Abstract
A variety of metal-binding compounds have been found to exert anti-cancer activity. We postulated that N-acetylcysteine (NAC), which is a membrane-permeable metal-binding compound, might have anti-cancer activity in the presence of metals. We found that NAC/Cu(II) significantly alters growth and induces apoptosis in human cancer lines, yet NAC/Zn(II) and NAC/Fe(III) do not. We further confirmed that this cytotoxicity of NAC/Cu(II) is attributed to reactive oxygen species (ROS). These findings indicate that the combination of Cu(II) and thiols generates cytotoxic ROS that induce apoptosis in cancer cells. They also indicate a fourth class of anti-neoplastic metal-binding compounds, the “ROS generator”.
Keywords: N-acetylcysteine, Copper, Metal binding, Anti-cancer, Hydrogen peroxide
1. Introduction
Some metal-binding compounds exhibit anti-cancer activity, due to three distinct mechanisms of action [1–3]. The most well-recognized is “chelation” (also called “sequestration”), whereby cells are killed because they are functionally deprived of a required metal [3,4]. The effects of a chelator are reversed by supplying the sequestered metal. Other metal-binding compounds kill cancer cells by transporting metals into cells, triggering apoptotic pathways [5–8]. Some act as “ionophores” and others as “shuttles”. An ionophore is a compound capable of sequentially transporting multiple metal ions into cells. A compound killing cells by acting as an ionophore can be recognized functionally by showing that the toxicity of a fixed concentration of the ionophore is potentiated by the addition of increasing concentrations of the effector metal. For example, the cytotoxicity of a zinc ionophore (clioquinol), is potentiated by increasing the concentration of zinc, while the toxicity of a zinc chelator (TPEN) is reduced by additional zinc [4]. Shuttles compose a third group of cytotoxic metal-binding compounds. By binding the metal, they facilitate the transport of the metal into the cell. Shuttles differ from ionophores in that their cytotoxic activity is not potentiated by adding excess metal [9,10]. For reasons yet to be fully elucidated, cancer cells are more susceptible to a number of metal-binding compounds than are non-malignant cells.
We postulated that an additional number of known metal- binding compounds might have unrecognized anti-cancer activity, by serving as chelators, ionophores, or shuttles. Active compounds would be expected to have both a metal-binding domain as well as domains capable of mediating passage across the plasma membrane. Identification of compounds with these properties and a record of safe human administration would provide new potential anti-cancer agents.
One well known metal-binding domain is the thiol (–SH) group, which is found in many small molecules, as well as proteins. We thus considered whether thiol-containing compounds that have been approved for clinical use have unrecognized anti-cancer properties, and began by examining N-acetylcysteine (NAC). NAC has been used for many years as an antioxidant in biomedical research as well as in clinical practice [11,12]. Glutathione is the major antioxidant of the cytoplasm and its synthesis can be limited in vivo by the availability of cysteine [13–15]. NAC is de-acetylated to cysteine on the cell surface or inside of the cell [16], thereby promoting glutathione formation when intracellular cysteine is limiting and enhancing the antioxidant activity of the cytoplasm. It is known that under certain circumstances, anti-oxidants may also serve as pro-oxidants, and it is not surprising that NAC has also been reported to promote DNA damage, suggestively via an increase in reactive oxygen species [17–19].
Given its chemical and biological profile, we hypothesized that NAC serves as either a metal ionophore or shuttle. We assayed its effect on cell viability in the presence or absence of metals. Unexpectedly, we found that NAC is cytotoxic only when administered with copper, and exerts its effects by a previously unreported mechanism.
2. Materials and methods
2.1. Materials
The (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent was purchased from Promega (Madison, WI). The FluoZin-3 probe was obtained from Invitrogen (Carlsbad, CA). Antibodies were obtained from the following sources: Poly (ADP-ribose) polymerase (PARP) from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA); caspase 3 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from Promab Biotechnologies, Inc. (Richmond, CA). All other reagents were analytic grade and purchased from Sigma–Aldrich (St. Louis, MO).
2.2. Cell culture and cell viability assay
A2780 cells were provided by Dr. Stephen Howell (University of California, San Diego). MCF-10A, MCF-7, MDAMB-231, T47D, and Panc-1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were routinely cultured in ATCC defined medium including RPMI 1640 for MCF-7, T47D, Panc-1, DMEM for MDA-MB231, and DMEM F12 for MCF-10A. Both mediums contain l-cystine (420 µM) but not l-cysteine. All the media were supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were grown in a humidified environment of 5% CO2 at 37 °C, and propagated once or twice a week. Depending on cell proliferation rate of the cell line, 3000 to 10,000 cells per well were seeded in a 96-well tissue culture plate with 100 µl appropriate medium and achieved 30–40% confluence within 24 h. After 24 h, the medium was replaced with 100 µl of fresh medium and the cells treated for 72 h. All compounds were dissolved in phosphate buffered saline without calcium and magnesium (PBS, pH 7.4) at room temperature and added sequentially to the medium at 1:100 ratio (PBS:medium). NAC and other thiol compounds were always added prior to addition of various metals. Cell viability was assessed by the MTS assay as described previously [6]. Briefly, 20 µl of MTS solution was added to each well and cells were incubated at 37 °C for 1 h. The optical density was recorded at 490 nm and data presented as a percentage of that found in untreated cells cultured simultaneously.
2.3. Western blot analysis
Western blot was performed as previously described [6,20]. Briefly, cell lysate was prepared with the lysis buffer, sonicated on ice, and centrifuged at 15,000g for 15 min to remove insoluble material. Thirty microgram of cell lysate from each sample was resolved in 10% SDS PAGE gel, transferred to PVDF membrane, and blotted with antibodies against human caspase 3, PARP, and GAPDH.
2.4. Hydrogen peroxide generation
Hydrogen peroxide concentrations were measured using a colorimetric assay according to the manufacturer’s protocol (BioVision, Mountain View, CA). Briefly, the compounds tested were first mixed in test tubes in PBS buffer (pH 7.4) at room temperature and 30 µl of the reaction mixtures were immediately added into each well of a 96-well plate, along with 20 µl of assay buffer. Standards were generated by adding reagent H2O2 in place of the reaction mixture at concentrations of 0–5 nmol/well. Fifty microliter of a reaction mixture, containing OxiRed™ Probe and horseradish peroxidase were then added and incubated for 10 min at room temperature after which time the optical density of the solution at 570 nm was measured.
