Abstract
Delirium is a frequently under-recognized complication in patients with advanced cancer. Uncontrolled delirium eventually leads to significant distress to patients and their families. However, delirium episodes can be reversed in half of these patients by eliminating precipitating factors and using appropriate interventions. The purpose of this narrative review is to discuss the most recent updates in the literature on the management of delirium in patients with advanced cancer. This article addresses the epidemiology, cause, pathophysiology, clinical characteristics, and assessment of delirium as well as various treatment options, including nonpharmacologic intervention and palliative sedation.
Keywords: Delirium, Advanced Cancer
Introduction
Delirium is one of the most frequent and distressing complications seen in patients with advanced cancer. Family caregivers experience high levels of distress from caring for delirious patients with terminal illness. Moreover, studies have shown that many patients who experienced a delirium episode recalled it vividly and rated it as a moderately to severely stressful experience.1, 2
Delirium impairs the communication ability of patients with advanced cancer and subsequently interferes with the appropriate assessment of physical symptoms such as pain. Delirium increases the duration of hospitalization and the risk for hospital-acquired complications. Delirium is also a negative prognostic indicator for survival in advanced cancer patients.3, 4 Thus, the proper management of delirium can be a challenging issue for physicians. The purpose of this review is to present a clinical update of the symptoms of delirium in advanced cancer patients and to discuss comprehensive approaches to managing delirium, including pharmacologic and nonpharmacologic interventions and palliative sedation.
Definition and Incidences
Delirium is not a disease but a clinical syndrome arising from multiple causes with similar symptoms. Delirium is a global brain dysfunction characterized by acute disruption of attention and cognition but without permanent organic changes in the brain; therefore, delirium may be reversible even in patients with terminal cancer.5
Delirium can be defined as a mental state in which a person is confused, disoriented, and unable to think or remember clearly.6 However, the characterization of delirium has evolved from a list of multiple symptoms to two imperative components, disordered attention and cognition.7 The American Psychiatric Association issued diagnostic criteria for delirium in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III, and those criteria have evolved into DSM-IV over the years. 8–10 The DSM-IV defines delirium as a disturbance of consciousness with inattention and problems in cognition and/or a disturbance in perception that develop over hours to days with organic causes.10, 11 Confusion is often used as a general term for incoherent thinking, and it is an essential component of delirium in terms of disordered cognition. Confused patients have problems thinking with normal speed, clarity, or coherence. Clinical diagnosis of delirium usually has been reserved for patients with obvious manifestation of disorientation and confusion. This clinical practice can cause mild delirium to be overlooked. However, there is no direct evidence that universal screening in asymptomatic individuals can directly improve patient outcomes.12
The definitions of prevalence and incidence for delirium and should be differentiated. Prevalence defines the number of cases of delirium that are present in a population at a specified time, whereas incidence represents the occurrence rate of delirium during a certain period in a population at risk. Many studies of delirium describe its prevalence at baseline and the incidence of new cases during the study period such as during hospitalization.
The incidence of delirium in patients with advanced cancer ranges from 6% to 68% depending on the health care setting, diagnostic tool, and disease status of the population (Table 1).13–19 The frequency increases up to 90% in cancer patients near the end of life.20 The frequency of delirium in outpatient clinics is little known. One small study reported that the incidence and prevalence of delirium were 45% and 7%, respectively, during outpatient treatment for head and neck cancer.21 However, delirium is often misunderstood or misdiagnosed as anxiety disorder or depression.22 Thus, the incidence or prevalence may be underreported.
Table 1.
Incidence of delirium in advanced cancer patients
| Incidence (No.) | Sample size (No.) | Study design | Diagnostic tool | Admitted health setting | |
|---|---|---|---|---|---|
| Chun-Kai F et al.13 | 46.9% (107) | 228 | prospective | DRS | Hospice and palliative care |
| Kim SY et al.14 | 30.2% (33) | 108 | prospective | CAM | Palliative care |
| Gaudreau JD et al.15 | 30.0% (31) | 107 | prospective | Nu-DESC | Oncology unit |
| Gagnon P et al.16 | 6.2% (N/A) | 2515 | prospective | CRS | Palliative care |
| Weckmann M17 | 38.6% (17) | 44 | retrospective | N/A | Academic medical center |
| Lawlor P et al.18 | 68.3% (71) | 104 | prospective | MDAS | Palliative care |
| Gagnon P et al.19 | 32.8% (21) | 64 | prospective | CAM | Hospice |
CAM, Confusion Assessment Method; DRS, Delirium Rating Scale; MDAS, Memorial Delirium Assessment Scale; Nu-DESC, Nursing Delirium Screening Scale; N/A, Not Available; CRS, Confusion Rating Scale
Precipitating Factors and Pathophysiology
Delirium is caused by diverse factors that present as global cerebral dysfunction. To prevent delirium, a balance is normally maintained between the inhibitory system and predisposing and precipitating factors for delirium. When these predisposing and precipitating factors disturb that balance, delirium can occur. Patients with multiple predisposing conditions are more vulnerable to the precipitating factors than are patients with only one predisposing factor.23 For example, elderly patients have a high prevalence of dementia and commonly have other coexisting conditions such as cancer. Patients who have had multiple chemotherapy regimens or cancer progression could also be more likely to experience delirium from minor factors such as hypnotics.24
Cancer is particularly common in the elderly population, and cancer and old age are major predisposing conditions for delirium. Thus, many elderly patients with cancer, especially those with advanced-stage disease, are highly vulnerable to and can easily develop delirium from minor precipitating factors. Precipitating factors for delirium include constipation, dehydration, hypoxia, immobility, infection, uncontrolled pain, bladder catheterization or outlet obstruction, and several medications—especially benzodiazepine or meperidine. The utilization of the acronym CHIMBOP (constipation, hypovolemia/hypoglycemia, infection, medications, bladder catheter /bladder outlet obstruction, oxygen deficiency, pain) can help medical staff to remember multiple precipitating factors.25
