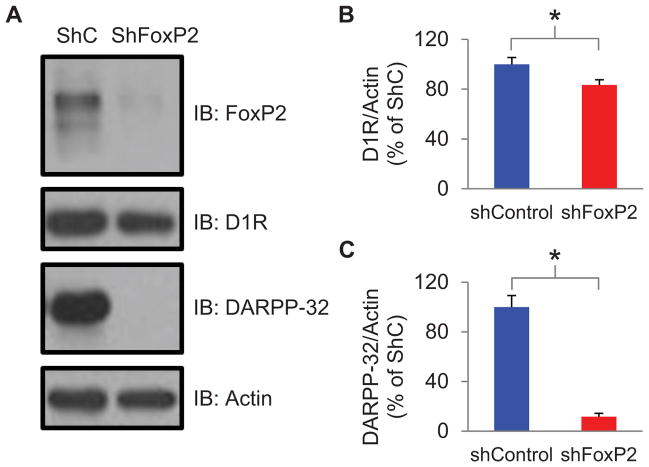
Knockdown of FoxP2 reduces levels of D1Rs and DARPP-32 in Area X
These electrophysiological findings raise the possibility that FoxP2 knockdown in Area X interferes with dopaminergic signaling by reducing levels of D1Rs and/or downstream molecules that play a critical role in signal transduction through this receptor. Consistent with this idea, FoxP2 knockdown reduces the density of dendritic spines on medium spiny neurons (MSNs), and many D1Rs are localized to MSN spines (Schulz et al., 2010; Levey et al., 1993; Yao et al., 2008). Furthermore, DARPP-32, a key component of the D1R signaling cascade, is a downstream target of FoxP2 (Vernes et al., 2011). To test whether FoxP2 knockdown affected levels of D1Rs and DARPP-32, we performed western blots of Area X tissue homogenates obtained from birds previously injected with LV-ShFoxP2 or LV-ShC (see Methods). Optical density measurements revealed that FoxP2 knockdown resulted in significantly lower levels of D1R and DARPP-32 (Figure 5A–C; D1R was reduced 16.6 ± 4.3% and DARPP-32 was reduced 88.4 ± 2.8%; p < 0.05). Therefore, FoxP2 knockdown reduces levels of D1R and a key molecule that links D1R receptor activation to intracellular signaling cascades, thus providing a molecular basis for the physiological and behavioral deficits we observed.
Figure 5. Knockdown of FoxP2 reduces levels of D1Rs and DARPP-32 in Area X.
A) A representative immunoblot (IB) showing FoxP2, D1R, DARPP-32 and actin protein levels in tissue punches from Area X of LV-ShC and LV-ShFoxP2 injected birds (>90 days after the injection). B) The Area X tissue samples from the LV-ShFoxP2-GFP injected animals had significantly lower levels of D1Rs compared to the control animals (mean 16% reduction; LV-ShFoxP2: n=11 birds; LV-ShC: n = 11; p < 0.05; DR1 levels were normalized to actin, mean ± s.e.m). C) The Area X tissue samples from the LV-ShFoxP2-GFP injected animals had significantly lower levels of DARPP-32 compared to the control animals (mean 88% reduction; LV-ShFoxP2: n=11 birds; LV-ShC: n = 10; p < 0.05; normalized to actin).
FoxP2 knockdown in Area X of adults abolishes context-dependent changes in LMAN activity
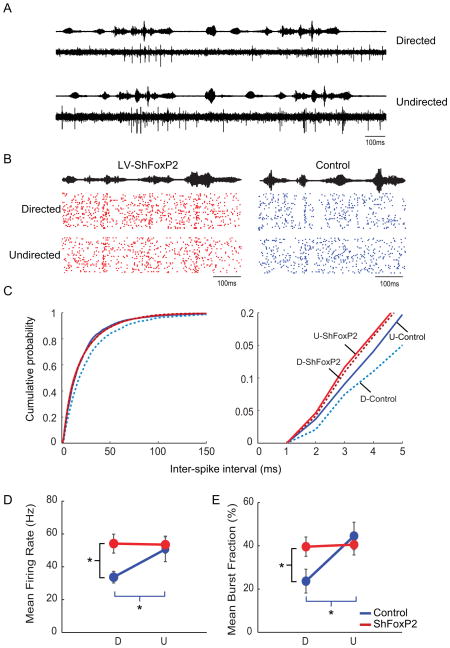
A remaining issue is how FoxP2 knockdown affects singing-related activity in the AFP. Prior electrophysiological studies in singing birds reveal that LMAN neurons exhibit higher firing rates, augmented bursting activity and greater trial-by-trial variability in spike timing during undirected singing relative to directed singing (Kao et al., 2008), which we confirmed here using chronic extracellular recordings of LMAN activity in adult male zebra finches (Figure 6A–E and S4 A–B, blue; n = 3 adult male zebra finches; 127 ± 26 dph). To test whether FoxP2 knockdown in Area X abolishes context-dependent changes in the singing-related activity of LMAN neurons, we recorded the singing-related activity of LMAN neurons in adult male zebra finches previously injected with LV-ShFoxP2 in Area X (n = 3 birds; 110 ± 8 dph at implantation; injections were made 32 ± 8 days prior to electrode implantation; see Methods). In contrast to LMAN neurons in control birds, the mean firing rate and levels of bursting activity in LMAN neurons remained elevated in LV-ShFoxP2 birds as they switched from undirected to directed singing (Figure 6B–E, red; these birds did not show context-dependent changes in song variability, Figure S4C). Consequently, the ISI distributions of LMAN neurons recorded in the directed state were left-shifted in LV-ShFoxP2 birds