2.5. Intracellular metals and ROS detection
Intracellular zinc and copper level was monitored with fluorescent tracers, as we have previously described ([7], the FluoZin-3® and Phen Green FL probes, excitation 490/20 nm and emission 528/38 nm, Invitrogen, Carlsbad, CA). Cells were treated with ZnCl2 or CuCl2 alone or in combination with NAC for 1 h at indicated concentrations. The medium was replaced with fresh medium containing 1 µM FluoZin-3, or 5 µM Phen Green FL. After incubating for 30 min, the medium was removed and the cells were washed three times with HBSS (Hanks balanced salt solution) and viewed on the Leica TCS NT Confocal Microscope using 63× Plan APO 1.2 NA. Copper and zinc were detected as a green color. Due to the nature of the probes, when the FluoZin-3 is used, enhanced fluorescence indicates an increase in intracellular zinc level, yet when the Phen Green FL is used, enhanced fluorescence indicates a decrease in intracellular copper level.
Intracellular ROS was detected with a carboxy-H2DCFDA indicator, following the manufacture’s protocol (Invitrogen, Carlsbad, CA). Briefly, A2780 cells in RPMI with supplements were plated at 2.5 × 105 cells per well in a 6-well plate. On the next day, cells were washed once with warm HBSS (Hanks balanced salt solution) and 25 µM of carboxy-H2DCFDA (final concentration) was loaded to cells in 1 ml of HBSS buffer. The cells were then treated with 20 µM CuCl2 or 2 mM NAC alone or in combination for 30 min, thoroughly washed with HBSS for three times, and immediately viewed under the Leica TCS NT Confocal Microscope using 63× Plan APO 1.2 NA (excitation/emission at 495/529 nm).
