Abstract
Aims
In addition to estrogen and progesterone receptors, gross cystic disease fluid protein-15 (GCDFP-15) and mammaglobin (MAM) are the most common markers to identify breast origin by immunohistochemistry (IHC). GCDFP-15 expression has been reported in approximately 60% of breast carcinomas and MAM expression in approximately 80%. Data on their expression in triple-negative breast cancer (TNBC) are very limited. In this study, we examined the expression of these markers in TNBC to determine their utility in pathologic diagnosis.
Methods and Results
We studied their IHC expression in 63 primary and 118 metastatic TNBCs. GCDFP-15 staining was present in 14% of primary and 21% of metastatic TNBCs. MAM staining was present in 25% of primary and 41% of metastatic TNBCs. The expression frequency of GCDFP-15 and/or MAM was 30% in primary and 43% in metastatic TNBCs, and many positive tumors had only focal staining.
Conclusion
Staining for GCDFP-15 and/or MAM in triple-negative carcinomas helps confirm breast origin, but most tumors in this subgroup of breast carcinomas lack expression of either marker.
Keywords: GCDFP-15, mammaglobin, triple-negative, immunohistochemistry
Introduction
Immunohistochemistry (IHC) has become an indispensable tool for aiding in pathologic diagnosis. Tissue-specific IHC markers, used in conjunction with histomorphology, are particularly helpful in characterizing tumors of unknown origin. For patients with a history of breast cancer, when carcinoma occurs at another organ site, it is clinically important to distinguish between metastatic breast cancer and a second primary, since the management and prognostic implications of these tumors can be markedly different. When a metastasis presents in a patient without a history of cancer, breast carcinoma may also enter the differential diagnosis. Furthermore, although the breast is an uncommon site to harbor metastatic disease, carcinomas from other organs such as the lung, ovary and gastrointestinal tract can infrequently involve the breast.1-4 Tissue-specific IHC markers may be helpful in these scenarios to determine the site of origin.
Estrogen receptor (ER) and progesterone receptor (PR) are frequently expressed in breast carcinoma but not entirely breast specific. Positive staining is frequently detected in both breast carcinoma and carcinomas from the gynecologic tract, therefore these markers cannot be used to distinguish between the two.5 Although hormone receptor staining has been reported in adenocarcinomas from other sites such as the lung and stomach, such staining appears to be limited to a small percentage of these tumors.6,7 Human epidermal growth factor receptor 2 (HER2) is not a breast specific marker, but in patients with a history of an HER2-positive breast carcinoma, positive HER2 staining in a second tumor may help confirm its breast origin.
Gross cystic disease fluid protein-15 (GCDFP-15, also known as BRST-2) is one of the proteins detected in the breast cyst fluid. Its monomer has a molecular weight of 15 kilodaltons.8 Mammaglobin A (MAM) is a 10.5-kilodalton secretory protein that shares homology with the uteroglobin family.9 GCDFP-15 and MAM are currently the most commonly used IHC markers to identify tumors of breast origin, the former being more specific.10,11 In addition to breast carcinoma, GCDFP-15 reportedly stains the salivary gland, sweat gland, and prostatic carcinomas.12 Rarely it stains other carcinomas as well.12-15 Reactivity for MAM has been identified in many endometrial endometrioid carcinomas and sweat gland and salivary gland tumors, as well as in gastric, pulmonary, colonic, and ovarian carcinomas and in some melanomas.13,16-19 Although there is some variation between studies, the expression frequency of MAM overall is approximately 80% in primary breast carcinoma.13,16,18,20-23 The expression frequency of GCDFP-15 in breast carcinoma appears to be somewhat lower, overall around 60%, and more variable between studies, ranging from 35% to 85% in a few large series.12,13,16,22-24 Positive staining for GCDFP-15 and/or MAM in a tumor of unknown origin favors breast carcinoma in the right clinical setting, although negative staining in a tumor does not exclude breast origin.
