Abstract
Background
Ligands binding the somatostatin receptor type 2 (SSTR2) are useful for imaging and treatment of neuroendocrine tumors (NETs), but not all tumors express high levels of these receptors. The aim of this study was to evaluate gene expression of new therapeutic targets in NETs relative to SSTR2.
Methods
RNA was extracted from 103 primary small bowel (SBNET) and pancreatic neuroendocrine tumors (PNET), matched normal tissue, and 123 metastases. Expression of 12 candidate genes was measured by quantitative PCR normalized to internal controls; candidate gene expression was compared to SSTR2.
Results
Relative to normal tissue, primary NET expression of SSTR2, GPR98, BRS3, GIPR, GRM1, and OPRK1 were increased by 3, 8, 13, 13, 17, and 20-fold, respectively. Similar changes were found in metastases. While most candidate genes showed lower absolute expression than SSTR2, absolute GIPR expression was closest to SSTR2 (mean dCT 3.6 vs. 2.7, p=0.01). Absolute OPRK1 and OXTR expression varied significantly by primary tumor type and was close to SSTR2 in SBNETs but not PNETs.
Conclusions
Compared to the current treatment standard SSTR2, GIPR has only somewhat lower absolute gene expression in tumor tissue but much lower expression in normal tissue, making it a promising new target for NET imaging and therapy.
Introduction
Small bowel and pancreatic neuroendocrine tumors (NETs) are rare tumors with a combined incidence of 0.8–1.2 cases per 100,000 per year1. SBNETs and PNETs together comprise around half of all gastroenteropancreatic neuroendocrine tumors (GEPNETs), and present with regional or distant metastasis in 50–85% of cases1, 2. When possible, surgery is effective for neuroendocrine tumors. Even metastatic NETs can be treated surgically, and retrospective studies report a survival benefit for resection of primary tumors and cytoreduction of liver metastases3–5. Still, most patients undergoing surgery will have recurrence6 and peptide receptor-directed strategies are recommended for most tumors4.
The utility of ligands binding the somatostatin receptor in neuroendocrine tumors has been long recognized7. Somatostatin analogues such as octreotide ameliorate symptoms, promote tumor regression or disease stabilization in 50–60% of patients, and are responsible for improvement in 5-year survival rates4, 6, 8. Somatostatin receptor scintigraphy (SRS) imaging with 111In-octreotide9, positron emission tomography with 68Ga-octreotide10 (PET/CT), and peptide-receptor radionuclide therapy (PRRT) with 90Y- or 177Lu-conjugated somatostatin analogues are also beneficial to NET patients11. Theranostic strategies rely on high expression of the target receptor in the NET with lower receptor expression in surrounding tissues to provide selective targeting to tumor cells. Five SSTR subtypes comprise the somatostatin-receptor family10. The most extensively expressed is the somatostatin type 2 receptor (SSTR2), which is found in 80–95% of GEPNETs12–15. While some newer somatostatin analogues show increased affinity for additional SSTR-types such as SSTR5, all use SSTR2 as their principal target10, 14. Effects of somatostatin analogues are mediated by anti-secretory activity through SSTR2, induction of apoptosis through SSTR5, and inhibition of angiogenesis through SSTR36, 13. By targeting these receptors, clinicians can achieve symptomatic improvement, image tumors, and potentially offer PRRT.
Despite these successes, many tumors do not respond adequately to SSTR2-based therapies. Somatostatin receptor-based imaging fails to detect primary tumors or nodes in over 25% of SRS cases, although sensitivity is improved using PET/CT16–18. Lack of uptake on imaging excludes patients from trials of PRRT11, which has reported complete response rates of 28–38% and disease stabilization in 50% of patients with GEPNETs6, 11. Perhaps most importantly, even patients who respond to treatment with octreotide develop increasing resistance to its effects over time15.
For these reasons, neuroendocrine tumor treatment requires new peptide receptor targets, which our group set out to identify using our collection of neuroendocrine tumor tissues. Our initial studies used exon and G-protein-coupled receptor (GPCR) microarrays to measure gene expression of many potential targets in a small number of tissue samples19. Of six genes selected for expression testing in additional tissue samples, the oxytocin receptor (OXTR) emerged as a strong candidate due to its dramatically elevated expression (15–90 fold) in tumor compared to normal tissues20. We set out to define additional receptor targets and compare their expression to the current standard for imaging and treatment, SSTR2, using an expanded 12-gene panel in a large set of GEPNETs and their metastases.
Methods
Patients
Since 2005, patients undergoing surgery for small bowel (SBNETs) and pancreatic NETs (PNETs) were enrolled under an IRB-approved protocol and provided informed consent. At surgery, tumor and corresponding normal tissues, involved lymph nodes, and liver metastases were collected and preserved in RNALater solution (Life Technologies, Grand Island, NY). Clinical correlations used our Neuroendocrine Tumor Registry Database, as described16.