3. Results
3.1. NAC and copper inhibit the viability and induce apoptosis of human cancer cell lines
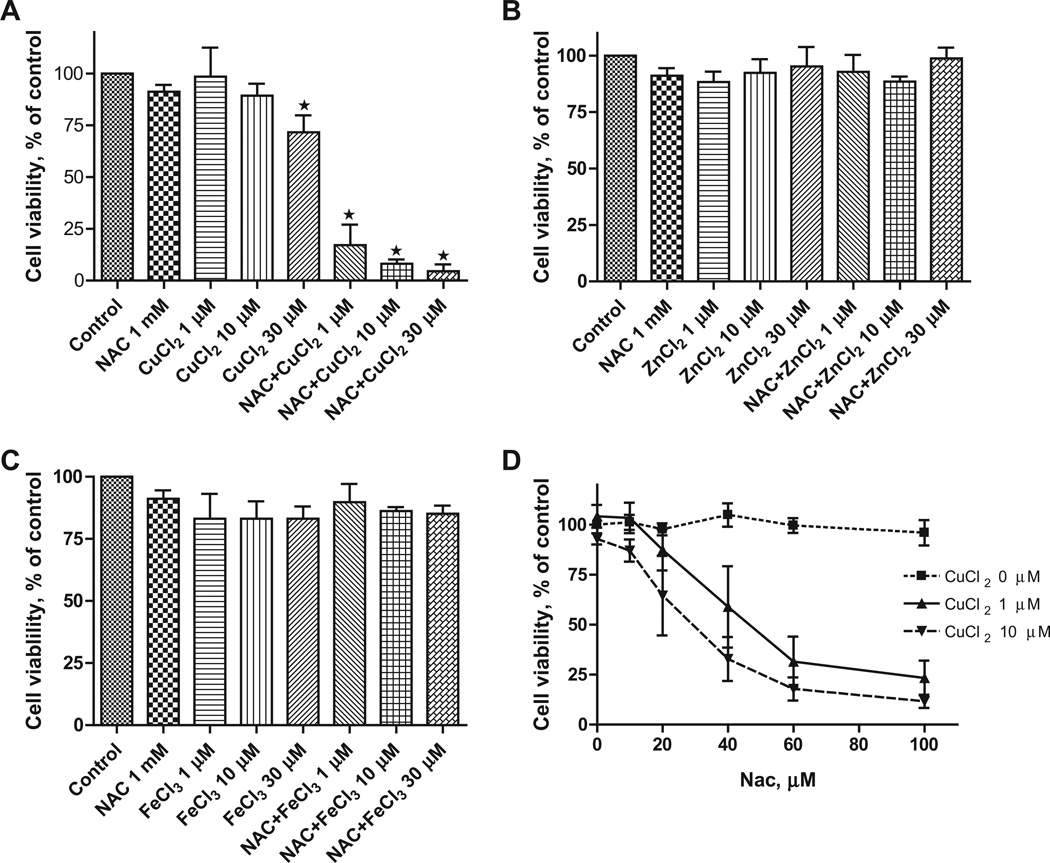
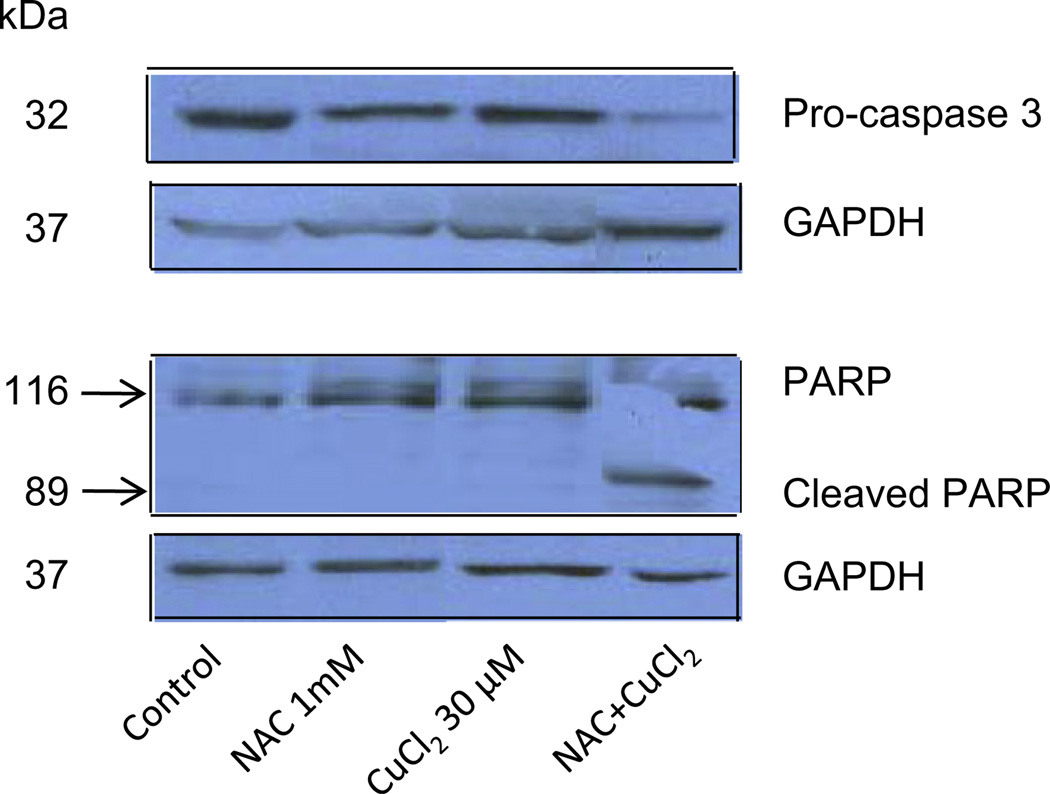
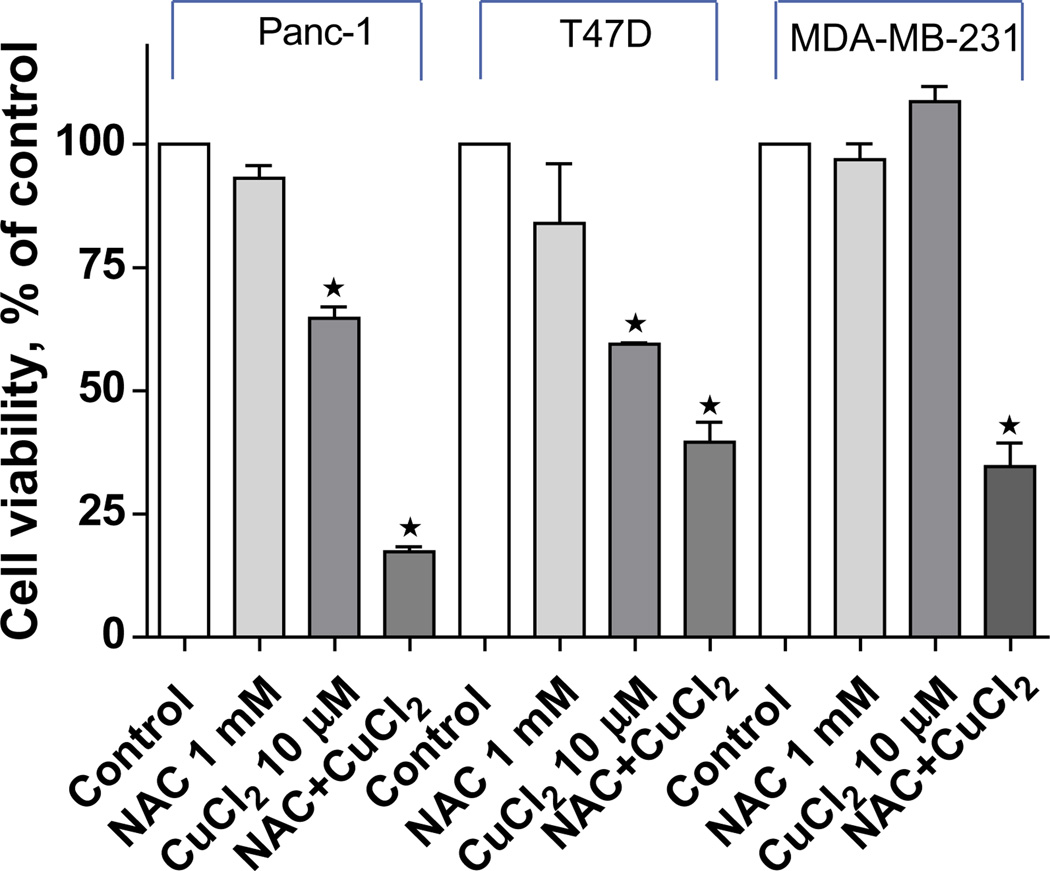
To evaluate NAC as a potential metal ionophore/shuttle, we treated A2780 human ovarian cancer cells with 1 mM NAC and various concentrations of Cu(II), Zn(II) or Fe(III), for 72 h after which time cell viability was determined using an MTS assay. As shown in Fig. 1A, NAC itself had no effect on cell viability but was toxic in the presence of Cu(II) in a concentration-dependent manner (1 µM caused 85 ± 10% inhibition, 10 µM 92 ± 3% inhibition and 30 µM 96 ± 4% inhibition). No cytotoxic effects of NAC were seen when Zn(II) or Fe(III) was substituted for Cu(II) (Figs. 1B and 1C). Cells treated with increasing concentrations of NAC (0, 10, 20, 40, 60 and 100 µM) in the absence or presence of copper showed a dose-dependent decrease in cell viability (Fig. 1D). The combination of NAC Cu(II) also induced apoptosis of A2780 cells as evidenced by caspase 3 activation and PARP cleavage (Fig. 2). To exclude the possibility that NAC selectively affects the A2780 cell line, three additional human cancer cell lines were treated with 1 mM NAC and 10 µM CuCl2. This fixed dose combination decreased the viability of a pancreatic cancer cell line (Panc-1) by 84 ± 2%, and two breast cancer lines MDA-MB-231 by 62 ± 4, and T47D 64 ± 6% (Fig. 3), paralleling the results obtained with the A2780 ovarian cancer line.