relative to controls (Figure 6C, K-S test, p = 4.4381e-19). Notably, the ISI distributions from LMAN neurons recorded in the undirected state were also left-shifted in LV-ShFoxP2 birds relative to controls (Figure 6C, p = 0.003, K-S test). Furthermore, FoxP2 knockdown abolished context-dependent changes in trial-by-variability of LMAN activity (p = 0.65), with activity patterns during directed singing becoming more variable compared to control birds (Figure S4A and B; p = 0.01). Finally, both the mean firing rates and levels of bursting activity of LMAN neurons recorded in the directed state were significantly higher in the LV-ShFoxP2 birds (Figure 7D and E; p = 0.006 and p = 0.013 respectively). Therefore, reducing FoxP2 levels in Area X abolishes context-dependent changes in both LMAN singing-related activity and song variability.
Figure 6. FoxP2 knockdown in Area X of adults abolishes context-dependent changes in LMAN activity.
A) An example of singing-related multiunit activity recorded in LMAN of a normal adult male zebra finch when he sings to a female (directed songs; top) and when he sings alone (undirected songs; bottom). Amplitude envelopes of the sound recordings are plotted above the neural traces; the bird sang two consecutive motifs in each context. Spike sorting of the multiunit records was used to isolate single units and measure mean firing rates and inter-spike intervals (see Methods). B) Example raster plots of singing related activity in sorted LMAN units from a LV-ShFoxP2 (left) and control animal (right) as they switched between social contexts (34 trials in each condition). Each dot signifies a spike and each line represents a trial. C) Cumulative probability distribution of inter-spike intervals (ISI) of singing-related activity of LMAN neurons recorded in LV-ShFoxP2 animals are indistinguishable between the directed (D, dotted) and undirected states (U, solid) (red: p = 0.127, K-S test). In contrast, the ISI distribution of LMAN neurons recorded in control animals is significantly left-shifted in the undirected state compared to the directed state (blue: p = 9.413 e-13, K-S test). The ISI distributions of LMAN neurons recorded from control and LV-ShFoxP2 animals are significantly different in the directed state (p = 4.4381 e-19, K-S test) and in the undirected state (p = .003, K-S test). The panel on the right shows ISI distributions over the interval that corresponds to bursting activity in LMAN neurons (i.e., 1–5 msec, or > 200 Hz). D) In control animals, the mean firing rate of LMAN neurons (n = 9 units, 3 birds) is significantly higher in the undirected state compared to the directed state (blue; p = 0.009; paired t-test). In contrast, in the LV-ShFoxP2 animals, LMAN neurons (n = 20 units, 3 birds) display comparable firing rates in the two states (red; p = 0.859; paired t-test). The mean firing rate of neurons in the LV- ShFoxP2 animals is significantly higher compared to control animals in the directed state (p = 0.006, mean ± s.e.m), while the mean firing rates in the undirected state are comparable (p = 0.707). E) In control animals, the mean burst fraction (%) is significantly higher in the undirected state compared to the directed state (blue; p = 0.002, paired t-test). This context-dependent difference in mean burst fraction was absent in LV-ShFoxP2 animals (red; p = 0.575, paired t-test). The mean burst fraction is significantly higher in the LV-ShFoxP2 animals compared to the control animals in the directed state (p = 0.01), but not the undirected state (p = 0.546). See also Figure S4 and Table S2.
