Abstract
Primary leiomyosarcoma of the breast is an extremely rare neoplasm. Only few cases have been reported in the literature. We report here a case of breast leiomyosarcoma in a 44-years-old female and we discuss the data of the existing literature.
1. Introduction
Breast sarcomas are rare nonepithelial malignancies that arise from the connective tissue within the breast [1]. They constitute about 1% of all breast malignancies [2]. Leiomyosarcoma subtype remains the least frequent. The average age of occurrence is ranging between 45 and 50 years [2–4]. In this case report, we present the clinical features of a 44-year-old woman with primary leiomyosarcoma of the breast, its pathological and therapeutic outcomes, and an up-to-date review of the literature on the topic.
2. Case Presentation
A 44-year-old Arab woman presented with a four-month history of rapidly increasing painless mass in her right breast. On clinical examination, the mass was lobulated and ulcerative taking almost all of the mammary gland and involving breast skin and the nipple areola (Figure 1). No axillary lymph nodes were palpable. An ultrasonography of the breast identified a hypoechoic and heterogeneous mass with an axillary lymphadenopathy measuring 9.2 cm × 7.6 cm × 6 cm and 1.3 cm, respectively. Core needle biopsy of the mass was performed. Pathology revealed tumor comprised of hyperchromatic spindle cells arranged in fascicles, with marked pleomorphism, atypical nuclei, and a moderate mitotic activity (6/10 high power field) (Figure 2). Immunohistochemistry showed that the tumor cells were positive for desmin and H-caldesmon while they were negative for cytokeratin (Figure 3).
Figure 1.
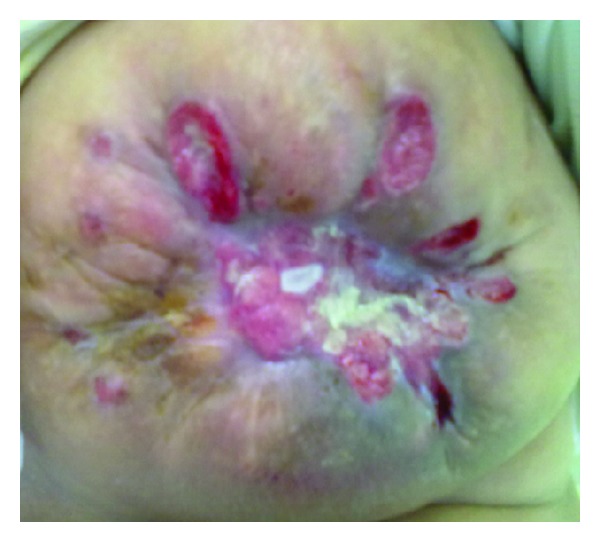
Photograph showing lobulated and ulcerative breast tumor.
Figure 2.
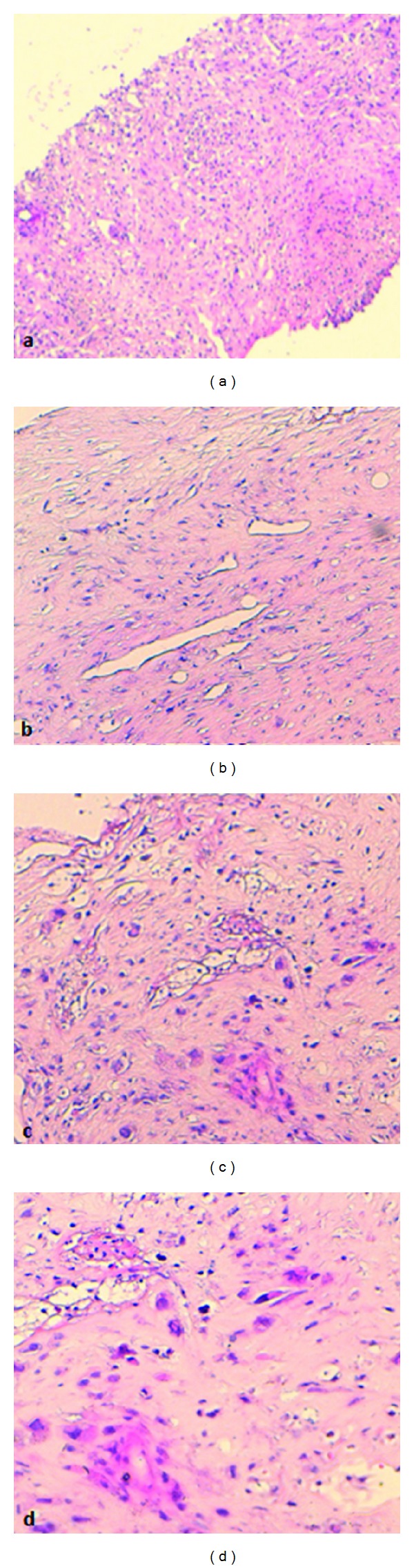
(a), (b), (c), and (d) Progressive increasing magnification of histology (HES 5, 10, 10, and 20, resp.) showing a tumor composed of hyperchromatic spindle cells with frequent atypical nuclei.