Selection of targets
Six target genes, ADORA1, SCTR, GPR113, MEP1B, MUC13, and OXTR were selected as previously described19, 20 (Table 1). GPCR microarray expression data were reanalyzed with normalization to POLR2A and GAPDH internal controls and gene expression in tumors was compared to normal tissues19. Six new targets, BRS3, DRD1, GIPR, GPR98, GRM1, and OPRK1 were selected based on high tumor expression relative to normal tissues, and also high expression relative to internal controls. All samples were then tested against the full 12-gene panel, SSTR2, and internal controls.
Table 1.
Gene targets and primers used for qPCR
| Gene | Name | Primer |
|---|---|---|
| SSTR2 | Somatostatin receptor type 2 | Hs00265624_s1 |
| BRS3 | Bombesin-like receptor 3 | Hs00179951_m1 |
| GIPR | Gastric inhibitory polypeptide receptor | Hs00609210_m1 |
| GPR98 | G-protein-coupled receptor 98 | Hs01022907_m1 |
| GRM1 | Glutamate receptor metabotropic 1 | Hs00168250_m1 |
| OPRK1 | Opioid receptor kappa 1 | Hs00175127_m1 |
| DRD1 | Dopamine receptor D1 | Hs00265245_s1 |
| OXTR | Oxytocin receptor | Hs00168573_m1 |
| GPR113 | G-protein-coupled receptor 113 | Hs00542378_m1 |
| SCTR | Secretin receptor | Hs01085380_m1 |
| ADORA1 | Adenosine A1 receptor | Hs00379752_m1 |
| MUC13 | Mucin 13 | Hs00217230_m1 |
| MEP1B | Meprin A beta | Hs00195535_m1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs02758991_g1 |
| POLR2A | Polymerase RNA polypeptide-2A | Hs00172187_m1 |
qPCR
Gene expression was measured by quantitative PCR (qPCR) as described19, 20. Briefly, total RNA was isolated from tissue harvested at surgery by the Trizol method (Life Technologies), and cDNA was prepared by reverse transcription. Taqman reagents and primers for target genes were obtained from Life Technologies (Table 1), and expression was measured in triplicate by qPCR on a StepOne-Plus RT-PCR System and a 384-well 7900 HT-Fast RT-PCR System (Applied Biosystems, Grand Island, NY). Gene expression in each tissue sample was determined by mean threshold cycle (Ct) normalized to average Ct of GAPDH and POLR2A internal controls. This gives the dCT, which is gene expression normalized to internal controls on a logarithmic scale. Expression relative to corresponding normal tissue (the ddCT) of tumors and metastases was computed by subtracting the dCT of tumor or metastatic tissue from the dCT of corresponding normal tissue. Fold-change relative to normal is given by 2(−ddCT).
Statistics
Welch’s two-sided t-test compared mean dCTs and ddCTs, and Fisher Exact test compared categorical variables. Multiple comparisons were p-value adjusted using the false discovery rate correction with significance set at p<0.01. All statistics used R v.2.15.2 (Vienna, Austria).
Results
Gene Expression Relative to Normal Tissue
Reasoning that for clinical utility a new target gene should have higher expression in tumor than normal tissue, gene expression was measured in 56 primary SBNETs (with 53 nodal and 32 liver metastases) and 47 PNETs (with 20 nodal and 18 liver metastases), as well as their corresponding normal tissues. Five of six new gene targets selected based on high expression in pilot experiments (n=26 primary tumors) showed high expression in this larger set of samples. Mean expression of BRS3, GIPR, GPR98, GRM1, and OPRK1 was significantly higher in primary tumors compared to normal tissues with measured mean ddCTs corresponding to 13, 13, 7.5, 17.1, and 19.7-fold increases, respectively (Table 2, Figure 1, p<0.0001 for all). Despite 14-fold increased expression in pilot experiments (not shown), expression of DRD1 was not significantly different from normal tissue in this larger sample (mean 1.2-fold higher, p=0.28).
Table 2.