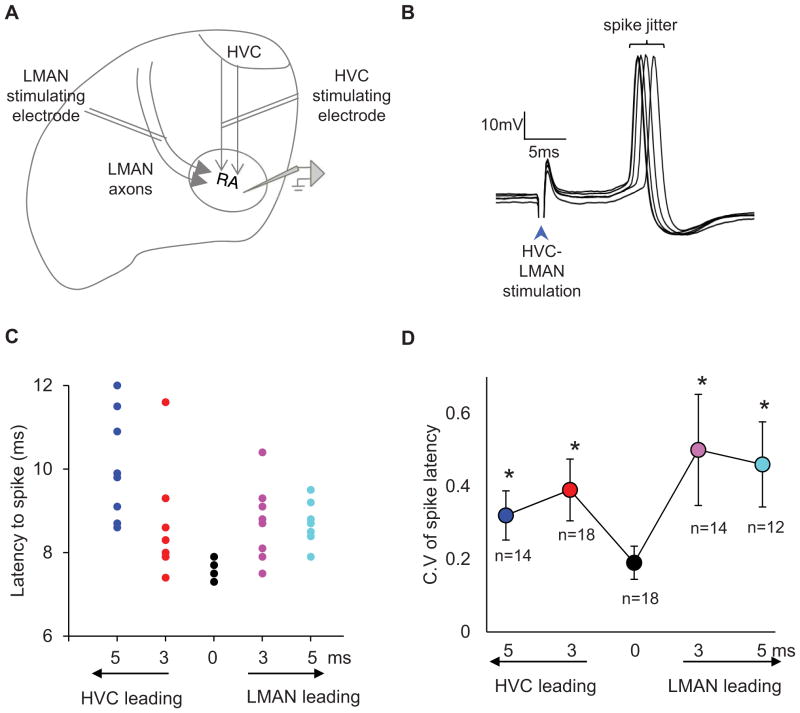
Figure 7. Millisecond differences in HVC and LMAN axon activity affect action potential timing variability in RA.
A) A schematic of a coronal brain preparation showing HVC and LMAN axon tracts in relation to RA and the relative placement of the stimulation and recording electrodes. B) Intracellular membrane potential recording from an RA neuron in a coronal brain slice preparation showing action potentials evoked by brief and simultaneous electrical stimulation of HVC and LMAN fiber tracts (HVC- 50 μA, LMAN-100 μA, 100 μs, bipolar electrodes). C) A raster plot of action potentials evoked from a RA neuron by stimulating HVC and LMAN axon tracts at varying relative latencies. Each color represents a different relative stimulation time of the axon tracts. D) The mean coefficient of variation in spike latency is at a minimum when HVC and LMAN axons tracts are simultaneously stimulated and is significantly higher when the absolute relative latencies in stimulation times are increased to 3 and 5 milliseconds (mean ± s.e.m, p < 0.05, n = number of RA neurons that contributed to each measurement).
Millisecond differences in AFP timing can affect spike timing variability in RA
We were also curious to know whether small differences in AFP signal propagation times could affect neural mechanisms important to song variability. Notably, the AFP affects syllable variability through the synapses that LMAN axons make on RA neurons (Olveczky et al., 2005), and the variability of action potential timing in RA neurons can account for a significant fraction of acoustic variation in the bird’s song (Sober et al., 2008). These observations along with our findings advance a model in which RA spike timing variability is affected by the relative latencies of singing-related activity arriving from LMAN and HVC. To begin to test this idea, we used sharp intracellular recordings in brain slices to measure the timing of action potentials in RA neurons evoked by electrically stimulating LMAN and HVC axons at different latencies (Figure 7A, n = 23 cells from brain slices from 5 adult male zebra finches, 120dph ± 10dph). Specifically, we measured the variability of RA action potential timing (i.e., “spike jitter”) when the interval between LMAN and HVC axon stimulation was varied from 0 to ± 5 ms, which encompasses the small but significant change in AFP signal propagation times in LV-ShFoxP2 birds (Figure 7B). We found that RA spike timing variability was significantly higher when the interval between LMAN and HVC axon stimulation was lengthened from 0 to ± 3 ms and ± 5 ms (Figures 7C, D; p < 0.05). Therefore, millisecond differences in the arrival times of signals from LMAN and HVC can affect RA spike timing variability, providing a mechanism by which millisecond changes in AFP signal propagation times, along with elevated bursting activity of LMAN neurons, could contribute to song variability.
Discussion
Here we found that lentiviral shRNA-mediated FoxP2 knockdown in Area X abolishes context-dependent modulation of song variability in adult birds and elevates song variability in juvenile birds. Using in vivo intracellular recordings in anesthetized adult birds, we also found that FoxP2 knockdown accelerates signal transmission from HVC through the AFP and renders this signal propagation time insensitive to modulation by dopamine receptors of the D1 subtype. Furthermore, we found that FoxP2 knockdown decreased levels of D1R and DARPP32, providing a plausible molecular basis for these physiological deficits. Using chronic recordings adult zebra finches, we also found that FoxP2 knockdown in Area X abolished context-dependent modulation of singing-related activity in LMAN, the output nucleus of the AFP that drives song variability through its synaptic connections to RA neurons. Finally, using intracellular recordings in brain slices, we demonstrated that small changes in signal propagation times through the AFP similar to those observed following FoxP2 knockdown in Area X are sufficient to drive increased variability in the action potential timing of RA song premotor neurons, an electrophysiological correlate of song variability (Sober et al., 2008). Taken together, these findings show that FoxP2 knockdown in Area X interferes with the D1R-dependent modulation of activity propagation in the AFP and the context-dependent modulation of singing-related activity in LMAN. These neuronal deficits disrupt the adult bird’s ability to modulate performance variability as a function of social context and could diminish the juvenile’s capacity to harness performance variability that is theorized to facilitate song copying.