Figure 3.
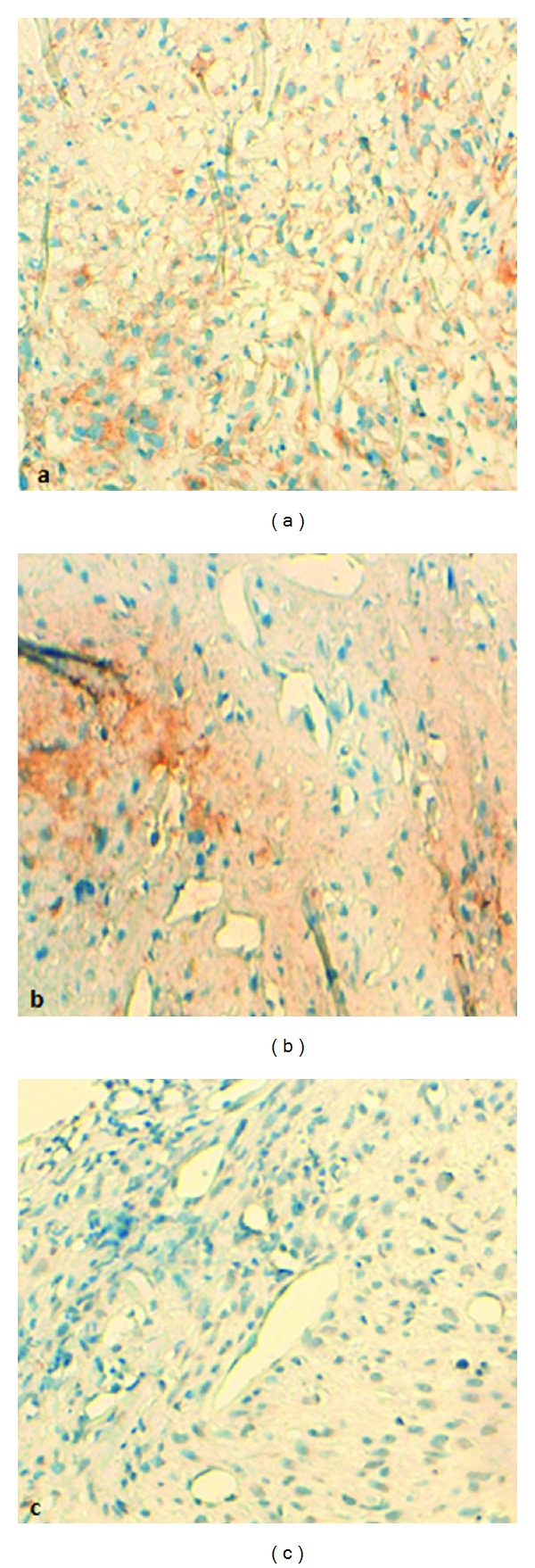
(a), (b) Immunochemistry positivity for desmin and H-caldesmon, respectively. (c) Negative immunohistochemical reactivity for cytokeratin.
The extent of disease was evaluated by systemic physical examination and chest and abdominopelvic computed tomography which revealed diffuse pulmonary metastasis.
Palliative chemotherapy based on doxorubicin (60 mg/m²) and ifosfamide (9 g/m²) every 3 weeks was indicated. After three cycles of this regimen, we noted a clear local and distant progression including the increase in the size of the mammary tumor and the occurrence of a well-circumscribed and ulcerative cutaneous nodule in the abdominal wall (Figure 4). Second line docetaxel (75 mg/m² day 8)—gemcitabine (900 mg/m² days 1 and 8) regimen was proposed, but the patient died of disease one month later.
Figure 4.