Fig. 1.
NAC specifically complexes with copper (Cu), but not zinc (Zn) or iron (Fe), to inhibit cell viability of the A2780 cells. A2780 cells were cultured in RPMI 1640 medium and treated with 0, 1, 10, and 30 µM of CuCl2 (A), ZnCl2 (B), or FeCl3 (C) in the absence or presence of NAC (1 mM) for 72 h and cell viability was examined by the MTS assay. (D) A2780 cells were treated with increasing concentrations of NAC ranging from 0 to 100 µM in the presence of CuCl2 (1 or 10 µM) for 72 h and cell viability was examined by the MTS assay. Data (means ± SD, n = 3) are expressed as percentages of the MTS level detected in untreated control cells.
Fig. 2.
Induction of apoptosis by NAC plus copper. A2780 cells were treated with NAC (1 mM) and CuCl2 (30 µM) alone, or in combination, for 72 h. Cell lysates were prepared and subjected to Western blot using antibodies against human pro-caspase 3, PARP, and GAPDH. Shown are representatives of two experiments.
Fig. 3.
Inhibitory effects of NAC plus copper on cell viability in MDA-MB-231, T47D, and Panc-1 cells. Cells were treated with NAC (1 mM) and CuCl2 (10 µM) alone, or in combination, for 72 h and cell viability was analyzed with the MTS assay. Data (mean ± SD, n = 3) are expressed as percentages of the MTS level detected in untreated control cells.
3.2. NAC does not act as a metal ionophore
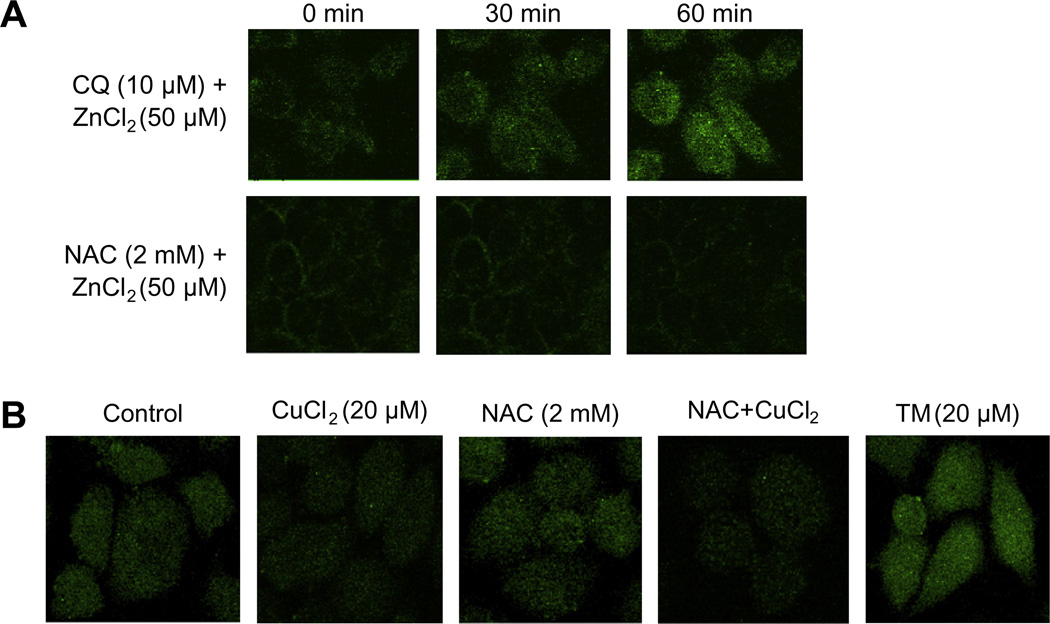
To date, the compounds identified as copper ionophores [6,8,21,22] have also been shown to be zinc ionophores. We therefore used a previously employed microscopic technique that assesses intracellular zinc concentrations to determine whether NAC was acting as an ionophore. A2780 cells were treated with NAC and zinc for 0.5–1 h, and intracellular zinc levels were then determined using a fluorescent zinc indicator, FluoZn-3 [2,7]. NAC did not increase the free intracellular zinc concentration, unlike the known zinc ionophore, clioquinol (Fig. 4A). We further tested whether NAC could act as a copper ionophore using a newly available fluorescent probe, Phen Green FL. While treatment with ammonium tetrathiomolybdate (TM, 20 µM), a well-established copper chelator [3], enhanced cellular fluorescence (indicating a reduction of intracellular free copper level), CuCl2 (20 µM) significantly quenched the fluorescence (suggesting an increase in intracellular free copper level). 2 mM NAC did not alter the cellular fluorescence level, nor did it further quench the fluorescence when combined with CuCl2 (Fig. 4B), indicating that NAC is not a copper ionophore nor is it a copper chelator [2]. Given that NAC did not appear to be a universal divalent metal transporter, we considered other means whereby NAC/Cu(II) could be cytotoxic to cells.
Fig. 4.
NAC is not a metal ionophore. A2780 cells were treated with 50 µM ZnCl2 or CuCl2 alone or in combination with 2 mM NAC for indicated times. Intracellular free zinc or copper level were assayed with the FluoZin-3® (A) and Phen Green FL (B) as described in Section 2. The cells were viewed on the Leica TCS NT Confocal Microscope using 63× Plan APO 1.2 NA (excitation 490/20 nm and emission 528/38 nm). Images are representatives of two experiments.