Relative gene expression in SBNET and PNET tissues compared to normal tissue
| Mean Fold-Change Compared to Normal | ||||
|---|---|---|---|---|
| Gene | Primary Tumors n=103 | Nodal Mets n=73 | Liver Mets n=50 | P-value Primary vs. Normal |
| SSTR2 | 3.0 | 3.7 | 4.3 | <0.0001 |
| BRS3 | 13.0* | 16.0* | 29.9* | <0.0001 |
| GIPR | 13.0* | 19.7* | 29.9* | <0.0001 |
| GPR98 | 7.5 | 13.0* | 12.1 | <0.0001 |
| GRM1 | 17.1* | 18.4* | 26.0* | <0.0001 |
| OPRK1 | 19.7* | 29.9* | 55.7* | <0.0001 |
| DRD1 | 1.2* | 0.8* | 0.7* | 0.28 |
| OXTR | 39.4* | 59.7* | 48.5* | <0.0001 |
| GPR113 | 4.9 | 7.0 | 11.3 | <0.0001 |
| SCTR | −7.5* | −17.1* | −4.3* | <0.0001 |
| ADORA1 | −3.0* | −2.8* | −4.6* | <0.0001 |
| MUC13 | 1.7 | −1.9* | 1.1 | 0.051 |
| MEP1B | 1.5 | −22.6* | −9.2* | 0.28 |
Indicates significantly different from SSTR2 at p<0.001
All p-values are false discovery rate (FDR) adjusted
Fold-changes for OXTR, GPR113, SCTR, ADORA1, MUC13, MEP1B are as reported20
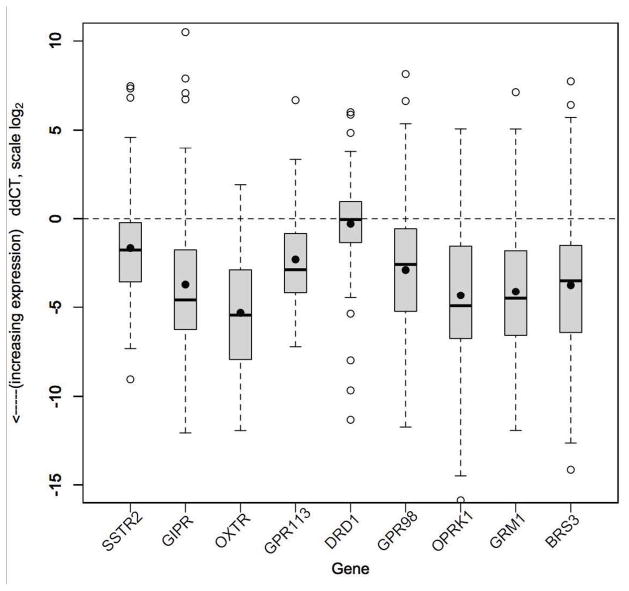
Figure 1.
Relative gene expression (ddCT) in SBNET and PNET primary tumors (n=103) compared to normal tissue. Lower ddCT indicates higher expression (log scale). Boxes show IQR, whiskers show 1.5*IQR, open circles show outliers, bar shows median, dot shows mean. Horizontal line indicates expression level equal to normal tissue. SSTR2, GIPR, OXTR, GPR113, GPR98, OPRK1, GRM1, and BRS3 all show significantly higher expression in tumors compared to their expression in normal pancreas or small bowel tissue. OXTR showed the greatest overexpression in tumor tissue compared to normal tissue as indicated by the lowest ddCT. Overexpression of GIPR, OXTR, OPRK1, GRM1, and BRS3 was significantly greater than that of SSTR2.
To ascertain whether these findings were present in associated neuroendocrine tumor metastases, their gene expression was determined. Among the five new genes (BRS3, GIPR, GPR98, GRM1, and OPRK1) found to have increased expression in primary tumors, both liver and lymph node metastases also showed significantly increased expression relative to normal tissues, with fold-increases trending higher from primary tumors to lymph node metastases to liver metastases (Table 2). Taken together, these results suggested that these five genes, in addition to OXTR and GPR113, which we previously reported to have elevated expression in NETs relative to normal tissue20, might show promise as therapeutic targets in NETs.
Increased Expression Relative to SSTR2
For assessment against a receptor protein of known clinical utility, expression of SSTR2 was measured and compared to the seven genes showing overexpression in tumor tissues (Table 2). As expected, primary tumors as well as nodal and liver metastases showed significantly higher SSTR2 expression than normal tissues (mean 3-fold higher in primaries, p<0.0001). Seven candidate genes showed greater overexpression than SSTR2. Five of these, BRS3, GIPR, GRM1, OPRK1, and OXTR had significantly greater overexpression than SSTR2 in primary tumors, nodal metastases, and liver metastases compared to normal tissue (p<0.001). GPR98 showed a trend towards higher relative expression compared to SSTR2 in primary tumors versus normal (7.5 vs. 3.0 fold, p=0.012), but this reached significance only for nodal metastases (p<0.001; p=0.016 in liver metastases). The significantly higher fold-increased expression as compared to SSTR2 of these five genes (BRS3, GIPR, GRM1, OPRK1, OXTR) versus normal tissue further supported them as potential new therapeutic targets.