Knockdown of FoxP2 in Area X affects song variability in adult and juvenile finches
A prior study demonstrated that FoxP2 knockdown affects the ability of juvenile zebra finches to accurately imitate their tutor’s song, although exactly how learning was affected remained unclear (Haesler et al., 2007). Here we found that shRNA-mediated reduction in FoxP2 protein levels in Area X affects the context-dependent modulation of song variability in adult birds and chronically elevates song variability in juvenile birds (see also Fig. 5b, Haesler et al, 2007), findings that could inform our understanding of how altered FOXP2 expression impacts vocal learning in humans. First, reducing FoxP2 levels in Area X affects adult song performance, ruling out a model where this gene only contributes to juvenile song learning and extending its role to encompass the adult’s ability to generate appropriate behavioral responses to salient social cues. Second, a diminished ability to control and modulate song variability could explain how decreased FoxP2 levels in Area X interfere with song copying. More specifically, decreased FoxP2 levels in Area X could prevent accurate imitation of a tutor song by disrupting the juvenile’s ability to efficiently generate and select those motor programs that produce the best match to the tutor song model. Consistent with this idea, juvenile birds that received LV-ShFoxP2 injections in Area X produced syllables that were more variable in pitch and noisier in comparison to those produced by their control-injected counterparts. However, we cannot exclude the possibility that decreased FoxP2 levels also affect mechanisms besides those that drive trial-by-trial variations in song performance, including those that are important to longer term synaptic and behavioral consolidation (Enard et al., 2009; Groszer et al., 2008).
Indeed, transcription factors such as FoxP2 can alter the expression of hundreds or thousands of downstream genes and thus have the potential to affect brain function and behavior through a wide variety of mechanisms (Hilliard et al., 2012; Vernes et al., 2011). However, several observations implicate FoxP2-mediated regulation of dopaminergic signaling in the learning and performance deficits that have been measured in zebra finches (Haesler et al., 2007; current findings). First, our findings along with gene expression studies show that FoxP2 regulates several molecules implicated in D1R-mediated signaling, including DARPP-32, which is co-expressed with FoxP2 in D1R-expressing MSNs in both zebra finches and mice (Vernes et al., 2011; Haesler et al., 2004; Heiman et al., 2008; Konopka et al., 2009). Furthermore, several genes implicated in neuromodulatory signaling pathways, including a gene that encodes a dopamine-metabolizing enzyme (monoamine oxidase B, MAOB), are differentially regulated by FOXP2 in humans versus chimps (i.e., FOXP2chimp) (Konopka et al., 2009; Spiteri et al., 2007; Vernes et al., 2007). Second, dopamine levels in Area X increase during directed singing and infusion of a D1R antagonist into Area X can abolish context-dependent changes in song variability, similar to the effects of FoxP2 knockdown observed here (Sasaski et al., 2006; Leblois et al., 2010). Third, we found that FoxP2 knockdown in Area X disrupts signal propagation through the AFP and renders this propagation insensitive to D1R-mediated signaling. Finally, dopaminergic signaling has been advanced as a key player in reinforcement learning, which is theorized to contribute to song motor learning (Reynolds et al., 2001; Doya and Sejnowski, 1995; Fee and Goldberg, 2011). Taken together, these findings support a model where FoxP2 is necessary for D1R-mediated control of striatal signaling important to effective vocal communication in adults and efficient vocal learning in juveniles.
Signal propagation deficits in the AFP and song variability
Disrupted FoxP2 expression can affect the structure and function of striatal MSNs in mice (Enard et al., 2009; Groszer et al., 2008) and can reduce spine density in MSNs in Area X of zebra finches (Schulz et al., 2010). Moreover, mice with FoxP2 mutations are impaired in learning motor skills and auditory-motor associations (Enard et al., 2009; Groszer et al., 2008; Kurt et al., 2010) and also display patterns of striatal activity that are abnormally high and aberrantly modulated during motor skill learning (French et al., 2012). Nonetheless, how these striatal deficits affect the function of circuits important to vocal performance and learning has remained unclear. By performing electrophysiological recordings in LMAN following FoxP2 knockdown in Area X, we were able to identify resultant circuit deficits, including more rapid propagation of activity from HVC to LMAN, diminished sensitivity of AFP signal propagation to D1R-mediated signaling, and loss of context-dependent modulation of singing-related activity in LMAN neurons. Notably, this latter deficit reflects persistently elevated bursting activity, greater trial-by-trial variability and elevated firing rates in LMAN neurons, all of which are electrophysiological signatures that are thought to drive song variability.