In patients with primary or metastatic triple-negative (ER negative, PR negative and HER2 negative) breast cancer (TNBC), ER, PR and HER2 are by definition of no utility in confirming breast origin. In this subgroup, GCDFP-15 and MAM are the only markers to rely upon. To date, most studies published on the IHC expression of these two markers in breast cancer have included breast carcinomas with various combinations of ER/PR/HER2 status. Some authors have attempted to find correlations between the expression of GCDFP-15/MAM and ER/PR/HER2,12,18,21,25,26 but only rarely have studies demonstrated an association between positive MAM staining and ER positivity in breast tumors.18,25 Little is known about the expression of GCDFP-15 and MAM in TNBC, yet this is the subgroup for which these two markers might be needed the most. It is important to know the sensitivity of an IHC marker in a particular subgroup before rendering a diagnostic interpretation. Our impression in routine practice has been that GCDFP-15 and MAM expression is lower in TNBC than that reported for breast carcinoma in general. In this study, we examined the expression of these two markers in primary and metastatic TNBC to determine their expression frequency in this important subgroup of invasive breast carcinomas.
Materials and methods
Identification of primary TNBC and Immunohistochemistry for GCDFP-15 and MAM
This project was approved by the institutional review board of MD Anderson Cancer Center. Patients with TNBC who had surgical resection between January 2006 and December 2011 and for whom there were available archival paraffin tissue blocks for the study were identified in our pathology database. ER, PR and HER2 IHC staining results and HER2 fluorescence in situ hybridization assay results were obtained from the pathology reports. ER-negative and PR-negative status was defined for this study as nuclear staining in no more than 5% of invasive tumor cells, as invasive tumors with minimal hormone receptor staining were regarded clinically as TNBC. HER2 negative status was defined according to the guideline recommendations of the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP).27,28
IHC staining for GCDFP-15 and MAM was performed using the polymeric biotin-free horseradish peroxidase (HRP) method on the Leica Microsystems Bond Max autostainer. In each case, one unstained tissue section of 4-μm thick that had been prepared from a representative paraffin block of a surgically excised TNBC was incubated at 60°C for 20 min. Following heat-induced epitope retrieval with citrate buffer for 20 min at 100 °C, slides were incubated with mouse monoclonal antibody to GCDFP-15 (clone D6, Covance, Princeton, NJ; 1:100) or MAM (clone 1A5, Biocare Medical, Concord, CA; pre-diluted). The Refine Polymer Detection kit was used to detect bound antibody, with 3,3-diaminobenzidine serving as the chromogen (Leica Microsystems). Slides were counterstained with Mayer's hematoxylin. Results were evaluated with known positive and negative tissue controls. Any cytoplasmic staining was considered positive. Staining in 5% of tumor cells or less was considered focal. The stains were reviewed by two pathologists (LH and HZ) independently, and a consensus was reached by re-reviewing the slides when there was any disagreement.
Identification of metastatic TNBC
Metastatic/recurrent TNBCs with available GCDFP-15 and MAM staining results from patients with diagnostic material obtained between January 2003 and June 2011 were identified by searching our pathology database. The inclusion criteria were triple-negative marker status of the primary breast cancer reported in the pathology database and/or medical records, triple-negative marker status in the metastasis/recurrence if the marker assays were performed, and histologic and clinical features consistent with breast origin. Any case with clinical uncertainty regarding site of tumor origin was excluded regardless of a history of primary TNBC. A reported negative result for ER/PR was accepted as negative if the percentage of invasive tumor cells with nuclear staining was not stated in the report. If the percentage was included in the report, up to 5% ER/PR staining was allowed in the primary or metastatic tumor if such tumors were considered clinically to be TNBCs. Unknown HER2 status in the primary breast tumor (for patients with a remote history of breast cancer) was accepted if the metastasis was documented to be HER2 negative. GCDFP-15 and MAM staining results were obtained from the pathology reports. The presence of any staining was considered positive. Focal staining if reported was noted. The location of the metastasis and the type of surgical procedure (core biopsy or excision) were recorded.
Statistical analysis
The association between the type of procedure and staining results in the metastatic/recurrent TNBCs was analyzed using Fisher's exact test. A P value of <0.05 indicated statistical significance.