Comparison of Absolute Expression
One limitation to the above findings is that genes with high expression in tumors relative to normal tissue may still have low absolute expression if expression in normal tissue is extremely low, and therefore they may not be effective as targets for therapy. Thus, we next evaluated absolute expression of all 12 genes based on dCT rather than relative expression versus normal, and compared these results to absolute expression of SSTR2 (Table 3). Expression of SSTR2 mRNA in primary tumor tissues was high, as indicated by a low dCT (mean 2.7, interquartile range 0.9–3.9). Only MUC13 showed higher expression than SSTR2 in primary tumors (mean dCT 1.1, IQR −0.4–2.4), but its expression in normal tissues was also high (mean dCT of 2.3 IQR −0.7–5.1). Among candidate genes BRS3, GIPR, GRM1, OPRK1, and OXTR, all showed lower absolute expression (higher dCT) than SSTR2, but with large differences in the degree to which expression was lower. BRS3, GRM1, and OPRK1 had significantly lower expression than SSTR2, with dCTs corresponding to −68.6, −48.5, and −9.8 fold lower expression than SSTR2, respectively (Figure 2, p<0.00001). Overall expression of OXTR was also significantly lower than SSTR2, but with a smaller fold-difference of −5.3 (mean dCT 5.1, IQR 2.9–7.1, p<0.0001 vs. SSTR2). Finally, mean GIPR expression was closest to SSTR2 at only −1.9 fold lower in primary tumors (mean dCT 3.6, IQR 2.1–4.9, p=0.01). Absolute GIPR expression in nodal and liver metastases was not significantly lower than SSTR2 (mean dCTs 3.3 and 2.9, p=0.03 and 0.07).
Table 3.
Absolute gene expression by mean dCT with interquartile range (IQR)
| All Primary Tumor Types | P-value Primary vs. SSTR2 | ||||
|---|---|---|---|---|---|
| Gene | Primary Tumors n=103 | Nodal Mets n=73 | Liver Mets n=50 | Normal Tissue n=103 | |
| SSTR2 | 2.7 (0.9–3.9) | 2.4 (1.1–3.0) | 2.3 (1.5–3.0) | 4.4 (3.4–5.9) | NA |
| BRS3 | 8.8 (5.8–11.5) | 9.2 (7.7–11.3) | 8.6 (5.6–11.0) | 12.7 (10.0–15.8) | <0.0001 |
| GIPR | 3.6 (2.1–4.9) | 3.3 (1.7–4.2) | 2.9 (1.9–3.8) | 7.3 (6.1–9.0) | 0.01 |
| GPR98 | 7.1 (5.9–7.1) | 6.8 (5.6–8.1) | 6.9 (5.7–7.5) | 10.0 (7.5–12.0) | <0.0001 |
| GRM1 | 8.3 (6.2–10.5) | 8.5 (6.7–10.5) | 8.0 (6.4–9.4) | 12.4 (10.5–14.3) | <0.0001 |
| OPRK1 | 6.0 (2.8–8.8) | 4.6 (1.4–6.9) | 4.0 (1.8–5.0) | 10.4 (7.6–13.7) | <0.0001 |
| DRD1 | 6.3 (4.7–8.1) | 6.7 (5.0–8.1) | 7.4 (5.7–8.5) | 6.5 (4.6–8.0) | <0.0001 |
| OXTR | 5.1 (2.9–7.1) | 4.6 (1.5–6.9) | 4.8 (2.4–6.9) | 10.5 (8.9–12.5) | <0.0001 |
| GPR113 | 5.6 (4.3–6.9) | 5.6 (4.0–7.1) | 5.1 (3.8–6.4) | 8.0 (6.9–9.3) | <0.0001 |
| SCTR | 7.7 (6.0–9.9) | 9.9 (9.1–11.7) | 7.9 (6.1–9.4) | 4.7 (0.7–8.6) | <0.0001 |
| ADORA1 | 8.7 (7.6–9.7) | 9.6 (8.5–11.0) | 9.8 (9.0–10.8) | 7.2 (5.5–9.1) | <0.0001 |
| MUC13 | 1.1 (−0.4–2.4) | 2.3 (0.1–3.5) | 1.9 (0.1–3.4) | 2.3 (−0.7–5.1) | <0.001 |
| MEP1B | 4.4 (1.6–7.2) | 8.0 (6.8–14.4) | 7.9 (5.9–10.4) | 5.3 (0.2–10.1) | <0.001 |
P-values are FDR-adjusted
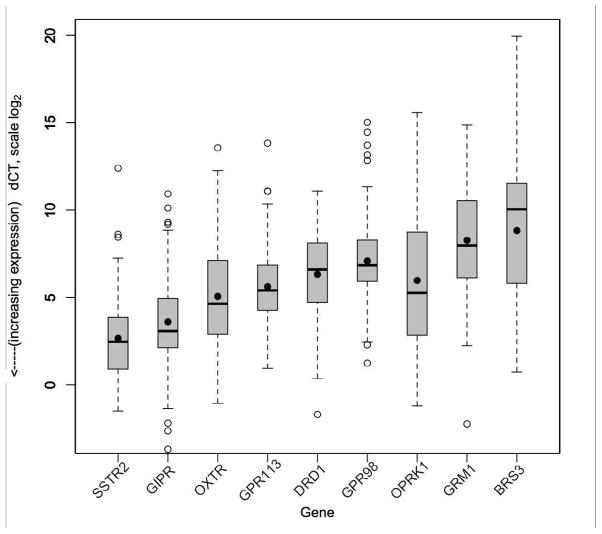
Figure 2.