Important goals of future studies will be to more fully resolve the origins and functional significance of FoxP2- and D1R-dependent changes in AFP propagation times, which were on the millisecond timescale. One possibility is that diminished signaling through the D1R+ pathway in FoxP2 knockdown birds unmasks or accentuates contributions from other pathways through Area X, including those involving synapses made by HVC axons onto pallidal neurons or D2R+ MSNs (Farries et al., 2005; Ding and Perkel 2002). Another possibility is that diminished signaling through D1R+ pathways reveals contributions from pathways that circumvent Area X altogether, such as the direct cortico-thalamocortical loop involving RA – DLM – LMAN (Goldberg and Fee 2012). Another issue is whether and how the small differences in AFP signal propagation times we observed following FoxP2 knockdown are linked to increases in song variability. One clue is that birdsong and the neural mechanisms that give rise to its generation display remarkable temporal precision. During singing, RA neurons produce bursts of action potentials with sub-millisecond precision and remarkably low trial-by-trial variability (Chi and Margoliash, 2001), and small variations in RA action potential timing can account for a significant portion of song variability (Sober et al., 2008). Here we provide evidence in brain slices that millisecond differences in the relative arrival time of signals from HVC and LMAN to RA can influence RA spike time variability, providing a plausible mechanism by which small timing differences in AFP signal propagation times could affect song variability.
However, the connection between altered AFP signal propagation times and song variability remains untested and thus an alternative is that faster AFP signal propagation times following FoxP2 knockdown in Area X reflect other pathological processes that interfere with the modulation of song variability. In fact, extracellular dopamine concentrations in Area X decrease and singing-related bursting activity in LMAN increases when male zebra finches sing more variable undirected songs (Sasaki et al., 2006; Kao et al., 2008). Notably, we found that FoxP2 knockdown in Area X renders the striatopallidal circuit insensitive to signaling through D1Rs, while abolishing context-dependent changes in LMAN bursting and song variability. These effects of FoxP2 knockdown are suggestive of dopamine hypofunction, which in rodents is associated with an increase in correlated corticostriatal activity (Costa et al., 2006; Wickens et al., 2007). Therefore, the diminished sensitivity to dopamine signaling following FoxP2 knockdown in Area X could trigger pathological levels of correlated corticostriatal (i.e., HVC – Area X) activity during singing, which could act through yet to be explored mechanisms within Area X to affect bursting activity in LMAN. Ultimately, heightened correlations in HVC – Area X activity could accelerate signal propagation through the AFP and augment bursting activity in LMAN, either or both of which could contribute to song variability.
Significance for the role of FOXP2 mutations in human speech deficits
In humans, FOXP2 mutations impair speech learning, result in an orofacial dyspraxia, and reduce the volume of the head of the caudate nucleus, a region important to the control of orofacial movements (Hurst et al., 1990; Watkins et al., 2002a; Vargha-Khadem et al., 2005; Watkins et al., 2002b; Belton et al., 2003; Jernigan et al., 1991). In particular, patients with FOXP2 mutations display severe deficits in articulation (Hurst et al., 1990) and trouble with fluent repetition of word and non-word sequences (Watkins et al., 2002a). Although birdsong and speech evolved independently, the extreme temporal demands of auditory-motor integration necessary to these learned vocal behaviors may require that striatal pathways operate at their performance limits, with the consequence that songbirds with diminished FoxP2 levels in Area X produce vocal deficits that resemble core aspects of those observed in humans with FOXP2 mutations. We found that FoxP2 knockdown in Area X increases LMAN bursting activity and song variability, directly opposite of the effects of Area X lesions (Kojima et al., 2013), suggesting that FOXP2 mutations may exert a gain of function effect on brain and behavior. Therefore, while FOXP2 may regulate many processes in the human brain necessary to normal language development, one way that FOXP2 mutations could interfere with speech learning is by disrupting dopaminergic signaling necessary to modulate and ultimately constrain vocal variability.
Experimental Procedures
All experimental procedures were conducted in accordance with National Institutes of Health guidelines and reviewed by Duke University’s Institutional Animal Care and Use Committee.
Virus injections
Birds were anaesthetized with 1–2% isoflurane and placed in a stereotaxic setup. Stereotaxic coordinates and multiunit recordings were used to localize Area X. A glass pipette attached to a Nanoject-II (Drummond Scientific) delivered ~1 μL of virus across ~5 sites per hemisphere spanning much of Area X (~200nl per site, 32nl every 30sec). Lentivirus (LV) particles expressing U6-shFoxP2-h-ubiquitin-gfp or U6-shFoxP2-f-ubiquitin-gfp (1×107 to 109 particles/ mL (Marguerita Klein, Duke University; Roberts et al., 2008) were used interchangeably to knockdown FoxP2 expression in Area X (Haesler et al., 2007). Control LV shRNA (shControl) particles (Santa Cruz Biotechnology, sc-108080) were used for the control experiments. All behavioral and electrophysiological experiments were conducted >15d post injection.