Results
GCDFP-15 and MAM expression in primary TNBC
Sixty-three primary invasive TNBCs from 63 patients with available paraffin blocks from the surgical resection were identified. All of the patients were women between the ages of 28 and 81 years (median, 54 years). Fifty patients had a diagnosis of invasive ductal carcinoma. Thirteen patients had a diagnosis of metaplastic carcinoma, including 9 with invasive ductal carcinoma and a spindle cell sarcomatoid component, 3 with invasive ductal carcinoma and a squamous carcinoma component, and 1 matrix-producing carcinoma.
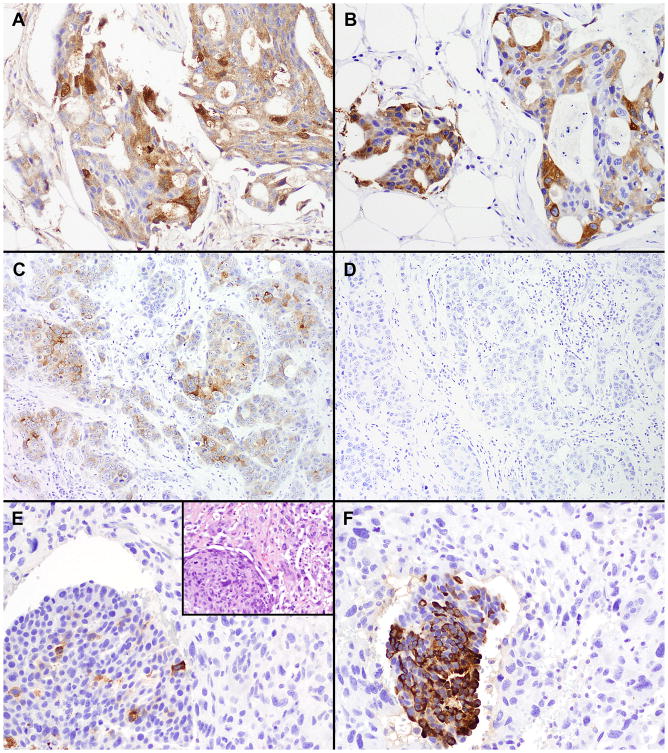
As summarized in Table 1, positive GCDFP-15 staining was observed in 9 (14%) of 63 primary TNBCs, including focal staining in 5 cases. Positive MAM staining was present in 16 (25%), including focal staining in 9. Staining for both markers was seen in 6 tumors (10%) and for either marker in an additional 13 tumors, resulting in 19 tumors (30%) with staining for GCDFP-15 and/or MAM (Figure 1, A-D).
Table 1.
Expression of GCDFP-15 and MAM in primary TNBC.
| MAM | Positive (>5%) | Focally positive (≤5%) | Negative | Total |
|---|---|---|---|---|
| GCDFP-15 | ||||
| Positive (>5%) | 2 | 0 | 2 | 4 |
| Focally positive (≤5%) | 4 | 0 | 1 | 5 |
| Negative | 1 | 9 | 44 | 54 |
| Total | 7 | 9 | 47 | 63 |
Figure 1.
Immunohistochemical staining in primary TNBC. (A) and (B), a carcinoma with positive staining for both GCDFP-15 (A) and MAM (B). (C) and (D), a carcinoma with positive staining for GCDFP-15 (C) and negative staining for MAM (D). (E) and (F), a metaplastic carcinoma with focal staining for GCDFP-15 (E) and positive staining for MAM (F) in the carcinoma component (lower left) but negative staining for both markers in the adjacent sarcomatoid areas. E inset, a hematoxylin and eosin stain of the tumor. (Original magnifications: A, B, E, E inset, F, ×200; C, D, ×100)
Among the metaplastic carcinomas, 1 of the 9 cases with a sarcomatoid component showed staining for MAM and focal staining for GCDFP-15 in the ductal carcinoma areas (Figure 1, E-F). None of the cases showed staining for either marker in the sarcomatoid areas. The 3 cases with a squamous component and the 1 case of matrix-producing carcinoma were negative for both markers.