Absolute gene expression (dCT) in SBNET and PNET primary tumors (n=103). Lower dCT indicates higher expression (log scale). Boxes show IQR, whiskers show 1.5*IQR, open circles show outliers, bar shows median, dot shows mean. SSTR2 has the highest absolute expression (mean dCT 2.7). GIPR expression (mean dCT 3.6) is closest to SSTR2, while mean expression of OXTR, GPR113, DRD1, GPR98, OPRK1, GRM1, and BRS3 is much lower than SSTR2, as indicated by higher dCT (p<.0001).
Expression by primary tumor type
To determine whether gene expression varied by the primary tumor site, dCTs of SBNET and PNET primary tumors were compared (Table 4). Again as expected, SSTR2 expression was high in both SBNETs and PNETs (mean dCT 3.1, IQR 2.0–3.8 and 2.1, IQR 1.6–3.8, p=0.09). GIPR expression was also high in both tumor types, and was not significantly different between SBNET and PNETs (mean dCT 3.9, IQR 2.4–5.3 and 3.2, IQR 1.9–4.1, p=0.18). These findings suggest that like SSTR2, GIPR could be an effective target for both tumor types. Four genes, BRS3, OPRK1, OXTR, and SCTR, were found to have significantly different absolute expression between SBNETs and PNETs (Figure 3, p<0.0001 for all). OXTR expression was low in PNETs (mean dCT 6.7, IQR 4.1–8.5) but close to SSTR2 in primary SBNETs (mean dCT 3.8, IQR 1.7–5.0, p=0.012 vs. SSTR2). Similarly, OPRK1 had higher expression in primary SBNETs (mean dCT 3.6, IQR 2.4–4.7, p=0.018 vs. SSTR2) compared to PNETs (mean dCT 9.3, IQR 7.7–10.8). Since levels of OXTR and OPRK1 expression were close to GIPR and SSTR2 in SBNETs, they also show promise as therapeutic targets in these tumors. Both BRS3 and SCTR had significantly higher expression in PNETs than SBNETs, but absolute expression was still low (mean PNET dCT 6.7 for BRS3, 5.6 for SCTR), suggesting that they may not be as useful to target as SSTR2 and GIPR. However, expression differences between tumor types seen in OXTR, OPRK1, BRS3, and SCTR may be useful for differentiating NETs of unknown origin.
Table 4.
Absolute gene expression by primary tumor type (mean dCT, IQR)
| Gene | SBNET Primary Tumors n=56 | PNET Primary Tumors n=47 | P-value SBNET vs. PNET |
|---|---|---|---|
| SSTR2 | 3.1 (2.0–3.8) | 2.1 (1.6–3.8) | 0.09 |
| BRS3 | 10.6 (10.0–12.2) | 6.7 (3.8–9.7) | <0.0001 |
| GIPR | 3.9 (2.4–5.3)* | 3.2 (1.9–4.1)* | 0.23 |
| GPR98 | 7.5 (5.9–8.6) | 6.6 (5.9–7.5) | 0.09 |
| GRM1 | 8.8 (6.5–11.6) | 7.6 (6.0–9.4) | 0.09 |
| OPRK1 | 3.6 (2.4–4.7)* | 9.3 (7.7–10.8) | <0.0001 |
| DRD1 | 6.9 (5.4–8.5) | 5.7 (3.8–7.6) | 0.056 |
| OXTR | 3.8 (1.7–5.0)* | 6.7 (4.1–8.5) | <0.0001 |
| GPR113 | 5.4 (4.5–6.3) | 5.9 (4.1–8.0) | 0.32 |
| SCTR | 9.4 (8.1–11.3) | 5.6 (6.2–7.6) | <0.0001 |
| ADORA1 | 8.8 (8.1–9.5) | 8.7 (7.0–10.8) | 0.89 |
| MUC13 | 1.1 (−0.4–2.3) | 1.2 (−0.5–2.7)* | 0.82 |
| MEP1B | 4.0 (1.5–5.5)* | 5.1 (2.4–7.7) | 0.21 |
Not significantly different from SSTR2 (p>0.01)
SBNET, small bowel neuroendocrine tumor; PNET, pancreatic neuroendocrine tumor
P-values are FDR-adjusted
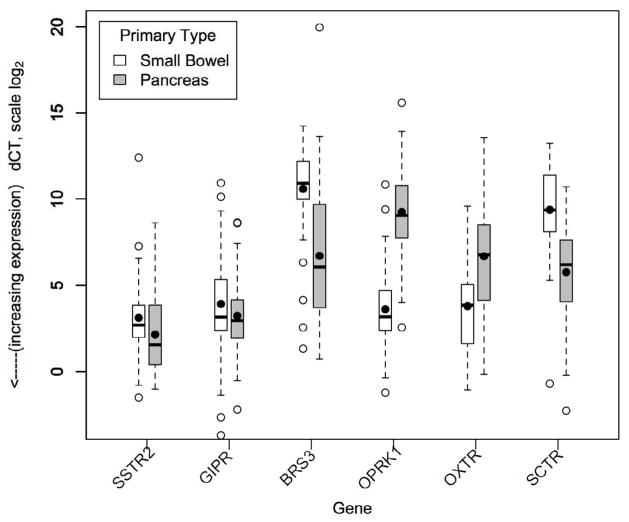
Figure 3.