The mechanism that causes delirium is poorly understood, and only hypothetical models exist. The two prevailing theories for delirium pathophysiology are neurotransmitter imbalance characterized by acetylcholine (Ach) deficiency and unbalanced inflammatory response.26–28
Neurotransmitters involving development of delirium include Ach, dopamine, serotonin, and γ-aminobutyric acid (GABA).29 Of these, Ach—which is involved in normal attention, memory, and arousal—is believed to be a key neurotransmitter in the final common pathway in the development of delirium. Dopamine and serotonin are another important neurotransmitters for attention and cognition. Activation of dopamine subreceptors (D2–D4) induces decreased Ach secretion, whereas serotonin is associated with secretion of Ach by interacting with the cholinergic and dopaminergic system.30
Any medical conditions that decrease cholinergic activity or increase dopamine production may result in delirium.26 For example, hypoxemia, infection, dehydration, and electrolyte imbalance commonly occur in advanced cancer patients and can be precipitating factors for delirium. In addition, polypharmacy and psychoactive medications such as opioids, benzodiazepines, and serotonin antagonists are widely used for supportive care and can influence the production of causative neurotransmitters in advanced cancer patients.31 Studies have shown that opioids, corticosteroids, or benzodiazepines are associated with increased risk of delirium in hospitalized cancer patients.15, 32 According to the unbalanced inflammatory response model, cytokines from acute systemic inflammation also contribute to delirium. Among these are proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, which are produced in the peripheral blood during infection.33 These peripheral cytokines can be transmitted to the brain and induce the activation of microglia, which subsequently create a neurotoxic response and eventually lead to delirium. The microglial response is normally under strict control of cholinergic inhibitory system.34 van Gool et al. suggested that impaired cholinergic inhibitory control of microglia in vulnerable patients contributes to uncontrolled neuroinflammation and ultimately causes delirium.35
Recent studies suggested that cortisol may also have a role in the development of delirium.36 Stress triggers the hypothalamic-pituitary-adrenal (HPA) axis and elicits production of cortisol. Repetitive and chronic stresses such as cancer could cause disruption of HPA axis homeostasis. It is hypothesized that cortisol, when secreted excessively and circulated in the blood at high levels, has harmful effects on hippocampal activity and results in cognitive dysfunction.
Clinical Characteristics
Disturbance of attention with abnormal consciousness and cognition are two key features needed to establish a diagnosis of delirium by DSM-IV criteria.11 The pace of symptom occurrence is important for differentiating from dementia. Delirium has an acute and rapid onset, whereas dementia develops more gradually.37 The severities of delirium’s symptoms are not constant but rather wax and wane over a 24-hour period with characteristic lucid intervals. For example, patients tend to be disoriented in the evening and become lucid the following morning. Delirium may present as complaints of fatigue, sleep disturbance, disinterest, and hypersensitivity to environment. Thus, physicians or nurses should be vigilant for early behavior changes indicating delirium and perform a clinical assessment to confirm the delirium. The clinical features of delirium are shown in Table 2.
Table 2.
| Frequency (%) | |
|---|---|
| Temporal factor | |
| Developed abruptly over hours or days | |
| Fluctuation of severity with characteristic lucid intervals | |
| Essential symptoms* | |
| Disturbance of consciousness (from hyperalertness to coma) | 65–100 |
| Impaired attention | 97–100 |
| Change in cognition | |
| Disorientation in time or place | 76–96 |
| Difficulty in recalling recent memory | 88–96 |
| Language problems in reading, writing, or speaking | 57–67 |
| Disorganized thinking such as incoherent speech | 54–79 |
| Perceptual abnormalities such as visual or auditory hallucinations | 46–63 |
| Delusion | 21–31 |
| Other symptoms | |
| Altered sleep-wake cycle | 75–97 |
| Psychomotor symptoms including agitation or lethargy | 62–75 |
Symptoms were classified by DSM-IV criteria
Disturbance of attention and consciousness
Disturbance of consciousness means that patients have a problem in their wakefulness or arousal. Such patients can be hypoalert or hyperalert, with mental statuses ranging from coma to hyperalertness marked by excess sensitivity to environmental stimuli and being easily startled. Disordered attention can be distinguished from disturbance of consciousness.38 Attention is defined as the ability to concentrate and shift from one subject to another, and patients with delirium have difficulty in focusing, sustaining, and shifting their attention. Patients with delirium easily forget a question being asked or repeatedly return to the previous question, which makes it difficult to have meaningful conversation.
Abnormalities in consciousness and attention are often subtle in the early stage of delirium, and their prodromal signs are sometimes overlooked or attributed to fatigue, annoying character, or old age.22 In one prospective study, all cancer patients with delirium had some intensity of inattention, and three-quarters had more than moderate intensity.39 Another study reported a similar frequency of inattention in patients with nonmalignant tumors who had delirium.38
Change in cognition or perception
The term of cognition is broadly used to describe the mental process of knowing, including memory, language, concept formation, perception, attention, and consciousness.40 In the diagnosis of delirium, cognition refers to problems in memory recall, orientation, concept formation, language, and perception (excluding problems in attention and consciousness).41 Cognitive impairment usually involves global or multiple deficits.24
Memory impairment usually is related to short-term memory. Family caregivers often describe patients’ forgetfulness as seeming like dementia. Disorientation, especially difficulty recognizing the current time, is a common prodromal sign of delirium. Patients subsequently manifest disorientation as to place, misidentifying location or recognizing it with laborious effort. Disorientation and memory impairment develop in 76% to 96% of delirious patients.42, 43 Many of these patients try to hide their inappropriate remarks about the time or place with humor or attribute them to mistakes.44 These patients’ responses to questions tend to be slow, and their speech often becomes sluggish. Patients might have marked difficulty properly phrasing answers to complex questions such as, “Describe your current medical condition.” Problems in concept formation can be detected during the interview by rambling, irrelevant, or incoherent answers.