3.3. Hydrogen peroxide is generated by NAC/Cu(II)
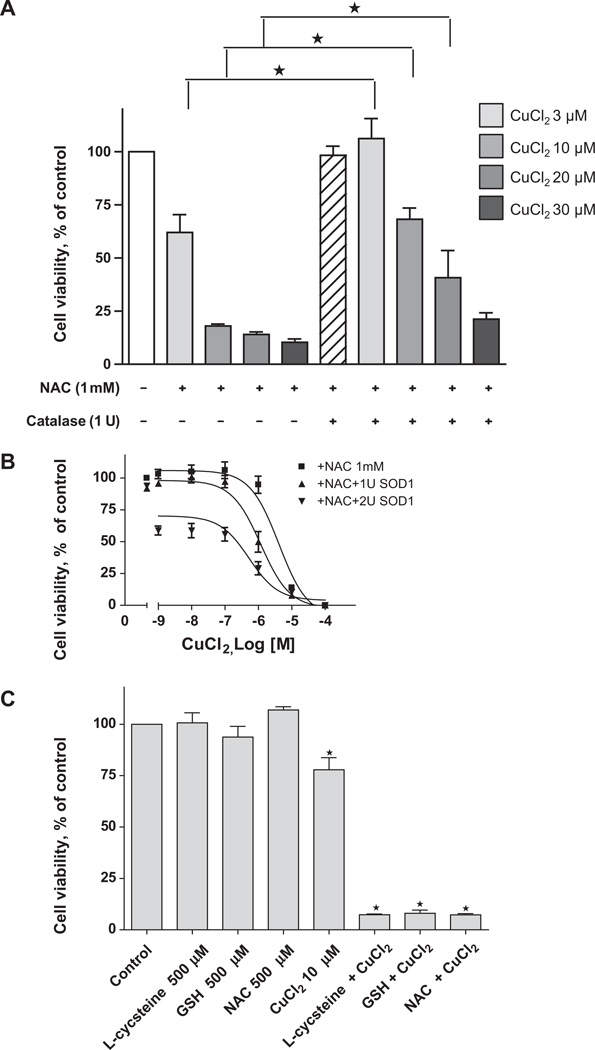
Since NAC caused cell killing in the presence of copper (a redox-active metal) but not zinc (a redox-inactive metal) we hypothesized that the combination of NAC and Cu(II) could induce an oxidative stress upon the cells. We therefore treated A2780 cells with NAC/Cu(II) in the presence of exogenous superoxide dismutase-1 (SOD1) and catalase, enzymes known to alter biological responses to reactive oxygen species. We found that catalase inhibited cytotoxicity (Fig. 5A), while SOD1 enhanced it (Fig. 5B). We postulated that these findings could be explained by the generation of superoxide by NAC/Cu(II) and its subsequent conversion to H2O2. We therefore combined NAC and CuCl2 and assayed for the production of H2O2. As shown in Table 1, H2O2 was generated in a dose-dependent manner when CuCl2 was added to a fixed concentration of NAC. NAC generated H2O2 in the presence of Cu(II), but not Zn(II) or Fe(III).
Fig. 5.
Catalase attenuates and SOD1 enhances the cytotoxic effects of NAC plus CuCl2. A2780 cells were treated with 1 mM NAC plus increasing concentrations of CuCl2 in the absence and presence of 1 unit of catalase (A) or 1–2 units of SOD1 (B). 500 µM GSH and l-Cysteine (both are reduced form) were also used to treat A2780 cells in the presence of 10 µMCuCl2 (C). After 72 h, cell viability was determined with the MTS assay. Data (means ± SD, n = 3) are expressed as percentages of the viability of untreated control cells.
Table 1.
Hydrogen peroxide is generated by thiols interacting with CuCl2. Thiols (NAC, GSH or l-cysteine) were mixed with Cu(II), Zn(II) or Fe(III) and the amount of hydrogen peroxide generated was measured after 30 min, as described in Section 2. Data are expressed as nmol of hydrogen peroxide generated in the reaction mixture (means ± SD, n = 3).
| Metals (µM) | H2O2 (nmol/well) | |||||
|---|---|---|---|---|---|---|
| Metals alone | ±NAC (2 mM) | ±GSH (2 mM) | ±l-Cysteine (2 mM) | |||
| CuCl2 | 0 | 0.03 ± 0.002 | 0.04 ± 0.004 | 0.05 ± 0.001 | 0.07 ± 0.001 | |
| 3 | 0.20 ± 0.02 | |||||
| 10 | 0.28 ± 0.01 | |||||
| 30 | 0.03 ± 0.01 | 0.47 ± 0.01 | 0.41 ± 0.01 | 0.25 ± 0.003 | ||
| 100 | 0.58 ± 0.007 | |||||
| 300 | 0.02 ± 0.01 | 0.63 ± 0.003 | ||||
| ZnCl2 | 30 | 0.02 ± 0.01 | 0.05 ± 0.005 | |||
| FeCl3 | 30 | 0.03 ± 0.004 | 0.04 ± 0.003 | |||
To determine if other thiols could generate oxidants in the presence of Cu(II), we substituted l-cysteine or GSH for NAC at comparable concentrations. As shown in Fig. 4C, reduced thiols were toxic in the presence of Cu(II). Each also generated H2O2 when added directly to Cu(II) (Table 1). Substitution of the oxidized forms of these thiols did not lead to the generation of H2O2 (data not shown).
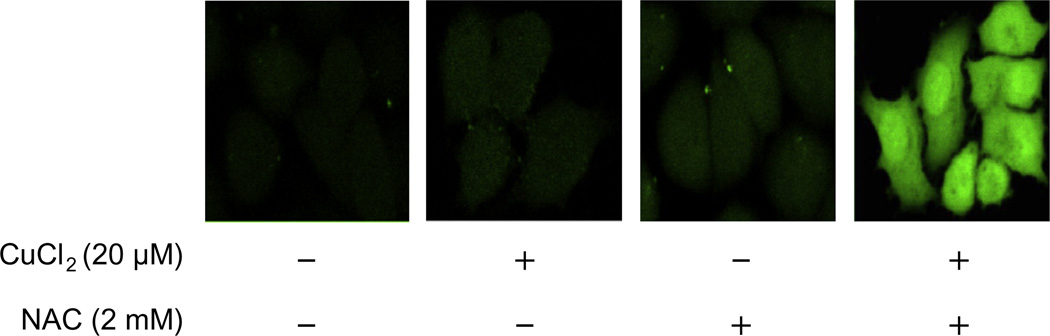
To understand whether intracellular ROS level is also increased by the treatment with NAC and Cu(II), we used a fluorescent ROS indicator to visualize ROS in living cells. While treatment of A2780 cells with 2 mM NAC or 20 µM Cu(II) alone for 30 min did not enhance intracellular ROS level, the combination led to a dramatic increase in intracellular ROS contents (Fig. 6), suggesting that the generation of H2O2 by NAC and Cu(II) leads to an enhanced cellular oxidative stress in these cells.