Absolute gene expression (dCT) by primary tumor type. Lower dCT indicates higher expression (log scale). Boxes show IQR, whiskers show 1.5*IQR, open circles show outliers, bar shows median, dot shows mean. When expression is separated by primary tumor type, BRS3, OPRK1, OXTR, and SCTR show significantly different expression in SBNETs (n=56) and PNETs (n=47), while expression of SSTR2 and GIPR is similar in both primary tumor types. Expression of OXTR and OPRK1 in SBNETs (mean dCT 3.8 and 3.6) is much higher than in PNETs (mean dCT 6.7 and 9.3), and is not significantly different from expression of SSTR2 (mean dCT 3.1).
Clinical Correlation of SSTR2 Expression
To determine whether SSTR2 expression levels correlated with successful peptide-receptor-based imaging, clinical data from the University of Iowa Neuroendocrine Tumor Registry Database were examined and patients from the highest and lowest quartiles of SSTR2 expression by dCT were compared (Table 5). Eighteen patients from the highest SSTR2 expression quartile underwent somatostatin receptor scintigraphy (SRS), which successfully identified the primary tumor site in 14 cases (77.7%). Among patients in the lowest quartile of SSTR2 expression who had SRS, the scan identified the primary in 7 of 11 cases (63.6%, p=0.4). In this limited sample, there was no significant difference between the highest and lowest SSTR2 expression quartiles in the rate of successful primary tumor site determination by somatostatin receptor-based imaging.
Table 5.
Somatostatin receptor scintigraphy success by SSTR2 expression
| Primary Tumor Visualized | ||
|---|---|---|
| SSTR 2 Expression | Yes (%) | No (%) |
| High (dCT <0.9) | 14 (77.7) | 4 (22.3) |
| Low (dCT >3.9) | 7 (63.6) | 4 (36.4) |
Discussion
We have shown that BRS3, GIPR, GPR98, GPR113, GRM1, OPRK1, and OXTR have high expression in PNET and SBNET tumors relative to their normal tissue counterparts, and that for five of these genes, this increase is significantly greater than that of the current standard for imaging and treatment, SSTR2. While absolute expression levels of these genes in primary tumors are lower than SSTR2, GIPR demonstrates expression closest to the high expression of SSTR2. At the same time, GIPR enjoys 13-fold higher expression in primary tumors compared to normal tissue, while SSTR2 expression in tumor versus normal tissue is only 3-fold higher. Taken together, this combination of high absolute expression of GIPR and greater differential expression between tumor and normal tissue compared to SSTR2 supports that GIPR ligands hold great potential for imaging and therapy of SBNETs and PNETs.
We previously determined that OXTR showed promise as a therapeutic target based on high expression relative to normal tissue20. A limitation of the ddCT method used in that study was that the level of absolute OXTR expression was not assessed, and its high expression fold-change relative to surrounding tissue could be due to either high expression in tumor or low expression in normal tissue. In this study, we resolve that limitation by comparing gene expression in tumor tissues to internal controls and a standard of known clinical value, SSTR2. By this method, while OXTR expression was more than 5-fold less than SSTR2 when averaged across all tumors. However, if separated by primary tumor type, OXTR expression in SBNETs was close to that of SSTR2 suggesting that OXTR might be a useful therapeutic target in SBNETs, and confirming our earlier enthusiasm for the receptor.
We succeeded in identifying new peptide-receptor targets in this study. The gastric inhibitory polypeptide receptor (GIPR), also known as the glucose-dependent insulinotropic polypeptide receptor, is a G-protein-coupled receptor related to the glucagon receptor. It binds the incretin hormone gastric inhibitory polypeptide (GIP) in response to glucose ingestion21. GIPR research has focused on its role in obesity and type 2 diabetes22. Mice with GIPR deletions do not become obese, and GIPR polymorphisms influence type 2 diabetes risk in humans21, 22. Consistent with this role, GIPR is expressed in neuroendocrine tissues including pancreatic β-cells, as well as adipose tissue and brain23. GIPR is also expressed in colorectal cancer (CRC). Prabakaran et al. detected GIPR expression in colorectal cancer specimens and cell lines by immunohistochemistry and RT-PCR. After treating CRC cells with GIP, they noted increased proliferation and activation of the MAP kinase and mTOR pathways, which was abolished by treatment with pathway inhibitors rapamycin and PD9805922. While GIPR is not the only receptor to activate these pathways, it is notable that clinical trials of NET treatment with the mTOR and MAPK pathway inhibitors everolimus and sunitinib are ongoing6. Interest in GIPR and diabetes has led to development of both agonists and antagonists to the receptor23. While our evidence of GIPR overexpression in neuroendocrine tumors compared to normal tissue supports its utility as an imaging target, this evidence of pro-malignant signaling mediated by the receptor in the setting of high absolute expression suggests that adaptation of GIPR antagonists to neuroendocrine cancer treatment could be beneficial.