Patients with delirium often hesitate during conversation while searching for proper words, have difficulty grasping the meaning of books, or commit spelling errors.
Patients also have perceptual disturbances such as visual or auditory hallucinations. However, these perception problems are more likely to be associated with incorrect cerebral processing of sensory data than pure hallucinations.45 Patients mistake simple shadows for real people or environmental sounds for voices with clear speech. These psychotic symptoms, including hallucinations or delusions, occur less frequently than cognitive symptoms in patients with delirium.31,32,41
Other symptoms
Although sleep-wake cycle disturbance is a cardinal manifestation of delirium, it is not evident whether sleep deprivation provokes delirium or delirium causes sleep deprivation.46 Patients may complain of difficulty in falling asleep or fragmented sleep in the night, or they may complain of frequent napping during the day or sleepiness. Other symptoms of delirium include psychomotor manifestations, such as agitation or lethargy, and emotional instability. Patients with delirium commonly show intermittent and labile symptoms of fear, anger, euphoria, or anxiety.24
Classification
Delirium can be categorized clinically into three subtypes—hyperactive, mixed, or hypoactive—according to the patients’ psychometric features.47 Hyperactive delirium is characterized by increased psychomotor activity such as loss of activity control, mood lability restlessness, and wandering. Hypoactive delirium, also called “quiet delirium,” is characterized by decreased or slow speech, reduced awareness of surroundings, and reduced activity. The mixed subtype is defined as having alternating clinical features between the hyperactive and hypoactive subtypes. Although this classification system is widely adopted in clinical use, no clear consensus for dividing subtype has been reached. Meagher et al. suggested simple checklists designed for use by nonpsychiatric specialists.48
Patients with hyperactive delirium are likely to attract medical staff’s or caregivers’ attention with their disruptive behavior, while the symptoms of hypoactive delirium are easy to miss without active monitoring.49 However, hypoactive or mixed delirium is more common in the general patient population.50, 51 In studies of cancer patients, the prevalence ranged from 20% to 86% for hypoactive delirium, 16% to 67% for mixed delirium, and 6% to 31% for hyperactive delirium.1, 52–54 These motor subtypes tend to be unchanged throughout an episode of delirium.14, 55 Patients with the hypoactive subtype have a poorer prognosis than those with the hyperactive subtype.56–58
Assessment and Diagnosis
All patients hospitalized for cancer treatment should be assessed for delirium. Once delirium is identified, physicians or nurses should identify and treat reversible precipitating factors. As the detection of delirium is based on clinical findings, careful bedside observation is of the utmost importance to establishing a diagnosis. However, because delirium develops over a short period of time and fluctuates during the course of the day, physicians and nurses often miss key symptoms.24, 59 Rather, family caregivers can notice the subtle changes in the patient, especially alterations of behavior, indicating hypoactive delirium. Hypoactive delirium in cancer patients often is misdiagnosed as other sudden causes of confusion such as depression, anxiety disorder, Wernicke aphasia, or dementia.22, 60 Agitation from delirium in dying cancer patients can be mistaken for pain, resulting in an increased dose of opioids, which exacerbates delirium. Conversely, uncontrolled pain, urinary retention, and constipation might be causes of agitated behavior in patients with dementia rather than cases of reversible delirium. Once the underlying discomfort is relieved, these patients will reverse back to dementia rather than to a normal cognitive status. Questions to family caregivers are needed to evaluate patients’ baseline cognition and recent changes in mental status. A single simple question like, “Do you think the patient has been more confused lately?” would be helpful to avoid missing a diagnosis.61
The Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MOCA) are the most common validated screening tools for cognitive dysfunction and are in available in various languages. These screening tools could be helpful for detecting early cognitive changes in cancer patients with delirium. The MOCA has advantages over the MMSE in briefness sensitivity, and is free of charge.62
Diagnosis of delirium can be made on the basis of DSM-IV or International Classification of Diseases–10 criteria. The DSM-IV criteria for delirium seem to be more inclusive, and thus are more widely used, than the International Classification of Diseases–10 criteria.63–65 To determine whether patients meet these criteria, patients can be evaluated with direct interviews or with established assessment tools. Table 3 describes the commonly used scales for delirium. Although none of the numerous assessment tools has been established as the standard scale for assessing delirium in advanced cancer patients, the Memorial Delirium Assessment Scale (MDAS) has some advantages: scores can be prorated in advanced cancer patients who commonly have problems answering all the questionnaires for reasons such as dyspnea or fatigue,66 and the tool’s use in cancer patients has been validated.66, 67
Table 3.
| Tool | Based criteria | Content | Evaluation time | Cutoff score | Sensitivity (%) | Specificity (%) | Rating Severity | Validation in cancer |
|---|---|---|---|---|---|---|---|---|
| CAM | DSM-III-R | 9 items | < 5 min | N/A | 88–100 | 90–100 | No | Yes |
| MDAS | DSM-IV | 10 items | 10 min | 7–13 | 68–97 | 93–95 | Yes | Yes |
| Nu-DESC | 5 items | < 2 min | 2 | 86–96 | 79–87 | Yes | No | |
| DRS | DSM-III | 10 items | 10 min | 10–12 | 80–98 | 61–98 | Yes | Yes |
| DRS R-98 | 16 items | > 10 min | 17.8–22.5 | 91–100 | 85–100 | Yes | No |
CAM, Confusion Assessment Method; DRS, Delirium Rating Scale; DRS R-98, Delirium Rating Scale Revised; MDAS, Memorial Delirium Assessment Scale; Nu-DESC, Nursing Delirium Screening Scale; N/A, not available
Management
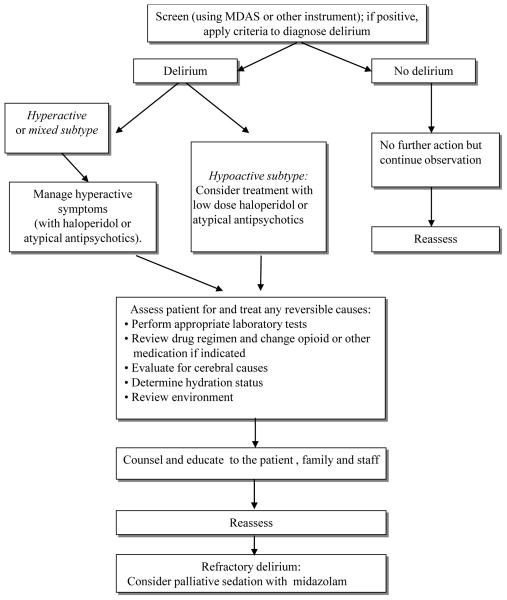
The assessment of delirium is crucial to its management because the assessment confirms the diagnosis and determines the severity of delirium (Figure 1). Various guidelines recommend that the precipitating factors be identified immediately and corrected if possible,68–70 since delirium is reversible in about 50% of patients with advanced cancer.18
Figure 1.