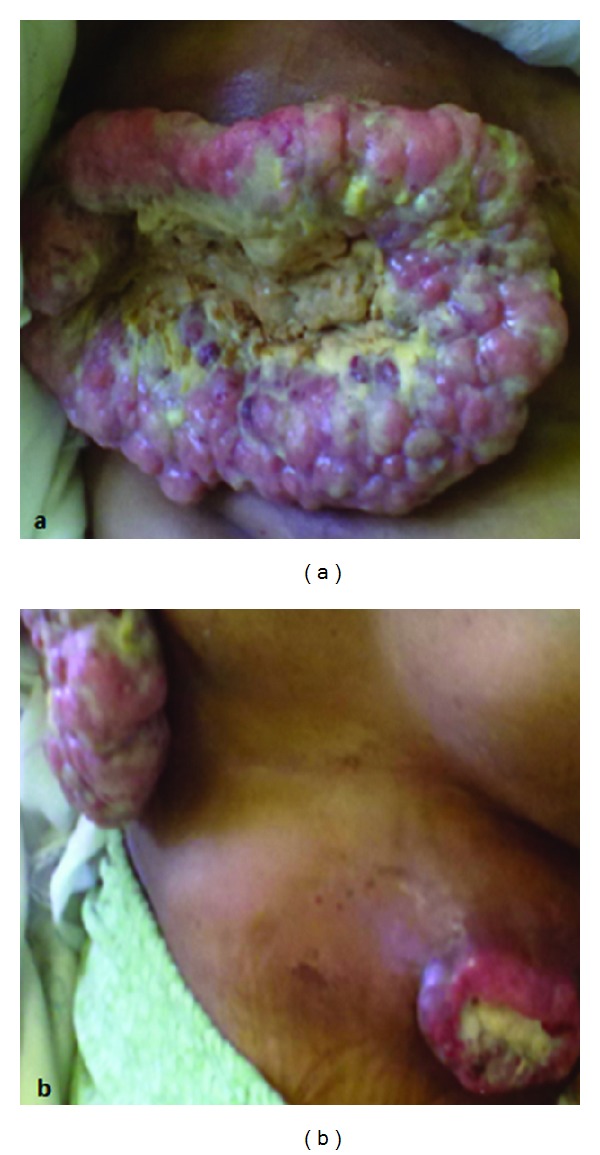
(a) Photograph of local progression of the breast tumor after 3 cycles of the first line chemotherapy and distant apparition of a cutaneous abdominal nodule.
3. Discussion
Leiomyosarcomas of the breast are uncommon neoplasms. The exact origin of these tumors is debated. They can arise either from the smooth muscle cells lining blood vessels or from stromal mesenchymal cells [5, 6]. Breast sarcoma often presents as palpable and well-circumscribed large tumors [3, 7]. The presence of clinical lymphadenopathy is exceptional, occurring in less than 10% [8, 9]. Breast skin and the nipple areola are rarely involved [10, 11], unlike our case where the tumor was very locally advanced involving the entire gland. Findings on mammography and ultrasound are nonspecific [12]. Core biopsy is the diagnostic procedure of choice if sarcomas are suspected [13]. Immunohistochemical staining is essential as adjuncts to differentiate leiomyosarcomas from other tumors and soft tissue sarcomas. These tumors are usually positive for desmin, smooth muscle actin, and muscle specific actin and negative for S100, cytokeratins, and epithelial markers [14, 15].
The limited number of cases treated at any one institution has hampered making specific recommendations regarding surgery and medical treatments for this pathological entity. Otherwise, treatment principles have been extrapolated from studies of nonbreast soft tissue sarcomas. Certainly, the cornerstone of treatment is complete excision with negative margins [11], although there is no consensus on the use of adjuvant chemotherapy or radiotherapy [16]. Patients with metastatic breast sarcoma are offered palliative chemotherapy; palliative surgery is proposed when local complications from primary breast sarcomas cause significant morbidity [17, 18]. However isolated and controlled metastatic disease may also be amenable to potentially curative resection [18, 19].
Most of the cases reported till date of primary breast leiomyosarcoma have undergone mastectomy without adjuvant treatment [20]. To the best of our knowledge, our patient is one of the rare cases of primary breast leiomyosarcoma to be diagnosed in an advanced stage of disease [21].
The anthracyclines based chemotherapy is the standard first line treatment [22]. Therefore, multiagent chemotherapy with adequate-dose anthracyclines plus ifosfamide may be indicated to offer advantage of potentially curative metastasectomy in patients with isolated pulmonary metastasis [19]. After failure of the first line chemotherapy, therapeutic options include gemcitabine alone or in association with dacarbazine or docetaxel [23, 24].
4. Conclusion
Primary leiomyosarcoma of the breast is a diagnostic challenge to clinicians as it is a rare entity which has no specific clinical or radiological features. A general agreement on treatment is still lacking, since there are no prospective trials to guide therapy.
Disclosure
All authors are aware of and agree on the content of the abstract and support of the data presented.