OPRK1, or the k-opioid receptor, is expressed in neural tissue. Its complex role in stress and reward pathways and impact on alcohol and opioid addiction has been studied extensively in behavioral science24. As with GIPR, its endogenous ligands, the dynorphins, are well described and agonist and antagonist molecules are available24. In addition to previous reports of OPRK1 expression in a breast adenocarcinoma cell line and small-cell lung cancer, it was recently reported that OPRK1 is overexpressed by gefitinib-sensitive and resistant non-small cell lung cancer cell lines as compared to normal lung fibroblasts25. In these cells, treatment with the agonist U50,488H caused markedly decreased growth and blocked phosphorylation of GSK-3β, particularly in gefitinib-resistant cells. Co-treatment with U50,488H and the OPRK1 antagonist nor-BNI restored cell growth. We found that neuroendocrine tumors also overexpress OPRK1 relative to normal tissue, and that expression is similar to SSTR2 in SBNETs. Whether drugs acting on this receptor will be useful for NET treatment also is deserving of further study.
Expression of DRD1 was not significantly higher in tumors than in normal tissue, despite our initial results indicating that it was overexpressed. The dopamine receptor, D2 (DRD2) has been previously proposed as a potential therapeutic target in GEPNETs, but expression in normal tissues was not measured14. We found that similar to DRD2, DRD1 is expressed in GEPNETs, but due to lower absolute expression and no difference in expression versus normal tissue, we doubt it will be as favorable a target for therapy. These results demonstrate the importance of measuring both absolute and relative expression, and of measuring expression in a large sample of tumors.
While SSTR2 expression is critical to the success of somatostatin-receptor scintigraphy, our finding that SRS is usually positive in NETs with both high and low SSTR2 expression agrees with the literature14. Interestingly, O’Toole et al. reported that while larger tumors were detected whether they had high or low SSTR2 expression, smaller tumors required higher levels of the receptor for visualization. Although we examined results in only a subset of tumors with the highest and lowest expression, a full comparison of positive and negative results by tumor size is possible in our population. As with previous reports, our sample size was small and we cannot account for biases in patterns of referral to our institution or SRS use. Whether these results obtained principally with 111In-pentetreotide will match findings using newer somatostatin analogues or 18F-PET DOTATOC is unknown.
Changes in gene expression in primary tumors were present in metastases as well, and tended to be more pronounced. We believe this is related to enrichment of malignant cells in metastases. We used a method of extracting RNA from whole tumors, and therefore the measured gene expression reflects a combination of both tumor and stromal cells. Microscopically, primary tumors tend to have a significant stromal component, which may be reduced in nodal and liver metastases. Finding that differences in gene expression are greater in these tumor-enriched tissues reassures us that observed changes are due to tumor rather than stromal gene expression, and suggests that therapeutics developed based upon gene expression levels in primary tumors should also be effective in their metastases. In future studies, we will test whether expression of these genes differs when the tumor cell population is further enriched using laser-capture microdissection.
One strength of this study is the large number of specimens used. Previous studies of gene expression or correlations of SSTR expression to clinical outcomes have generally reported results of specimens from 25–35 or fewer patients and only a few have reported expression in metastases12–14. While our pilot studies used a limited number of specimens19, the present study had 56 primary SBNETs and 47 PNETs, as well as 123 metastases from the same patients, allowing robust estimates of the true means and ranges of gene expression in these tumor populations.
A limitation of this study is that results in clinical practice may not match extrapolations from gene expression data. A study using similar methodology reported no correlation between qPCR-defined expression of SSTR2 or SSTR5 and disease stabilization or survival after treatment with somatostatin analogues, but unexpectedly found that high SSTR4 expression correlated with significantly worse outcomes12. We likewise speculated that OXTR might prove less useful than SSTR2 due to its lower absolute expression, but early immunohistochemistry results using an OXTR antibody have shown good staining for the receptor in neuroendocrine tumor samples (M.S.O., unpublished observation). So while results are not always predictable, gene expression data are likely to provide insight and generate useful hypotheses.
Perhaps the best evidence of gene expression data as a starting point for therapeutic development is the efficacy of octreotide in GEPNETs, which overexpress SSTR2. Not only have somatostatin analogues improved survival in patients with metastatic midgut carcinoids8, but they have also allowed significant response rates with PRRT11. Similar to these SSTR-based methods, radiolabeled ligands binding GIPR have successfully imaged adrenal glands with aberrant GIPR expression in the past26. These observations highlight the potential for clinical relevance and feasibility of drugs targeting highly expressed cell-surface receptors. We hope that ligands to the receptors we describe will prove similarly beneficial, but it is critical to first further evaluate these gene expression results in the context of protein expression, immunohistochemistry, and in cell culture, testing the effects of ligands on tumor growth.