GCDFP-15 and MAM expression in metastatic TNBC
One hundred eighteen pathologic specimens in 116 patients with GCDFP-15 and/or MAM IHC performed on metastatic/recurrent TNBC at the time of diagnosis were identified. Of these 118, 109 specimens contained metastatic carcinoma and 9 contained locally recurrent invasive carcinoma. All of the patients were women between the ages of 20 and 84 years (median, 56 years). The primary tumor was invasive ductal carcinoma in 112 patients, pleomorphic invasive lobular carcinoma in 1 patient and metaplastic carcinoma in 3 patients. Two patients each had two specimens. One had brain and bladder metastases, and the other had lung and bone metastases.
The results of GCDFP-15 and MAM IHC staining in the different organ sites are summarized in Table 2. GCDFP-15 IHC was available for 110 specimens; staining was present in 23 (21%), including focal staining in 10. MAM IHC was available in 64 specimens; staining was present in 26 (41%), including focal staining in 20.
Table 2.
GCDFP-15 and MAM expression in metastatic/recurrent TNBC at different organ sites.
| Site | No. of cases | GCDFP-15 | MAM | ||
|---|---|---|---|---|---|
|
| |||||
| Positive | Negative | Positive | Negative | ||
|
| |||||
| Chest wall soft tissue recurrence | 5 | 2 | 2 | 1 | 3 |
|
| |||||
| Chest wall/breast skin recurrence | 4 | 2 | 2 | 0 | 2 |
|
| |||||
| Regional lymph node metastasis | 14 | 3 | 10 | 4 | 5 |
|
| |||||
| Distant metastasis | |||||
| Lung | 44 | 3 | 37 | 10 | 13 |
| Liver | 17 | 3 | 12 | 7 | 5 |
| Soft tissuea | 8 | 0 | 8 | 0 | 3 |
| Bone | 7 | 3 | 4 | 1 | 2 |
| Distant lymph node | 5 | 2 | 3 | 0 | 1 |
| Brain | 4 | 3 | 1 | 2 | 0 |
| Ovary | 3 | 1 | 2 | 0 | 1 |
| Bladder | 2 | 0 | 2 | 0 | 2 |
| GI tract | 2 | 0 | 2 | 0 | 1 |
| Skin (abdomen) | 1 | 0 | 1 | 0 | 0 |
| Trachea | 1 | 0 | 1 | 1 | 0 |
| Uterus | 1 | 1 | 0 | 0 | 0 |
|
| |||||
| Total | 118 | 23 (21%) | 87 | 26 (41%) | 38 |
Soft tissue sites include neck (×2), mediastinum (×2), pelvis, back, shoulder, and axilla.
Results for staining of metastatic/recurrent tumors with GCDFP-15 and/or MAM are summarized in Table 3. For the entire cohort, staining for one or both markers was seen in 42 specimens (36%), including 25 specimens with only focal positivity. Staining for both markers was performed on 56 specimens. Twenty-four of them (43%) had positive staining for either or both markers, including 18 with only focal positivity. In 7 specimens (13%), there was staining for both markers, including focal staining for both in 4 specimens. No association was observed between these staining results and the type of surgical procedure (core biopsy versus excision) performed (Table 4).
Table 3.
Expression of GCDFP-15 and MAM in metastatic TNBC.
| MAM | Positivea (focal) | Negative | Not performed | Total |
|---|---|---|---|---|
| GCDFP-15 | ||||
| Positive (focal) | 7 (4)b | 3 (3) | 13 (3) | 23 (10) |
| Negative | 14 (11) | 32 | 41 | 87 |
| Not performed | 5 (4) | 3 | 0 | 8 |
| Total | 26 (20) | 38 | 54 | 118 |
Positive includes focal staining.
Among the seven cases that were positive for both markers, four cases had focal staining for both mammaglobin and GCDFP-15, and one case had staining for GCDFP-15 and focal staining for mammaglobin.
Table 4.
GCDFP-15 and MAM expression in metastatic TNBC according to type of specimen.
| Type | GCDFP-15 | MAM | GCDFP-15 and/or MAM | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Biopsy | 17 (21%) | 65 | 18 (38%) | 30 | 31 (35%) | 58 |
| Excision | 6 (21%) | 22 | 8 (50%) | 8 | 11 (38%) | 18 |
| P value | 1.00 | 0.40 | 0.82 | |||
The metaplastic carcinomas in this cohort consisted of two metastases and one local recurrence. Both metastatic metaplastic carcinomas had negative staining for GCDFP-15. Staining for MAM was not performed in these two cases. In the locally recurrent metaplastic carcinoma, GCDFP-15 was focally positive and MAM was negative. In the single metastatic pleomorphic lobular carcinoma to the bone, focal staining for both GCDFP-15 and MAM was observed.