Fig. 6.
NAC plus copper enhances intracellular ROS level. A2780 cells were loaded with a carboxy-H2DCFDA indicator (final concentration: 25 µM) and treated with 20 µM CuCl2 or 2 mM NAC alone or in combination for 30 min. Images were captured under the Leica TCS NT Confocal Microscope using 63× Plan APO 1.2 NA (excitation/emission at 495/529 nm). Shown are representative images from two experiments.
3.4. Modulation of the cytotoxic effects of the NAC/copper combination
The effects of two well-established copper chelators, tetrathiomolyb-date (TM) [3] and bathocuproinedisulfonic acid (BCS) [23] were then examined. High concentrations (30–300 µM) of TM alone were toxic to cancer cells (Table 2) as shown by a significant reduction of cell viability, consistent with a previous report [24]. Despite the intrinsic toxicity of TM, it was able to ameliorate the toxicity of NAC/Cu(II). BCS itself had no toxic effects on the cells (presumably because it did not enter the cytosol), but also attenuated the cytotoxic effects of NAC/Cu(II). These data indicate the potential for modulation of NAC/Cu(II) toxicity by copperbinding compounds. Measurement of H2O2 generation in vitro confirmed the ability of these copper-binding compounds to decrease H2O2 generation (Table 2).
Table 2.
Copper binding compounds attenuate the cytotoxicity of NAC/Cu(II). A2780 cells were treated with increasing concentrations of tetrathiomolybdate (TM) or bathocuproinedisulfonic acid (BCS) in the presence or absence of 1 mM NAC and 10 µM CuCl2 (dissolved in PBS buffer, added sequentially) for 72 h. Cell viability was determined by the MTS assay. Data (means ± S.D, n = 3) are expressed as percentages of the viability of untreated control cells.
| Chelators (µM) | Cell viability, % of untreated control | ||
|---|---|---|---|
| Chelator alone | +NAC (1 mM)/CuCl2 (10 µM) | ||
| TM | 0 | 100 | 2 ± 1 |
| 1 | 90 ± 11 | 2 ± 1 | |
| 3 | 78 ± 9 | 3 ± 1 | |
| 10 | 80 ± 3 | 41 ± 2 | |
| 30 | 63 ± 2 | 52 ± 5 | |
| 100 | 45 ± 7 | 57 ± 8 | |
| 300 | 35 ± 2 | 40 ± 2 | |
| BCS | 1 | 84 ± 15 | 2 ± 1 |
| 3 | 85 ± 21 | 3 ± 1 | |
| 10 | 91 ± 15 | 5 ± 1 | |
| 30 | 102 ± 20 | 9 ± 1 | |
| 100 | 110 ± 5 | 101 ± 2 | |
| 300 | 117 ± 4 | 111 ± 15 | |
3.5. Neoplastic cells are more sensitive to NAC/Cu(II)
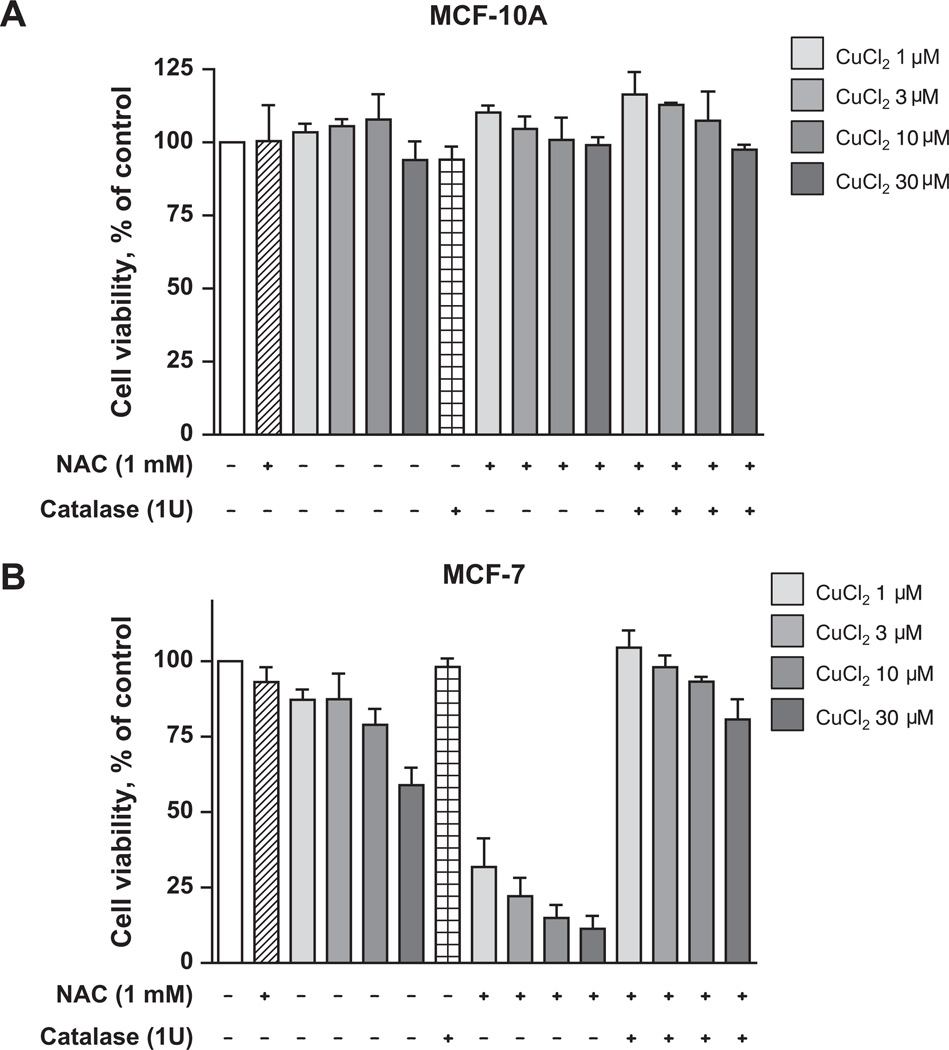
Cancer cells are more vulnerable to oxidative stress than normal cells [25]. We therefore examined the effect of NAC/Cu(II) on MCF-10A, an immortalized (non-neoplastic) breast epithelial cell line [26], and MCF-7, a well-established neoplastic breast epithelial cancer line. As shown in Fig. 7, the viability of MCF-10A cells was not altered by NAC/Cu(II). In contrast, NAC/Cu(II) showed a concentration-dependent toxicity towards the MCF-7 cancer cell line. This toxicity was blocked by catalase.