In summary, molecules binding neuroendocrine tumor receptor proteins have proven effective in symptom management, imaging, and radionuclide therapy, but additional drug targets are needed. From its high expression in tumors and low expression in normal tissues, GIPR shows great promise for both PNETs and SBNETs, as do OPRK1 and OXTR in SBNETs. These findings suggest that further evaluation of potential ligands to these targets represent the next important step towards improving our therapeutic armamentarium against GEPNETs.
Acknowledgments
We extend our gratitude to the patients participating in this research. This work was supported by NIH 5T32 #CA148062-03 (S.K.S. and J.C.C.), and by a grant from the University of Iowa Institute for Clinical and Translational Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 3.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–36. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124–30. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 6.Ganetsky A, Bhatt V. Gastroenteropancreatic neuroendocrine tumors: update on therapeutics. Ann Pharmacother. 2012;46:851–62. doi: 10.1345/aph.1Q729. [DOI] [PubMed] [Google Scholar]
- 7.Long RG, Barnes AJ, Adrian TE, Mallinson CN, Brown MR, Vale W, et al. Suppression of pancreatic endocrine tumour secretion by long-acting somatostatin analogue. Lancet. 1979;2:764–7. doi: 10.1016/s0140-6736(79)92115-9. [DOI] [PubMed] [Google Scholar]
- 8.Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: Experience of two decades of cancer registry. Eur J Cancer. 2013;12:01031–3. doi: 10.1016/j.ejca.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 10.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Prasad V, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–68. doi: 10.1007/s00259-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 11.Zaknun JJ, Bodei L, Mueller-Brand J, Pavel ME, Baum RP, Horsch D, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013 doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaby O, Sachlova M, Bednarikova M, Fabian P, Svoboda M, Vytopilova S, et al. Gene expression of somatostatin receptor 4 predicts clinical outcome of patients with metastatic neuroendocrine tumors treated with somatostatin analogs. Cancer Biother Radiopharm. 2010;25:237–43. doi: 10.1089/cbr.2009.0708. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama Y, Wada R, Yajima N, Hakamada K, Yagihashi S. Profiling of somatostatin receptor subtype expression by quantitative PCR and correlation with clinicopathological features in pancreatic endocrine tumors. Pancreas. 2010;39:1147–54. doi: 10.1097/MPA.0b013e3181e78120. [DOI] [PubMed] [Google Scholar]
- 14.O’Toole D, Saveanu A, Couvelard A, Gunz G, Enjalbert A, Jaquet P, et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol. 2006;155:849–57. doi: 10.1530/eje.1.02307. [DOI] [PubMed] [Google Scholar]
- 15.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 16.Dahdaleh FS, Calva-Cerqueira D, Carr JC, Liao J, Mezhir JJ, O’Dorisio TM, et al. Comparison of clinicopathologic factors in 122 patients with resected pancreatic and ileal neuroendocrine tumors from a single institution. Ann Surg Oncol. 2012;19:966–72. doi: 10.1245/s10434-011-1997-4. [DOI] [PubMed] [Google Scholar]
- 17.Dahdaleh FS, Lorenzen A, Rajput M, Carr JC, Liao J, Menda Y, et al. The Value of Preoperative Imaging in Small Bowel Neuroendocrine Tumors. Ann Surg Oncol. 2013 doi: 10.1245/s10434-012-2836-y. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 19.Carr JC, Boese EA, Spanheimer PM, Dahdaleh FS, Martin M, Calva D, et al. Differentiation of small bowel and pancreatic neuroendocrine tumors by gene-expression profiling. Surgery. 2012;152:998–1007. doi: 10.1016/j.surg.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr JC, Sherman SK, Wang D, O’Dorisio MS, O’Dorisio TM, Howe JR. Overexpression of Membrane Proteins In Primary and Metastatic Gastrointstinal Neuroendocrine Tumors. Submitted to Annals of Surgical Oncology. 2013 doi: 10.1245/s10434-013-3318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–8. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabakaran D, Wang B, Feuerstein JD, Sinclair JA, Bijpuria P, Jepeal LI, et al. Glucose-dependent insulinotropic polypeptide stimulates the proliferation of colorectal cancer cells. Regul Pept. 2010;163:74–80. doi: 10.1016/j.regpep.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin N, Flatt PR. Therapeutic potential for GIP receptor agonists and antagonists. Best Pract Res Clin Endocrinol Metab. 2009;23:499–512. doi: 10.1016/j.beem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–96. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzumaki N, Suzuki A, Narita M, Hosoya T, Nagasawa A, Imai S, et al. Effect of kappa-opioid receptor agonist on the growth of non-small cell lung cancer (NSCLC) cells. Br J Cancer. 2012;106:1148–52. doi: 10.1038/bjc.2011.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix A, Bolte E, Tremblay J, Dupre J, Poitras P, Fournier H, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion--a new cause of Cushing’s syndrome. N Engl J Med. 1992;327:974–80. doi: 10.1056/NEJM199210013271402. [DOI] [PubMed] [Google Scholar]