Immunohistochemistry
Birds were anesthetized with 0.08 ml equithesin (i.m. injection) and perfused with 0.025 M phosphate-buffered saline (PBS), followed by fixation with 4% paraformaldehyde in PBS (PFA). Brains were removed and post-fixed in 4% PFA with 30% sucrose overnight at 4°C. Parasagittal sections were cut on a freezing microtome (Reichert) at 50–75 μm. Antibody staining was run to enhance visualization of co-expressed GFP (mouse monoclonal anti-GFP primary (Invitrogen, 1:1000) followed by goat anti-mouse secondary coupled to Alexa 488 (Invitrogen, 1:500).
Western Blot
~ 90d following LV injections, birds were placed in a darkened chamber at ~ noon. 2h later, the birds were anesthetized with 5% isoflurane, decapitated, and their brains were removed and mounted on a vibratome (Leica, VT 1000s). 100 μm sections were cut until Area X was visible (~ 1200 μm from the rostral tip of the brain; Miller et al., 2008). A 0.8mm tissue punch was used to remove much of the tissue encompassing Area X, which was localized by GFP fluorescence; samples were from the left hemisphere. Blots were incubated with FoxP2 (1:500, Sigma-Aldrich), D1R (1:1000, Abcam), DARPP-32 (1:1000, Abcam) and actin (1:10000, Sigma-Aldrich) primary antibodies for at least 12 hours at 4°C followed by incubation with secondary antibodies (1:5000, Jackson Labs) for at least 1 hour at RT. For quantitative analysis, gels were digitally scanned and the optical band density was quantified using ImageJ. Optical densities for FoxP2, D1R, and DARPP-32 were normalized to corresponding actin levels, shown as mean ± SEM and significance was assessed with Student’s t-test.
Song recordings and analysis
Songs were recorded in sound attenuation chambers and signals were bandpass filtered (0.4 – 10 kHz) and stored to a hard drive using Sound Analysis Pro (SAP) (Tchernichovski et al., 2000), which was also used for all song analyses.
Adult experiments
Songs were recorded from adult (> 120 dph) male zebra finches 2 ± 1 d before and 27 ± 1 d after LV injection. Undirected songs were recorded from isolated males. Directed songs were elicited by presenting one or two female birds (limited to a 2 min window and repeated at 1h intervals) and were classified as those produced when the male faced the female(s), as monitored with a webcam. Only syllables with constant frequency components (i.e., harmonic stacks) were included in the analysis (Kao and Brainard, 2006). The syllable’s fundamental frequency (FF) was measured for directed and undirected songs and the CV was estimated from 20 syllable renditions in each context. Multifactor ANOVA followed by post-hoc t-tests was used to test for significance.
Juvenile experiments
Juvenile (~20 dph) male zebra finches raised with their nesting group were injected with LV-ShFoxP2 (n = 4) or LV-ShC (n = 2), then returned to a sound-attenuating chamber with an adult male tutor (their father) until 45 dph. A subset of juvenile male zebra finches were isolated from adult male birds between 7–10 dph, injected with LV-ShFoxP2 (n = 2) or LV-ShC (n = 2) at ~20 dph and housed with an adult male zebra finch between 45–50dph. Following tutor experience, juveniles were housed individually and their songs recorded till 95 dph. Both groups showed comparable learning in their respective categories (ShFoxP2 groups, p = 0.4 and ShC groups, p = 0.2, t-test) and were grouped together for all subsequent analysis.
To measure song copying across development, 50 renditions of a pupil’s motif on a given day were compared to a single representative motif from his tutor to generate a similarity score (asymmetric comparisons). Syllable level analysis was restricted to those that were copied by both the FoxP2 KD juveniles and their control siblings. The FF and Wiener entropy of syllables were calculated at multiple time points through development and the entropy at any time point was normalized to the entropy of the same syllable at 45 dph. Multifactor ANOVA followed by post-hoc t-tests were used to test for significance.
In vivo and in vitro sharp intracellular recordings
All electrophysiological data were collected in a sound-attenuating chamber on an air table using a data acquisition board (National Instruments) controlled by custom Labview or MATLAB (K. Hamaguchi) software. Sharp intracellular membrane potential recordings of LMAN and RA neurons were made with glass electrodes (80–150 MΩ, 2M KAc, BF100-50-10, Sutter instruments) and amplified with an Axoclamp 2B amplifier (Axon Instruments) in bridge mode, low-pass filtered at 3 kHz, and digitized at 11 kHz (10 kHz for slice data).