Fig. 7.
The neoplastic breast cancer line MCF-7 is more sensitive to NAC/Cu(II) than the immortalized breast line MCF-10A. MCF-10A (A) and MCF-7 (B) cells were treated with 1 mM NAC and increasing concentrations of CuCl2, ranging from 1 µM to 30 µM, in the presence or absence of catalase. Cell viability was determined by the MTS assay. Data (means ± SD, n = 3) are expressed as percentages of the level detected in untreated control cells.
4. Discussion
A number of studies have reported that cancer cells are sensitive to metal-binding compounds, which operate by several different mechanisms. Although “sequestrants” are the best recognized, others act as “ionophores” or “shuttles” (2). We postulated that other metal-binding compounds would have unrecognized anti-neoplastic properties, and might be identified by selecting those with properties of the recognized metal shuttles and ionophores. Specifically, we considered compounds that could both bind metals and cross cell membranes, preferably with a track record of safe human administration. We identified NAC as a compound with the required properties and therefore explored its anti-cancer activity in the presence of three metals. We found that NAC, in the presence of Cu(II), but not Zn(II) or Fe(III), was able to alter cellular viability of several human cancer lines. The millimolar concentrations of NAC used in this study are based on previous reports in which 1–10 mM NAC were applied to reduce cytotoxicity of pro-oxidants in cultured cells [27,28].
Because Cu(II) is redox active, we postulated that the activity of the NAC/Cu(II) combination was due to the generation of reactive oxygen species (ROS). Evidence for this mechanism was obtained by finding that the extracellular addition of enzymes (SOD and catalase) known to alter ROS modified the cytotoxicity of NAC/Cu(II). SOD (which catalyzes H2O2 generation from superoxide) increased cytotoxicity, while catalase (which hydrolyzes H2O2) decreased cytotoxicity, implicating H2O2 as a key effector molecule. This assumption was strengthened by finding that H2O2 was generated by NAC/Cu(II), but not by NAC/Zn(II) or NAC/Fe(III). The protection afforded by catalase suggested that the oxidative stress was being initiated in the extra-cellular space, and implied that other thiols might be substituted for NAC, without a loss of efficacy. Experiments using l-cysteine and GSH confirmed this assumption.
We also demonstrated that the immortalized, non-neoplastic, breast-derived epithelial line MCF-10A was not sensitive to NAC/Cu(II), unlike the neoplastic line, MCF-7. These results are in accord with previous studies which have collectively suggested that neoplastic cells are more vulnerable to oxidative stress than normal cells [25]. Oxidative stress is often cited as accounting for the anti-cancer properties of radiation, as well as a number of drugs. It is commonly believed that the oxidative stress is imposed in the intracellular space. We found that H2O2 generated by NAC and Cu(II) in the extracellular space triggers a dramatic increase in intracellular ROS level, thus leading to reduced cell viability and apoptotic cell death. A similar mechanism has recently been proposed to account for neoplastic cell killing by ascorbate, which is not thought to operate via metal-binding [29,30]. It is instructive to note that both NAC and ascorbate exhibit anti-tumorigenic behaviors by serving as anti-oxidants [31], but also have growth inhibitory effects when they act as pro-oxidants.
Our finding that SOD1 potentiates NAC/Cu(II) toxicity is important, because a functionally similar protein (extracellular SOD, SOD3) is found in the plasma. Although circulating SOD3 concentrations are relatively low (about 1 U/ml), much of this enzyme is sequestered on the endothelial surface and can be mobilized by injecting heparin, resulting in plasma levels that are 25-fold increased [32]. Thus, the plasma level of NAC required to effect cancer cell killing may not be as high as that required in typical cell culture experiments. Similar considerations may apply to ascor-bate-mediated cytotoxicity. Moreover, high-dose NAC has been administrated to humans [33] and animals [34], which indicate that millimolar plasma concentrations can be achieved without undue toxicity.
Finally, these experiments provide evidence for a fourth mechanism whereby metal-binding compounds kill cancer cells. By serving as “ROS-generants”, compounds such as the thiols identified here may prove to have clinical efficacy. Of note is the fact that serum copper levels are elevated in animals and humans with a variety of types of cancer [35–37], probably due to the elevation of plasma ceruloplasmin. The plasma copper level may thus prove to be a determinant of thiol effectiveness in vivo. It may also play a role in the in vivo effectiveness of ascorbate, which is known to oxidatively inactivate proteins in the presence of copper [38]. Because free copper is required for NAC to generate H2O2, the availability of free copper in vivo could play a critical role in NAC’s cytotoxicity. This needs to be directly investigated using in vivo tumor model systems.
The findings reported here support the evolving view that the imposition of oxidative stress upon cancer cells may have therapeutic and/or preventative potential [39]. While the explanations for the apparent resistance of normal tissues to oxidant stress remains incomplete, these findings provide an impetus for both identifying and targeting compounds capable of generating oxidants in a physiological milieu as well as the further study of metal-binding compounds.
Acknowledgments
Financial support was obtained from the American Cancer Society (CNE-117557), the Suzan G. Komen for the Cure Foundation (KG081083), the NIH OK-INBRE program (3P20RR016478-09S2), and the Oklahoma Center for the Advancement of Science and Technology (HR09-025).
Abbreviations
- BCS
bathocuproinedisulfonic acid
- NAC
N-acetylcysteine
- MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- PARP
poly (ADP-ribose) polymerase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TM
tetrathiomolybdate
- TPEN
(N,N,N′N′-tetrakis(–)[2–pyridylmethyl]-ethylenediamine
Footnotes
Conflicts of interest
None declared.