Treatment of delirium includes the elimination of its underlying causes with concurrent management of its symptoms. Clinicians should maximize nonpharmacologic interventions before initiating pharmacologic management. Patients’ safety must be ensured because behavioral changes from delirium often endanger the patients or clinic staff. Physicians also should support caregivers by educating them about the etiology and clinical course of delirium and by urging them to consider involving family or close friends to minimize their distress.
Nonpharmacologic management
Nonpharmacologic interventions are recommended for all the patients with delirium.70 For example, precipitating factors for delirium—including dehydration, infection, pain, hypoxia, medications such as opioids and sleeping aids—should be evaluated and corrected, if possible. Although little evidence exists that these interventions would be helpful for prolonging survival, studies have shown that nonpharmacologic interventions may help expedite the improvement of delirium and slow the deterioration of cognitive function.71, 72
It is important to create a relaxing environment for the patients. Inadequate lighting, which may cause illusions or hallucinations, should be avoided; a well-lit room is preferable. Other environmental strategies to help manage delirium include reducing noise and using a clock or calendar to provide appropriate orientation. In addition, the removal of annoying stimuli such as urinary catheters and intravenous pumps should be considered. Disruption of patients’ normal sleep/wake cycle, such as awaking patients at night for medication or vital sign monitoring, should be minimized.
Pharmacologic intervention
To our knowledge, no randomized controlled trials for delirium treatment have been conducted in the advanced cancer patient population. Thus, delirium treatment for patients with advanced cancer is usually derived from studies of delirium treatment for patients with different conditions. Table 4 presents a summary of randomized controlled trials for delirium. No antipsychotic drugs have been approved by the U.S. Food and Drug Administration (FDA) for the management of delirium, despite the reality that antipsychotics are routinely used in the management of symptoms related to delirium. The lack of approved drugs and large studies warrants a need for clinical trials in cancer patients. Clinicians often select antipsychotics on the basis of their sedative effects rather than their efficacy for treating cognitive disturbances.54 Some studies have suggested that patients with hypoactive delirium may require different treatment than those with the mixed or hyperactive subtypes because patients with hypoactive delirium are more vulnerable to side effects from sedation and have a poorer prognosis.73 In fact, clinicians tend to avoid the use of antipsychotics in patients with the hypoactive subtype.54 However, the antidelirium effect of antipsychotics does not appear to correlate with the motor subtypes of delirium. Studies have suggested that haloperidol or atypical antipsychotics were equally effective in cancer patients with hypoactive and other subtypes of delirium.73–75
Table 4.
Randomized trials about pharmacologic treatment for delirium
| Author | Population | Size | Intervention | Results |
|---|---|---|---|---|
| Edward et al. 94 | Delirious patients after hip fracture | 16 | Donepezil vs placebo | No differences regarding presence (odd ratio = 0.9) and severity (effect size = −0.2 on 30-point MDAS) over time. |
| Tagarakis et al. 95 | Delirious patients after cardiac surgery | 80 | Ondasetron vs haloperidol | Effective with no differences between ondasetron (61%) and haloperidol (58%) |
| Tahir et al.96 | General patients with diagnosed delirium | 42 | Quetiapine vs placebo | Quetiapine improved 83% (p = .026) faster than placebo by DRS-R-98 severity |
| van Eijk et al. 97 | Delirious patients diagnosed in intensive care unit | 104 | Haloperidol rivastigmine | Rivastigmine increased mortality (22% vs 5%, p = 0.07) with no decrease duration of delirium |
| Devlin et al.98 | Delirious patients diagnosed in | 36 | Quetiapine vs placebo | Quetiapine added to as-needed haloperidol results in faster resolution (1 vs 4.5 days, p = 0.001) |
| Kim et al.84 | Delirious patients with mostly malignancy | 32 | Risperidone vs olanzapine | Equally effective (65% vs 73%) with no differences(p = 0.71) |
| Overshott et al. 99 | Various patients with diagnosed delirium | 15 | Rivastigmine vs placebo | No difference in duration of delirium between two group(6.3 vs 9.9 days, p = 0.5) |
| Hu et al.100 | Various patients with diagnosed delirium | 175 | Haloperidol vs olanzapine vs placebo | Effective rates of olanzapine, haloperidol and placebo were 82%, 88%, and 31% (p < 0.01 vs placebo), respectively |
| Breitbart et al. 101 | Hospitalized AIDS patients with delirium | 30 | Haloperidol vs chlorpromazine vs lorazepam | DRS scores improved significantly in haloperidol and chlorpromazine (p < 0.001) but not in lorazepam |
| Grover et al.102 | Various patients with delirium | 64 | Haloperidol vs olanzapine vs risperidone | Significant (p < 0.001) reduction in severity scores but no differences between agents |
| Han et al.103 | Various patients with delirium | 28 | Haloperidol vs risperidone | No significant difference in the mean MDAS scores between the two groups (p = 0.51) |
DRS, Delirium Rating Scale; DRS R-98, Delirium Rating Scale Revised; MDAS, Memorial Delirium Assessment Scale
A small prospective study reported that psychostimulants such as methylphenidate may be beneficial in advanced cancer patients with hypoactive delirium.76, 77 However, methylphenidate should be used with caution because high doses can result in agitation, and the drug’s potential role in treating hypoactive delirium has not been studied formally.