For the two patients for whom there were two metastases each, one had negative staining for both markers in both the lung and bone metastases, and the other had focal staining for both markers in the brain metastasis but negative staining for both markers in the bladder metastasis.
Discussion
In the current study we found that expression of GCDFP-15 and MAM occurs in a smaller proportion of both primary and metastatic/recurrent TNBCs than that reported in the literature for breast carcinoma in general. In our study, GCDFP-15 expression was detected in 14% of primary TNBCs and 21% of metastatic TNBCs, and MAM expression was detected in 25% of primary TNBCs and 41% of metastatic/recurrent TNBCs. The expression frequency of GCDFP-15 and/or MAM was slightly higher, reaching 30% in primary TNBCs and 43% in metastatic TNBCs. For both markers, we noted that a large proportion of the positive cases had only focal staining in both primary and metastatic TNBCs. When IHC markers are used to classify tumors, diffuse staining is more likely to be considered supportive of a diagnosis than focal staining which needs to be interpreted more cautiously.
To our knowledge, this is the first study to evaluate the staining frequency of GCDFP-15 and MAM in metastatic TNBC. Only rarely have published studies reported the expression of both of these markers in primary TNBC. One study using tissue microarrays, in which any staining was considered a positive result, showed expression in 1 (3%) of 33 primary TNBCs for GCDFP-15 and in 7 (17%) of 41 TNBCs for MAM.22 In that study, non-TNBCs showed staining for GCDFP-15 in 25 (66%) of 38 cases and staining for MAM in 31 (82%) of 38 cases. Another study using tissue microarrays, in which ≥5% tumor staining was considered a positive result, reported MAM staining in 7 (21%) of 33 primary TNBCs compared with 107 (52%) of 205 non-TNBCs.18 In contrast to the low expression rates reported in the above two studies, a third study,23 which used whole slide sections and considered any cytoplasmic staining as positive, detected positive GCDFP-15 expression in 14 (61%) of 23 ER-negative, HER2-negative primary breast carcinomas, and MAM expression in 11 (58%) of 19. Positive staining was reported as 82% for GCDFP-15 and 78% for MAM in the ER- and/or HER2-positive cases in the same study. As ER-negative, PR-positive breast carcinoma is infrequent, most of the ER-negative, HER2-negative cases were most likely TNBCs. However, in the third study, ER positivity was defined such that moderate staining in less than 10% of the tumor and weak staining in less than 50% of the tumor would be considered ER negative. It is possible that some of the ER-negative cases in that study would be considered ER positive using our criteria or those of others. Nevertheless, the results of this third study are markedly different from the first two and from the current study.
Compared with the first two studies mentioned above,18,22 expression of GCDFP-15 and MAM was slightly higher in our primary TNBC cohort. This may be due in part to the differences in the cutoff for positive staining and/or the use of whole slide sections compared with tissue microarrays. For both markers, we considered any cytoplasmic staining as positive, and we used whole slide sections of the primary carcinomas. Both GCDFP-15 and MAM often stain breast carcinomas in a patchy pattern.16,23 It has been shown that the sensitivity of detecting these markers can be lower in tissue microarrays than in whole slide sections, likely owing to the limited sampling of the tumors in tissue microarrays.16
Among the few published studies of GCDFP-15 and/or MAM expression in metastatic breast carcinoma, most have found higher expression than in our study, varying from 44% to 76% for GCDFP-1512,13,16,29 and from 58% to 84% for MAM.13,16,19,30 Because these studies included cases with various combinations of ER/PR/HER2 status, our results from the metastatic cohort support our hypothesis that expression of GCDFP-15 and MAM is lower in TNBC. One exception is a study including 115 recurrent and metastatic breast carcinomas that showed an expression frequency of 11% for GCDFP-15 and 48% for MAM, with no association between the expression of these markers and ER, PR or HER2 status.26 There was no direct comparison between TNBC and non-TNBC tumors in that study.