References
- 1.Huang R, Wallqvist A, Covell DG. Anticancer metal compounds in NCI’s tumor-screening database: putative mode of action. Biochem. Pharmacol. 2005;69:1009–1039. doi: 10.1016/j.bcp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ding WQ, Lind SE. Metal ionophores – an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 3.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin. Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 4.Ding WQ, Yu HJ, Lind SE. Zinc-binding compounds induce cancer cell death via distinct modes of action. Cancer Lett. 2008;271:251–259. doi: 10.1016/j.canlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak PK, Wang X, Lynch V, Wei W, Csokai V, Hacia JG, Sessler JL. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68:5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Zhou Y, Lind SE, Ding WQ. Clioquinol targets zinc to lysosomes in human cancer cells. Biochem. J. 2009;417:133–139. doi: 10.1042/BJ20081421. [DOI] [PubMed] [Google Scholar]
- 8.Zhai S, Yang L, Cui QC, Sun Y, Dou QP, Yan B. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxyquinoline and clioquinol requires their capabilities to bind copper and transport copper into cells. Biol. J. Inorg. Chem. 2010;15:259–269. doi: 10.1007/s00775-009-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecane PS, Karaman MW, Sirisawad M, Naumovski L, Miller RA, Hacia JG, Magda D. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 10.Sahoo SK, Sawa T, Fang J, Tanaka S, Miyamoto Y, Akaike T, Maeda H. Pegylated zinc protoporphyrin: a water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjugate Chem. 2002;13:1031–1038. doi: 10.1021/bc020010k. [DOI] [PubMed] [Google Scholar]
- 11.Atkuri KR, Mantovani JJ, Herzenberg LA. N-Acetylcysteine – a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millea PJ. N-acetylcysteine: multiple clinical applications. Am. Fam. Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 13.Aitio ML. N-acetylcysteine – passe-partout or much ado about nothing? Brit J. Clin. Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32:6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- 15.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 16.Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology. Cerebellum. 2007:1–7. doi: 10.1080/14734220601142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikawa S, Yamada K, Yamashita N, Tada-Oikawa S, Kawanishi S. N-acetylcysteine, a cancer chemopreventive agent, causes oxidative damage to cellular and isolated DNA. Carcinogenesis. 1999;20:1485–1490. doi: 10.1093/carcin/20.8.1485. [DOI] [PubMed] [Google Scholar]
- 18.Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, van Oirschot JF, van der Bruggen T, van Asbeck BS. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am. J. Respir. Crit. Care Med. 1998;157:1283–1293. doi: 10.1164/ajrccm.157.4.9508063. [DOI] [PubMed] [Google Scholar]
- 19.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radical Biol. Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 20.Tuller ER, Beavers CT, Lou JR, Ihnat MA, Benbrook DM, Ding WQ. Docosahexaenoic acid inhibits superoxide dismutase 1 gene transcription in human cancer cells: the involvement of peroxisome proliferator-activated receptor alpha and hypoxia-inducible factor-2alpha signaling. Mol. Pharmacol. 2009;76:588–595. doi: 10.1124/mol.109.057430. [DOI] [PubMed] [Google Scholar]
- 21.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CH, Kim JH, Moon SJ, Chung KC, Hsu CY, Seo JT, Ahn YS. Pyrithione, a zinc ionophore, inhibits NF-kappaB activation. Biochem. Biophys. Res. Commun. 1999;259:505–509. doi: 10.1006/bbrc.1999.0814. [DOI] [PubMed] [Google Scholar]
- 23.Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O’Halloran TV. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter A, Rassam A, Jennings MH, Robinson-Jackson S, Alexander JS, Erkuran-Yilmaz C. Effects of ammonium tetrathiomolybdate, an oncolytic/angiolytic drug on the viability and proliferation of endothelial and tumor cells. Inflamm. Res. 2007;56:515–519. doi: 10.1007/s00011-007-7025-2. [DOI] [PubMed] [Google Scholar]
- 25.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 27.Winter K, Pagoria D, Geurtsen W. The effect of antioxidants on oxidative DNA damage induced by visible-light-irradiated camphorquinone/N,N-dimethyl-p-toluidine. Biomaterials. 2005;26:5321–5329. doi: 10.1016/j.biomaterials.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 28.Pagoria D, Geurtsen W. The effect of N-acetyl-l-cysteine and ascorbic acid on visible-light-irradiated camphorquinone/N,Ndimethyl-p-toluidine-induced oxidative stress in two immortalized cell lines. Biomaterials. 2005;26:6136–6142. doi: 10.1016/j.biomaterials.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte TL, Almeida GM, Jones GD. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol. Lett. 2007;170:57–65. doi: 10.1016/j.toxlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 33.Prescott L. Oral or intravenous N-acetylcysteine for acetaminophen poisoning? Ann Emerg. Med. 2005;45:409–413. doi: 10.1016/j.annemergmed.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RE, Hawkins HC, Weikel JH., Jr The toxicity of N-acetylcysteine in laboratory animals. Semin. Oncol. 1983;10:17–24. [PubMed] [Google Scholar]
- 35.Majumder S, Chatterjee S, Pal S, Biswas J, Efferth T, Choudhuri SK. The role of copper in drug-resistant murine and human tumors. Biometals. 2009;22:377–384. doi: 10.1007/s10534-008-9174-3. [DOI] [PubMed] [Google Scholar]
- 36.Mazdak H, Yazdekhasti F, Movahedian A, Mirkheshti N, Shafieian M. The comparative study of serum iron, copper, and zinc levels between bladder cancer patients and a control group. Int. Urol. Nephrol. 2009 doi: 10.1007/s11255-009-9583-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Milacic V, Frezza M, Dou QP. Metal complexes, their cellular targets and potential for cancer therapy. Curr. Pharm. Des. 2009;15:777–791. doi: 10.2174/138161209787582183. [DOI] [PubMed] [Google Scholar]
- 38.Lind SE, McDonagh JR, Smith CJ. Oxidative inactivation of plasmin and other serine proteases by copper and ascorbate. Blood. 1993;82:1522–1531. [PubMed] [Google Scholar]
- 39.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliver. Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]