All antipsychotics affect delirium by acting as antagonists to the neurotransmitter (ie, dopaminergic, serotonergic, muscarinic, histaminic, adrenergic) receptors. The action mechanisms of these medications vary according to their binding affinities to various receptors.
Haloperidol is the most widely used antipsychotic for delirium treatment in advanced cancer patients and is recommended as the drug of choice in the various guidelines.69, 70 Haloperidol is a strong antagonist acting at the dopamine D2 receptor, which may explain its efficacy against delirium. The advantages of haloperidol are diverse routes of administration, wide therapeutic safety margin, and minimal anticholinergic effects; risks at low doses include mild cardiopulmonary adverse events.53 Intravenous administration is preferred to intramuscular injection, despite the lack of FDA approval for the intravenous administration of haloperidol. Subcutaneous administration is another well-established route for haloperidol in palliative care and could be considered as an useful alternative. 78 The optimal dose range of haloperidol for patients with delirium has not been investigated. Guidelines recommend initial doses in the range of 0.5 mg to 10 mg every 2 to 4 hours as needed, with titration to higher doses for patients who continue to be agitated.69, 70 The motor subtype of delirium is a major determinant of the dose for haloperidol, creating a possibility of underdosing in the treatment of hypoactive subtype.79 A meta-analysis of delirium treatment studies revealed that the median doses of haloperidol per day ranged from 2.5 mg to 3.2 mg, which was significantly lower than the guidelines’ recommended doses; however, controversy remains over the optimal dose of haloperidol because lower doses of haloperidol have been reported to be effective.80 In addition, adverse events and extrapyramidal symptoms can occur more frequently at doses greater than 4.5 mg per day.81 The FDA issued a warning in 2007 about the risk of QT prolongation or torsades de pointes in patients treated with intravenous haloperidol.82 However, cardiac arrhythmias usually are associated with cumulative doses of haloperidol higher than 5 mg and in the setting of risk factors such as underlying cardiac disease or concomitant proarrhythmic agents.83 Thus, the FDA warning should not inhibit clinicians from prescribing intravenous haloperidol to delirious patients without these risk factors.
Atypical antipsychotics, including olanzapine, risperidone, and aripiprazole, have been found to be effective.75, 84, 85 Atypical antipsychotics are less likely than haloperidol to cause extrapyramidal side effects. The difference in side effects is the result of differences in the drugs’ binding affinities. None of the atypical antipsychotics have proven superior to haloperidol or the other agents in same class. For atypical antipsychotics except olanzapine, the lack of a parenteral form limits their usefulness as a treatment for agitated, delirious patients.
Benzodiazepines are generally ineffective in the treatment of delirium associated with advanced cancer despite their wide use in cancer patients. Lorazepam is associated with the adverse effects of oversedation, ataxia, and increased confusion.
Palliative sedation for refractory delirium
Palliative sedation could be indicated for patients who have refractory delirium and expected survival of hours to days. Fainsinger et al. defined palliative sedation as “patients deliberately sedated by increasing doses to control delirium or observed to be reduced to a clearly unresponsive condition by pharmacological management”,86 and delirium is the most common cause of palliative sedation. A recent study found that palliative sedation did not appear to have a detrimental effect on survival when appropriately used to relieve uncontrolled symptoms.87 Thus, it is not unethical to provide palliative sedation for dying patients with uncontrolled agitation from delirium. Clinicians should clearly explain patients’ disease status and prognosis along with the goals and expected outcomes of palliative sedation to patients’ families or surrogates.
Midazolam is the most prescribed medication for palliative sedation, followed by haloperidol, chlorpromazine, other benzodiazepines, morphine, methotrimeprazine, propofol, and phenobarbital. The National Comprehensive Cancer Network guidelines recommend giving midazolam infusion at an initial rate of 0.4 mg to 0.8 mg per hour.69
Summary.
Delirium is a frequent and underdiagnosed complication in patients with advanced cancer. Regular screening for delirium with a validated assessment tool is recommended. Delirium in cancer patients can be improved in about 50% of cases by eliminating precipitating factors and using appropriate interventions. Palliative sedation can be considered for refractory delirium in dying cancer patients after consultation with a palliative care specialist or psychiatrist.
Acknowledgments
We appreciate Bryan Tutt in the Department of Scientific Publications at MD Anderson Cancer Center for valuable editorial assistance. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672. The sponsors have no involvement in the article design, collection of literature, interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
All authors declare no potential conflicts of interest.