In vivo experiments
A day prior to the experiment a stainless steel post was mounted on the bird’s skull with dental cement under 2% isofluorane anesthesia. The next day, birds were anaesthetized with either 20% urethane (30–40 μL / 30 min – 3 does) or diazepam (50 μl, 2.5 mg/mL) injected into their pectoral muscles. The average latencies obtained using both anesthetic agents were comparable (mean latency ± s.e.m, ms: ShFoxP2diaz: 12.5 ± 0.3, ShFoxP2ure: 12.4 ± 0.4, ShCdiaz: 15.4 ± 0.3, ShCure: 16.4 ± 0.7 ms). Sharp intracellular recordings of LMAN neurons were obtained while HVC was electrically stimulated (Biphasic; 400 μs duration; 40 μA current strength) using bipolar tungsten electrodes (0.1 MΩ) with the tips spaced ~300 μm. Synaptic latency and amplitude of postsynaptic potentials (PSPs) of LMAN responses to HVC stimulation were measured from median filtered traces using custom MATLAB software. The mean spontaneous firing rates and ISIs were measured from recordings of spontaneous activity during a 1s baseline period prior to HVC stimulation. ANOVA followed by post hoc Student t-tests were used to test for significance.
Pharmacology experiments
A glass pipette (tip diameter 15–20 μm) was attached to a tungsten electrode (0.5–1.0 MΩ, Microprobes). The glass pipette was filled with either 0.5 mM SKF 38393 hydrobromide (Tocris Bioscience) or 5 mM SCH 23390 (Sigma) in .9% saline and ~30–60 nl was injected using a Picospritzer II (General Valve) in 50–150 ms pulses at 30 psi (Leblois et al., 2010). A multiunit neural activity and robust responses in to HVC stimulation was used to confirm the location of Area X. The glass pipette-tungsten electrode into Area X was lowered at a 25–30° and a more anterior coordinate (6.3 mm rostral, 1.6 mm lateral or 5.3 mm rostral and .5 mm lateral) to avoid passing through LMAN. Control birds used for pharmacological experiments received no viral injections. Sharp intracellular recordings of LMAN neurons with HVC stimulation were performed in the above section. Each hemisphere was limited to a single instance of drug delivery. Data are presented as mean ± s.e.m. A two-factor ANOVA with repeated measures on one factor was used to test for statistical significance in latency measurements followed by post hoc two-tailed t-tests for correlated samples.
In vitro slice experiments
Birds (> 90 dph) were anaesthetized with isoflurane (5%) and decapitated. The brain was removed and moved into a solution of ice-cold aCSF. 400 μm coronal brain slices including RA were cut using a vibratome (Leica, VT 1000s). Concentric tungsten bipolar stimulation electrodes (FHC) were placed ≤ 1mm lateral and dorsal to RA to stimulate (A360 Stimulus Isolators, World Precision Instruments; 30–200 μA current strength; 100–200 μs duration) either the LMAN or HVC axon tracts respectively while intracellular sharp recordings were obtained from RA neurons. 23 cells from 5 normal birds that responded to both HVC and LMAN stimulation were included in the analysis. A subset of the 23 cells contributed to each stimulation parameter, all birds contributed to each parameter, and trial blocks were pseudo randomized to avoid bias. A Master-8 (A.M.P.I) controlled by custom LABVIEW software was used to allow either HVC or LMAN stimulation to lead the other by 0, 3 or 5ms. Spike latency was determined using custom software (MATLAB).
In vivo extracellular recordings in singing birds
A custom built manually (threaded rod) operated microdrive carrying an insulated platinum electrode (1–5 MΩ) was stereotaxically implanted 200 μm dorsal to LMAN in an anaesthetized adult male zebra finch. A reference electrode (uninsulated platinum wire) was positioned 1mm posterior to the recording electrode and a ground electrode (uninsulated silver wire) was placed over the cerebellum. The microdrive, electrodes and ground wire were secured to the bird’s skull using dental cement. All recordings were limited to the right hemisphere. After the animal was fully recovered the electrode was lowered slowly (~25 μm/ 1 h) until LMAN activity was detected. The electrode signal was amplified through a JFET on the cable attached to the bird’s head and an op-amp followed by an instrumentation amplifier (Brownlee Precision, Model 440), filtered (200 Hz-10 kHz), digitized and saved using MATLAB software. A webcam was used to observe the bird’s behavior. Multiunit data were collected in interleaved trials of directed and undirected singing. Trials with movement artifact were excluded. Wave clus was used to spike sort the multiunit data (Quiroga et al., 2004; Spike detection threshold: 3 standard deviations from baseline; 2ms detector dead time). The waveforms of the sorted clusters were tested for refractory period violations (<1% of interspike intervals 1 ms; Control: n = 9 units from 3 animals; LV-ShFoxP2: n = 20 units from 3 animals). All sorted single unit data were aligned to the onset of the first syllable of the song motif without time warping for measuring burst fraction and firing rates. Bursts were defined as spikes separated by less than 5ms. Burst fraction was defined as the fraction of the total spikes that occur as bursts. Mean firing rate and burst fraction were calculated over the entire duration of the motif. Data were analyzed using custom software (MATLAB).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003;18:194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron. 2001;32:899–910. doi: 10.1016/s0896-6273(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova T, Cyr M, Gainetdinov R, Caron M, Nicolelis MAL. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. J Neurosci. 2002;22:5210–5218. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28:353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Doya K, Sejnowski T. A novel reinforcement model of birdsong vocalization learning. Adv Neural Information Processing Syst. 1995;7:101–108. [Google Scholar]