References
- 1.Berg JW, Hutter RVP. Breast cancer. Cancer. 1995;75(1):257–269. doi: 10.1002/1097-0142(19950101)75:1+<257::aid-cncr2820751311>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.McGowan TS, Cummings BJ, O’Sullivan B, Catton CN, Miller N, Panzarella T. An analysis of 78 breast sarcoma patients without distant metastases at presentation. International Journal of Radiation Oncology Biology Physics. 2000;46(2):383–390. doi: 10.1016/s0360-3016(99)00444-7. [DOI] [PubMed] [Google Scholar]
- 3.Zelek L, Llombart-Cussac A, Terrier P, et al. Prognostic factors in primary breast sarcomas: a series of patients with long-term follow-up. Journal of Clinical Oncology. 2003;21(13):2583–2588. doi: 10.1200/JCO.2003.06.080. [DOI] [PubMed] [Google Scholar]
- 4.Pandey M, Mathew A, Abraham EK, Rajan B. Primary sarcoma of the breast. Journal of Surgical Oncology. 2004;87(3):121–125. doi: 10.1002/jso.20110. [DOI] [PubMed] [Google Scholar]
- 5.Cameron HM, Hamperl H, Warambo W. Leiomyosarcoma of the breast originating from myothelium (myoepithelium) Journal of Pathology. 1974;114(2):89–92. doi: 10.1002/path.1711140206. [DOI] [PubMed] [Google Scholar]
- 6.Pardo Mindan J, Garcia Julian G, Eizaguirre Altuna M. Leiomyosarcoma of the breast. Report of a case. American Journal of Clinical Pathology. 1974;62(4):477–480. doi: 10.1093/ajcp/62.4.477. [DOI] [PubMed] [Google Scholar]
- 7.Gutman H, Pollock RE, Ross MI, et al. Sarcoma of the breast: implications for extent of therapy. The M.D. Anderson experience. Surgery. 1994;116(3):505–509. [PubMed] [Google Scholar]
- 8.Gullett NP, Delman K, Folpe AL, Johnstone PAS. National surgical patterns of care: regional lymphadenectomy of breast sarcomas. American Journal of Clinical Oncology. 2007;30(5):461–465. doi: 10.1097/COC.0b013e31804b40f4. [DOI] [PubMed] [Google Scholar]
- 9.North JH, Jr., Mcphee M, Arredondo M, Edge SB. Sarcoma of the breast: implications of the extent of local therapy. American Surgeon. 1998;64(11):1059–1061. [PubMed] [Google Scholar]
- 10.Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer. 1990;66(5):941–944. doi: 10.1002/1097-0142(19900901)66:5<941::aid-cncr2820660522>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Shabahang M, Franceschi D, Sundaram M, et al. Surgical management of primary breast sarcoma. American Surgeon. 2002;68(8):673–677. [PubMed] [Google Scholar]
- 12.Smith TB, Gilcrease MZ, Santiago L, Hunt KK, Yang WT. Imaging features of primary breast sarcoma. American Journal of Roentgenology. 2012;198(4):W386–W393. doi: 10.2214/AJR.11.7341. [DOI] [PubMed] [Google Scholar]
- 13.Rashmi C, Michael SS, Mary F. Breast sarcoma: epidemiology, risk factors, clinical presentation, diagnosis, and staging. Uptodate. In press. [Google Scholar]
- 14.Lawrence W, Jr., Donegan WL, Natarajan N. Adult soft tissue sarcomas: a pattern of care survey of the American College of Surgeons. Annals of Surgery. 1987;205(4):349–359. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arista-Nasr J, Gonzalez-Gomez I, Angeles-Angeles A, Illanes-Baz E, Brandt-Brandt H, Larriva-Sahd J. Primary recurrent leiomyosarcoma of the breast. Case report with ultrastructural and immunohistochemical study and review of the literature. American Journal of Clinical Pathology. 1989;92(4):500–505. doi: 10.1093/ajcp/92.4.500. [DOI] [PubMed] [Google Scholar]
- 16.Fields RC, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Treatment and outcomes of patients with primary breast sarcoma. American Journal of Surgery. 2008;196(4):559–561. doi: 10.1016/j.amjsurg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Rashmi C, Michael SS, Mary F. Breast sarcoma treatment. Uptodate. In press. [Google Scholar]
- 18.Raut CP, George S, Demetri GD. Surgical treatment and other localized therapy for metastatic soft tissue sarcoma. Uptodate. In press. [Google Scholar]
- 19.Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Annals of Oncology. 2012;23(supplement 7):vii92–vii99. doi: 10.1093/annonc/mds253. [DOI] [PubMed] [Google Scholar]
- 20.Swapnil UR, Charu B, Uma NS. Primary leiomyosarcoma of breast in an adolescent girl: a case report and review of the literature. Case Reports in Pathology. 2012;2012:5 pages. doi: 10.1155/2012/491984.491984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciatto S, Bonardi R, Cataliotti L, Cardona G. Sarcomas of the breast: a multicenter series of 70 cases. Neoplasma. 1992;39(6):375–379. [PubMed] [Google Scholar]
- 22.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. Journal of Clinical Oncology. 1993;11(7):1276–1285. doi: 10.1200/JCO.1993.11.7.1276. [DOI] [PubMed] [Google Scholar]
- 23.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. Journal of Clinical Oncology. 2007;25(19):2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 24.García-del-Muro X, López-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish group for research on sarcomas study. Journal of Clinical Oncology. 2011;29(18):2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]