Authorship Contribution
Conception and design of the study: Jung Hun Kang, Seong Hoon Shin, Eduardo Bruera
Drafting the article: Jung Hun Kang, Seong Hoon Shin, Eduardo Bruera
Revising the manuscript critically for important intellectual content: Jung Hun Kang, Eduardo Bruera
Final approval of the manuscript: Jung Hun Kang, Seong Hoon Shin, Eduardo Bruera
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruera E, Bush SH, Willey J, Paraskevopoulos T, Li Z, Palmer JL, et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer. 2009;115:2004–12. doi: 10.1002/cncr.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium- related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics. 2002;43:183–94. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 3.Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–8. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 4.Scarpi E, Maltoni M, Miceli R, Mariani L, Caraceni A, Amadori D, et al. Survival prediction for terminally ill cancer patients: revision of the palliative prognostic score with incorporation of delirium. Oncologist. 2011;16:1793–9. doi: 10.1634/theoncologist.2011-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer. 1997;79:835–42. [PubMed] [Google Scholar]
- 6.Institute NC. Dictionary of Cancer Terms. [Google Scholar]
- 7.Del Fabbro E, Dalal S, Bruera E. Symptom control in palliative care--Part III: dyspnea and delirium. J Palliat Med. 2006;9:422–36. doi: 10.1089/jpm.2006.9.422. [DOI] [PubMed] [Google Scholar]
- 8.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 3. Washinton, DC: American Psychiatic Association; 1980. [Google Scholar]
- 9.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Revised. 3. Washinton, DC: American Psychiatic Association; 1987. [Google Scholar]
- 10.Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- 11.Tucker G. The diagnosis of delirium and DSM-IV. Dementia and geriatric cognitive disorders. 1999;10:359–63. doi: 10.1159/000017171. [DOI] [PubMed] [Google Scholar]
- 12.Greer N, Rossom R, Anderson P, MacDonald R, Tacklind J, Rutks I, et al. Delirium: Screening, Prevention, and Diagnosis – A Systematic Review of the Evidence [Internet] 2011 [PubMed] [Google Scholar]
- 13.Fang CK, Chen HW, Liu SI, Lin CJ, Tsai LY, Lai YL. Prevalence, detection and treatment of delirium in terminal cancer inpatients: a prospective survey. Jpn J Clin Oncol. 2008;38:56–63. doi: 10.1093/jjco/hym155. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreau J-DGP, Roy M-A, Radtke FM. Delirium subtypes in hospitalized cancer patients: Prevalence and evolution. IPOS 12th World Congress of Psycho-Oncology; Quebec City, QC Canada. 2010. p. s276. [Google Scholar]
- 15.Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23:6712–8. doi: 10.1200/JCO.2005.05.140. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon PAP, Gagnon B, Merette C, Jomphe V, Mond C, Tardif F. Delirium phenomenology in terminal cancer. 11th World Congress of Psycho-Oncology of the International Psycho-Oncology Society(IPOS); Vienna Austria. 2009. p. 18.p. S300. [Google Scholar]
- 17.Weckmann M. Delirium Incidence and Cause in Younger Hospitalized Patients With Advanced Cancer. Journal of Pain and Symptom Management. 2012;43:470. [Google Scholar]
- 18.Lawlor PG, Gagnon B, Mancini IL, Pereira JL, Hanson J, Suarez-Almazor ME, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: a prospective study. Arch Intern Med. 2000;160:786–94. doi: 10.1001/archinte.160.6.786. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon P, Allard P, Masse B, DeSerres M. Delirium in terminal cancer: a prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage. 2000;19:412–26. doi: 10.1016/s0885-3924(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 20.Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135:32–40. doi: 10.7326/0003-4819-135-1-200107030-00011. [DOI] [PubMed] [Google Scholar]
- 21.Bond SM, Dietrich MS, Shuster JL, Jr, Murphy BA. Delirium in patients with head and neck cancer in the outpatient treatment setting. Support Care Cancer. 2011 doi: 10.1007/s00520-011-1174-0. [DOI] [PubMed] [Google Scholar]
- 22.Wada T, Wada M, Onishi H. Characteristics, interventions, and outcomes of misdiagnosed delirium in cancer patients. Palliat Support Care. 2010;8:125–31. doi: 10.1017/S1478951509990861. [DOI] [PubMed] [Google Scholar]
- 23.Laurila JV, Laakkonen ML, Tilvis RS, Pitkala KH. Predisposing and precipitating factors for delirium in a frail geriatric population. J Psychosom Res. 2008;65:249–54. doi: 10.1016/j.jpsychores.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 25.White J, Hammond L. Delirium assessment tool for end of life: CHIMBOP. J Palliat Med. 2008;11:1069. doi: 10.1089/jpm.2008.9851. [DOI] [PubMed] [Google Scholar]
- 26.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–72. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–54. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 28.Cerejeira J, Nogueira V, Luis P, Vaz-Serra A, Mukaetova-Ladinska EB. The Cholinergic System and Inflammation: Common Pathways in Delirium Pathophysiology. J Am Geriatr Soc. 2012 doi: 10.1111/j.1532-5415.2011.03883.x. [DOI] [PubMed] [Google Scholar]
- 29.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5:132–48. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 30.Steiner LA. Postoperative delirium. Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–36. doi: 10.1097/EJA.0b013e328349b7f5. [DOI] [PubMed] [Google Scholar]
- 31.Agar M, Lawlor P. Delirium in cancer patients: a focus on treatment-induced psychopathology. Curr Opin Oncol. 2008;20:360–6. doi: 10.1097/CCO.0b013e328302167d. [DOI] [PubMed] [Google Scholar]
- 32.Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109:2365–73. doi: 10.1002/cncr.22665. [DOI] [PubMed] [Google Scholar]