Most metaplastic carcinomas are triple negative and our study of TNBCs included a relatively large proportion of metaplastic carcinomas. In our primary TNBC cohort, there was very low expression of GCDFP-15 and MAM. Only 1 of the 13 cases had staining for GCDFP-15 and MAM, and the staining was present in the invasive ductal carcinoma component of the tumor. In the 9 cases with a sarcomatoid component, both GCDFP-15 and MAM were negative in the sarcomatoid areas. The other 4 cases (1 matrix-producing carcinoma and 3 carcinomas with a squamous component) were also negative for both markers. Therefore, GCDFP-15 and MAM are not likely to be helpful in confirming the site of origin for metaplastic carcinomas of the breast.
Although ours is the largest study to date on the expression of GCDFP-15 and MAM in TNBC, our study is nevertheless limited because of its retrospective nature and potential selection bias. Metastatic tumors that were not confirmed clinically to be of breast origin were excluded, and this may have selected for a disproportionate number of metastatic tumors with either GCDFP-15 or MAM expression. In spite of this, however, the expression of each marker in TNBCs was quite low, supporting our hypothesis for this subgroup of breast carcinomas.
In summary, our study showed that the expression of GCDFP-15 and MAM in primary and metastatic TNBCs is lower than that reported for breast carcinomas in general. While positive staining supports breast origin, negative staining for each marker is to be expected in the majority of the cases. Therefore, the clinical utility of IHC staining for GCDFP-15 and MAM in TNBCs is limited to a small proportion of this subgroup of breast carcinomas. When new breast-specific markers are developed, their utility specifically in TNBC should be clinically validated.
Acknowledgments
The authors would like to thank Ariana Trevino for her excellent clerical assistance and Kim-Anh Vu for her assistance with the manuscript. We would also like to thank Michael Worley for editing the manuscript.
This project is supported in part by the MDACC institutional start-up funds to L.H and in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
References
- 1.Chaignaud B, Hall TJ, Powers C, Subramony C, Scott-Conner CE. Diagnosis and natural history of extramammary tumors metastatic to the breast. J Am Coll Surg. 1994;179:49–53. [PubMed] [Google Scholar]
- 2.Hadju SI, Urban JA. Cancers metastatic to the breast. Cancer. 1972;29:1691–1696. doi: 10.1002/1097-0142(197206)29:6<1691::aid-cncr2820290637>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.McCrea ES, Johnston C, Haney PJ. Metastases to the breast. AJR Am J Roentgenol. 1983;141:685–690. doi: 10.2214/ajr.141.4.685. [DOI] [PubMed] [Google Scholar]
- 4.Sanderson AT. Metastatic tumours in the breast. Br J Surg. 1959;47:54–58. doi: 10.1002/bjs.18004720111. [DOI] [PubMed] [Google Scholar]
- 5.Lee BH, Hecht JL, Pinkus JL, Pinkus GS. WT1, estrogen receptor, and progesterone receptor as markers for breast or ovarian primary sites in metastatic adenocarcinoma to body fluids. Am J Clin Pathol. 2002;117:745–750. doi: 10.1309/QLV6-HH0H-UCTF-WEF6. [DOI] [PubMed] [Google Scholar]
- 6.Ollayos CW, Riordan GP, Rushin JM. Estrogen receptor detection in paraffin sections of adenocarcinoma of the colon, pancreas, and lung. Arch Pathol Lab Med. 1994;118:630–632. [PubMed] [Google Scholar]
- 7.Yokozaki H, Takekura N, Takanashi A, Tabuchi J, Haruta R, Tahara E. Estrogen receptors in gastric adenocarcinoma: a retrospective immunohistochemical analysis. Virchows Arch A Pathol Anat Histopathol. 1988;413:297–302. doi: 10.1007/BF00783021. [DOI] [PubMed] [Google Scholar]
- 8.Haagensen CD, editor. Diseases of the breast. 3. Philadelphia: Saunders; 1986. Biochemistry and immunohistochemistry of gross cystic disease fluid proteins of the breast; pp. 474–500. [Google Scholar]