- Enard W, Gehre S, Hammerschmidt K, Hölter S, Blass T, Somel M, Brückner M, Schreiweis C, Winter C, Sohr R, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–71. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Farries MA, Ding L, Perkel DJ. Evidence for “direct” and “indirect” pathways through the song system basal ganglia. J Comp Neurol. 2005;484:93–104. doi: 10.1002/cne.20464. [DOI] [PubMed] [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–170. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SE, Scharff C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009;25:166–177. doi: 10.1016/j.tig.2009.03.002. [DOI] [PubMed] [Google Scholar]
- French CA, Jin X, Campbell TG, Gerfen E, Groszer M, Fisher SE, Costa RM. An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol Psychiatry. 2011;17:1077–1085. doi: 10.1038/mp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci. 2011;31:10023–33. doi: 10.1523/JNEUROSCI.1606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nat Neurosci. 2012;15:620–627. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Keays D, Deacon R, de Bono J, Prasad-Mulcare S, Gaub S, Baum M, French C, Nicod J, Coventry J, et al. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–62. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey E, Lints T, Jarvis E, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–75. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson J, Day M, Ramsey K, Suárez-Fariñas M, Schwarz C, Stephan D, Surmeier D, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard AT, Miller JE, Fraley ER, Horvath S, White SA. Molecular microcircuitry underlies the functional specification of a basal ganglia circuit dedicated to vocal learning. Neuron. 2012;73:537–552. doi: 10.1016/j.neuron.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JA, Baraitser M, Auger E, Graham F, Norell S. An extended family with a dominantly inherited speech disorder. Dev Med Child Neurol. 1990;32:352–355. doi: 10.1111/j.1469-8749.1990.tb16948.x. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge, UK: Cambridge University Press; 1969. pp. 61–74. [Google Scholar]
- Jernigan TL, Hesselink JR, Sowell E, Tallal P. Cerebral structure on magnetic resonance imaging in language and learning-impaired children. Archives of Neurology. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise ring and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kao MH, Doupe AJ. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci USA. 2013;110:4756–4761. doi: 10.1073/pnas.1216308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- Konopka G, Bomar JM, Winden K, Coppola G, Jonsson ZO, Gao F, Chen L, Wang GZ, Luo R, Preuss TM, Geschwind DH. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009;462:213–217. doi: 10.1038/nature08549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Kurt S, Fisher SE, Ehret G. Foxp2 mutations impair auditory-motor association learning. PloS one. 2012;7:e33130. doi: 10.1371/journal.pone.0033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. A comparative approach to vocal learning: song development in white-crowned sparrows. J Comp Physiol Psychol. 1970;71:1–25. [Google Scholar]
- Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA. Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J Neurophysiol. 2008;100:2015–25. doi: 10.1152/jn.90415.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ölveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106:386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural computation. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- Reimers-Kipping S, Hevers W, Pääbo S, Enard W. Humanized Foxp2 specifically affects cortico-basal ganglia circuits. Neurosci. 2011;175:75–84. doi: 10.1016/j.neuroscience.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci. 2008;28:3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra nch. Genes Brain Behav. 2010;9:732–740. doi: 10.1111/j.1601-183X.2010.00607.x. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Wohlgemuth MJ, Brainard MS. Central contributions to acoustic variation in birdsong. J Neurosci. 2008;28:10370–10379. doi: 10.1523/JNEUROSCI.2448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra nch (Poephila guttata castanotis) Z Tierpsychol. 1980;53:123–132. [Google Scholar]
- Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, Vernes SC, Fisher SE, Ren B, Geschwind DH. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007;81:1144–1157. doi: 10.1086/522237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol. 2010;104:2474–2486. doi: 10.1152/jn.00977.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I, Poopatanapong A, Torrisi S, White SA. Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS One. 2010;5(1):e8548. doi: 10.1371/journal.pone.0008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I, White SA. FoxP2 regulation during undirected singing in adult songbirds. J Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Vernes S, Oliver P, Spiteri E, Lockstone H, Puliyadi R, Taylor J, Ho J, Mombereau C, Brewer A, Lowy E, et al. Foxp2 regulates gene networks implicated in neurite outgrowth in the developing brain. PLoS Genetics. 2011;7:e1002145. doi: 10.1371/journal.pgen.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, Geschwind DH, Fisher SE. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007;81:1232–1250. doi: 10.1086/522238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002a;125:452–464. doi: 10.1093/brain/awf058. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RS, Mishkin M, Gadian DG. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002b;125:465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–3. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochem Pharmacol. 2008;75:2055–2069. doi: 10.1016/j.bcp.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.