- 33.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–73. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–37. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 35.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 36.Kazmierski J, Kloszewska I. Is cortisol the key to the pathogenesis of delirium after coronary artery bypass graft surgery? Crit Care. 2011;15:102. doi: 10.1186/cc9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304:779–86. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 38.Bhat R, Rockwood K. Delirium as a disorder of consciousness. J Neurol Neurosurg Psychiatry. 2007;78:1167–70. doi: 10.1136/jnnp.2007.115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosisio M, Caraceni A, Grassi L. Phenomenology of delirium in cancer patients, as described by the Memorial Delirium Assessment Scale (MDAS) and the Delirium Rating Scale (DRS) Psychosomatics. 2006;47:471–8. doi: 10.1176/appi.psy.47.6.471. [DOI] [PubMed] [Google Scholar]
- 40.Freedheim DK. Handbook of psychology: History of psychology. Donald K. Freedheim: Wiley; 2003. [Google Scholar]
- 41.Caraceni A, Simonetti F. Palliating delirium in patients with cancer. Lancet Oncol. 2009;10:164–72. doi: 10.1016/S1470-2045(09)70018-X. [DOI] [PubMed] [Google Scholar]
- 42.Gupta N, de Jonghe J, Schieveld J, Leonard M, Meagher D. Delirium phenomenology: what can we learn from the symptoms of delirium? J Psychosom Res. 2008;65:215–22. doi: 10.1016/j.jpsychores.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Meagher DJ, Moran M, Raju B, Gibbons D, Donnelly S, Saunders J, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190:135–41. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 44.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16:526–38. doi: 10.1176/jnp.16.4.526. [DOI] [PubMed] [Google Scholar]
- 45.Kamholz B. Update on Delirium: Diagnosis, Management, and Pathophysiology. Psychiatric Annals. 2010;40:52. [Google Scholar]
- 46.Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–12. [PubMed] [Google Scholar]
- 47.Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843–5. doi: 10.1192/bjp.161.6.843. [DOI] [PubMed] [Google Scholar]
- 48.Meagher D, Moran M, Raju B, Leonard M, Donnelly S, Saunders J, et al. A new databased motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci. 2008;20:185–93. doi: 10.1176/jnp.2008.20.2.185. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan NM, Palmer BF, Roche V. Etiology and management of delirium. The American journal of the medical sciences. 2003;325:20. doi: 10.1097/00000441-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–84. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 51.Stagno D, Gibson C, Breitbart W. The delirium subtypes: a review of prevalence, phenomenology, pathophysiology, and treatment response. Palliat Support Care. 2004;2:171–9. doi: 10.1017/s1478951504040234. [DOI] [PubMed] [Google Scholar]
- 52.Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20:17–23. doi: 10.1191/0269216306pm1097oa. [DOI] [PubMed] [Google Scholar]
- 53.Moyer DD. Review article: terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Care. 2011;28:44–51. doi: 10.1177/1049909110376755. [DOI] [PubMed] [Google Scholar]
- 54.Meagher D. Motor subtypes of delirium: past, present and future. Int Rev Psychiatry. 2009;21:59–73. doi: 10.1080/09540260802675460. [DOI] [PubMed] [Google Scholar]
- 55.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: Frequency and stability during episodes. J Psychosom Res. 2012;72:236–41. doi: 10.1016/j.jpsychores.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62:174–9. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 57.Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50:248–54. doi: 10.1176/appi.psy.50.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res. 2011;71:395–403. doi: 10.1016/j.jpsychores.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Steis MR, Fick DM. Are nurses recognizing delirium? A systematic review. J Gerontol Nurs. 2008;34:40–8. doi: 10.3928/00989134-20080901-12. [DOI] [PubMed] [Google Scholar]
- 60.Somes J, Donatelli NS, Barrett J. Sudden confusion and agitation: causes to investigate! Delirium, dementia, depression. J Emerg Nurs. 2010;36:486–8. doi: 10.1016/j.jen.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 61.Sands MB, Dantoc BP, Hartshorn A, Ryan CJ, Lujic S. Single Question in Delirium (SQiD): testing its efficacy against psychiatrist interview, the Confusion Assessment Method and the Memorial Delirium Assessment Scale. Palliat Med. 2010;24:561–5. doi: 10.1177/0269216310371556. [DOI] [PubMed] [Google Scholar]
- 62.Larner AJ. Screening utility of the Montreal Cognitive Assessment (MoCA): in place of--or as well as--the MMSE? Int Psychogeriatr. 2012;24:391–6. doi: 10.1017/S1041610211001839. [DOI] [PubMed] [Google Scholar]
- 63.Cole MG, Dendukuri N, McCusker J, Han L. An empirical study of different diagnostic criteria for delirium among elderly medical inpatients. J Neuropsychiatry Clin Neurosci. 2003;15:200–7. doi: 10.1176/jnp.15.2.200. [DOI] [PubMed] [Google Scholar]
- 64.Kazmierski J, Kowman M, Banach M, Fendler W, Okonski P, Banys A, et al. The use of DSM-IV and ICD-10 criteria and diagnostic scales for delirium among cardiac surgery patients: results from the IPDACS study. J Neuropsychiatry Clin Neurosci. 2010;22:426–32. doi: 10.1176/jnp.2010.22.4.426. [DOI] [PubMed] [Google Scholar]
- 65.Kazmierski J, Kowman M, Banach M, Fendler W, Okonski P, Banys A, et al. Clinical utility and use of DSM-IV and ICD-10 Criteria and The Memorial Delirium Assessment Scale in establishing a diagnosis of delirium after cardiac surgery. Psychosomatics. 2008;49:73–6. doi: 10.1176/appi.psy.49.1.73. [DOI] [PubMed] [Google Scholar]
- 66.Lawlor PG, Nekolaichuk C, Gagnon B, Mancini IL, Pereira JL, Bruera ED. Clinical utility, factor analysis, and further validation of the memorial delirium assessment scale in patients with advanced cancer: Assessing delirium in advanced cancer. Cancer. 2000;88:2859–67. [PubMed] [Google Scholar]
- 67.Fadul N, Kaur G, Zhang T, Palmer JL, Bruera E. Evaluation of the memorial delirium assessment scale (MDAS) for the screening of delirium by means of simulated cases by palliative care health professionals. Support Care Cancer. 2007;15:1271–6. doi: 10.1007/s00520-007-0247-6. [DOI] [PubMed] [Google Scholar]
- 68.Young J, Murthy L, Westby M, Akunne A, O’Mahony R. Diagnosis, prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. doi: 10.1136/bmj.c3704. [DOI] [PubMed] [Google Scholar]
- 69.Levy MH, Back A, Benedetti C, Billings JA, Block S, Boston B, et al. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009;7:436–73. doi: 10.6004/jnccn.2009.0031. [DOI] [PubMed] [Google Scholar]