- 9.Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uterglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 10.Sapino A, Cassoni P, Bussolati G. Gross cystic disease fluid protein (GCDFP-15) in the breast: past and present. J Biol Regul Homeost Agents. 2000;14:259–262. [PubMed] [Google Scholar]
- 11.Zehentner BK, Carter D. Mammaglobin: a candidate diagnostic marker for breast cancer. Clin Biochem. 2004;37:249–257. doi: 10.1016/j.clinbiochem.2003.11.005. Review. [DOI] [PubMed] [Google Scholar]
- 12.Wick MR. Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. 1989;20:281–287. doi: 10.1016/0046-8177(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 13.Han JH, Kang Y, Shin HC, et al. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330–1334. doi: 10.5858/2003-127-1330-MEILNI. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654–661. doi: 10.1038/modpathol.2010.38. Epub 2010 Feb 19. [DOI] [PubMed] [Google Scholar]
- 15.Wang LJ, Greaves WO, Sabo E, et al. GCDFP-15 positive and TTF-1 negative primary lung neoplasms: a tissue microarray study of 381 primary lung tumors. Appl Immunohistochem Mol Morphol. 2009;17:505–511. doi: 10.1097/PAI.0b013e3181a8e809. [DOI] [PubMed] [Google Scholar]
- 16.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–113. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 17.Zafrakas M, Petschke B, Donner A, et al. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer. 2006;6:88. doi: 10.1186/1471-2407-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki E, Tsunoda N, Hatanaka Y, Mori N, Iwata H, Yatabe Y. Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol. 2007;20:208–214. doi: 10.1038/modpathol.3800731. Epub 2006 Dec 22. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Spaulding B, Sienko A, et al. Mammaglobin, a valuable diagnostic marker for metastatic breast carcinoma. Int J Clin Exp Pathol. 2009;2:384–389. Epub 2008 Dec 1. [PMC free article] [PubMed] [Google Scholar]
- 20.Watson MA, Dintzis S, Darrow CM, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59:3028–3031. [PubMed] [Google Scholar]
- 21.Al-Joudi FS, Kaid FA, Ishak I, Mohamed N, Osman K, Alias IZ. Expression of human mammaglobin and clinicopathologic correlations in breast cancer: the findings in Malaysia. Indian J Pathol Microbiol. 2011;54:284–289. doi: 10.4103/0377-4929.81596. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GH, Subhawong AP, Nassar H, et al. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587–591. doi: 10.1309/AJCPMFR6OA8ICHNH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritzsche FR, Thomas A, Winzer KJ, et al. Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer. Histol Histopathol. 2007;22:1221–1230. doi: 10.14670/HH-22.1221. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007;131:1561–1567. doi: 10.5858/2007-131-1561-POIMHD. [DOI] [PubMed] [Google Scholar]
- 25.Guan XF, Hamedani MK, Adeyinka A, et al. Relationship between mammaglobin expression and estrogen receptor status in breast tumors. Endocrine. 2003;21:245–250. doi: 10.1385/ENDO:21:3:245. [DOI] [PubMed] [Google Scholar]
- 26.Chia SY, Thike AA, Cheok PY, Tan PH. Utility of mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15) in confirming a breast origin for recurrent tumors. Breast. 2010;19:355–359. doi: 10.1016/j.breast.2010.02.007. Epub 2010 Mar 26. [DOI] [PubMed] [Google Scholar]
- 27.Hammond ME, Hayes DF, Dowsett M, et al. Arch Pathol Lab Med. 134. 2010. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer; pp. 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka K, Ohsumi S, Takashima S, Saeki T, Aogi K, Mandai K. Occult breast carcinoma presenting with axillary lymph node metastases: follow-up of eleven patients. Breast Cancer. 2003;10:330–334. doi: 10.1007/BF02967653. [DOI] [PubMed] [Google Scholar]
- 30.Raica M, Cîmpean AM, Meche A, Alexa A, Suciu C, Mureşan A. Analysis of the immunohistochemical expression of mammaglobin A in primary breast carcinoma and lymph node metastasis. Rom J Morphol Embryol. 2009;50:341–347. [PubMed] [Google Scholar]