- 70.Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1–20. [PubMed] [Google Scholar]
- 71.Cole MG, McCusker J, Bellavance F, Primeau FJ, Bailey RF, Bonnycastle MJ, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167:753–9. [PMC free article] [PubMed] [Google Scholar]
- 72.Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61:176–81. doi: 10.1093/gerona/61.2.176. [DOI] [PubMed] [Google Scholar]
- 73.Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43:175–82. doi: 10.1176/appi.psy.43.3.175. [DOI] [PubMed] [Google Scholar]
- 74.Boettger S, Friedlander M, Breitbart W, Passik S. Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry. 2011;45:477–82. doi: 10.3109/00048674.2011.543411. [DOI] [PubMed] [Google Scholar]
- 75.Boettger S, Breitbart W. An open trial of aripiprazole for the treatment of delirium in hospitalized cancer patients. Palliat Support Care. 2011;9:351–7. doi: 10.1017/S1478951511000368. [DOI] [PubMed] [Google Scholar]
- 76.Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci. 2005;30:100–7. [PMC free article] [PubMed] [Google Scholar]
- 77.Elie D, Gagnon P, Gagnon B, Giguere A. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: A literature review. Can J Psychiatry. 2010;55:386–93. doi: 10.1177/070674371005500608. [DOI] [PubMed] [Google Scholar]
- 78.Barcia E, Reyes R, Luz Azuara M, Sanchez Y, Negro S. Compatibility of haloperidol and hyoscine-N-butyl bromide in mixtures for subcutaneous infusion to cancer patients in palliative care. Support Care Cancer. 2003;11:107–13. doi: 10.1007/s00520-002-0415-7. [DOI] [PubMed] [Google Scholar]
- 79.Hui D, Reddy A, Palla S, Bruera E. Neuroleptic prescription pattern for delirium in patients with advanced cancer. J Palliat Care. 2011;27:141–7. [PubMed] [Google Scholar]
- 80.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007:CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Breitbart W, Alici Y. Evidence-Based Treatment of Delirium in Patients With Cancer. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.39.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Administration UFaD. Information for Healthcare Professionals. Haloperidol. 2007 [Google Scholar]
- 83.Meyer-Massetti C, Cheng CM, Sharpe BA, Meier CR, Guglielmo BJ. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5:E8–16. doi: 10.1002/jhm.691. [DOI] [PubMed] [Google Scholar]
- 84.Kim SW, Yoo JA, Lee SY, Kim SY, Bae KY, Yang SJ, et al. Risperidone versus olanzapine for the treatment of delirium. Hum Psychopharmacol. 2010;25:298–302. doi: 10.1002/hup.1117. [DOI] [PubMed] [Google Scholar]
- 85.Elsayem A, Bush SH, Munsell MF, Curry E, 3rd, Calderon BB, Paraskevopoulos T, et al. Subcutaneous olanzapine for hyperactive or mixed delirium in patients with advanced cancer: a preliminary study. J Pain Symptom Manage. 2010;40:774–82. doi: 10.1016/j.jpainsymman.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 86.Fainsinger RL, Waller A, Bercovici M, Bengtson K, Landman W, Hosking M, et al. A multicentre international study of sedation for uncontrolled symptoms in terminally ill patients. Palliat Med. 2000;14:257–65. doi: 10.1191/026921600666097479. [DOI] [PubMed] [Google Scholar]
- 87.Maltoni M, Scarpi E, Rosati M, Derni S, Fabbri L, Martini F, et al. Palliative Sedation in End-of-Life Care and Survival: A Systematic Review. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.37.3795. [DOI] [PubMed] [Google Scholar]
- 88.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–37. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 89.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 90.Ryan K, Leonard M, Guerin S, Donnelly S, Conroy M, Meagher D. Validation of the confusion assessment method in the palliative care setting. Palliat Med. 2009;23:40–5. doi: 10.1177/0269216308099210. [DOI] [PubMed] [Google Scholar]
- 91.Gaudreau JD, Gagnon P, Harel F, Tremblay A, Roy MA. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage. 2005;29:368–75. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Adamis D, Sharma N, Whelan PJ, Macdonald AJ. Delirium scales: A review of current evidence. Aging Ment Health. 2010;14:543–55. doi: 10.1080/13607860903421011. [DOI] [PubMed] [Google Scholar]
- 93.Grassi L, Caraceni A, Beltrami E, Borreani C, Zamorani M, Maltoni M, et al. Assessing delirium in cancer patients: the Italian versions of the Delirium Rating Scale and the Memorial Delirium Assessment Scale. J Pain Symptom Manage. 2001;21:59–68. doi: 10.1016/s0885-3924(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 94.Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 (Suppl 2):S282–8. doi: 10.1111/j.1532-5415.2011.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tagarakis GI, Voucharas C, Tsolaki F, Daskalopoulos ME, Papaliagkas V, Parisis C, et al. Ondasetron versus haloperidol for the treatment of postcardiotomy delirium: a prospective, randomized, double-blinded study. J Cardiothorac Surg. 2012;7:25. doi: 10.1186/1749-8090-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tahir TA, Eeles E, Karapareddy V, Muthuvelu P, Chapple S, Phillips B, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69:485–90. doi: 10.1016/j.jpsychores.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 97.van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–37. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 98.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–27. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 99.Overshott R, Vernon M, Morris J, Burns A. Rivastigmine in the treatment of delirium in older people: a pilot study. Int Psychogeriatr. 2010;22:812–8. doi: 10.1017/S1041610209991359. [DOI] [PubMed] [Google Scholar]
- 100.Hu HDW, Yang H, Liu Y. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chin J Clin Rehab. 2006;10:188–90. [Google Scholar]
- 101.Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153:231–7. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 102.Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71:277–81. doi: 10.1016/j.jpsychores.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301. doi: 10.1016/S0033-3182(04)70170-X. [DOI] [PubMed] [Google Scholar]