Abstract
Purpose
Recognition of microbes is important to trigger the innate immune system. Mycolic acid (MA) is a component of the cell walls of mycobacteria such as Mycobacterium bovis Bacillus Calmette-Guerin. MA has immunogenic properties, which may modulate the innate and adaptive immune response. This study aimed to investigate whether a novel synthetic MA (sMA) inhibits allergic inflammatory responses in a mouse model of asthma.
Methods
BALB/c mice were injected intraperitoneally with sMA followed by sensitization and challenge with ovalbumin (OVA). Mice were examined for bronchial hyperresponsiveness (BHR), the influx of inflammatory cells into the lung tissues, histopathological changes in the lungs and CD4+CD25+Foxp3+ T cells in the spleen, and examined the response after the depleting regulatory T cells (Tregs) with an anti-CD25mAb.
Results
Treatment of mice with sMA suppressed the asthmatic response, including BHR, bronchoalveolar inflammation, and pulmonary eosinophilic inflammation. Anti-CD25mAb treatment abrogated the suppressive effects of sMA in this mouse model of asthma and totally depleted CD4+CD25+Foxp3+ T cells in the spleen.
Conclusions
sMA attenuated allergic inflammation in a mouse model of asthma, which might be related with CD4+CD25+Foxp3+ T cell.
Keywords: Mycolic acid, asthma, allergic inflammation, regulatory T cells, mice
INTRODUCTION
Asthma is a chronic inflammatory disease, characterized by the infiltration of inflammatory cells (e.g., eosinophils) in the airway or lung tissues, airway hypersensitivity, and remodeling.1 In recent decades, allergic diseases such as asthma have shown a rapidly increasing trend.2-4 The hygiene hypothesis suggests that a Westernized life style coupled with improved sanitation has reduced microbial exposure, thereby curtailing Th1 immune responses and exacerbating Th2 immune responses.5-7
Mycolic acid (MA), major components of the cell walls of Mycobacterium tuberculosis and related species, is β-hydroxy-α-alkyl branched fatty acids comprising about 60-90 carbon atoms. Their structural uniqueness provides the microorganisms with certain benefits, such as low permeability to hydrophobic compounds, resistance to dehydration, and the capacity to survive attacks by macrophages. MA has immunomodulatory activity, which is associated with the regulation of allergic inflammatory responses.8 Recently, a negative correlation between a positive tuberculin skin test and the development of allergic rhinitis was reported.9 In addition, MA isolated from Mycobacteria may modulate experimental asthma by inducing alveolar macrophages into foamy cell morphotype.10 This modulation prevents the onset of allergic airway inflammation by inducing of regulatory T cells (Tregs) which express markers, including Forkhead box p3 (Foxp3), neuropilin-1, and glucocorticoid-induced tumor necrosis factor receptor-related protein.10 Therefore, we postulated that MA may serve as a potential therapeutic molecule for allergic diseases. However, MA is produced by mycobacteria in small amounts, and its use as a therapeutic agent is difficult and not cost-effective. Hence, a synthetic form of MA (sMA) was developed by Neopharm Co., Ltd using a new process involving a simple and cost-effective production pathway.11
Here, we aimed to use a mouse model of asthma to examine whether this sMA could inhibit allergic inflammatory responses in a manner similar to natural MA derived from Mycobacteria.
MATERIALS AND METHODS
Animals
Female BALB/c mice were purchased from OrientBio (Seongnam, Korea) and cared for and used in accordance with the guidelines set down by the Institutional Animal Care and Use Committee (IACUC) at Asan Medical Center and Ulsan University College of Medicine. The mice were housed in a specific pathogen-free facility.
Antigen sensitization and challenge
The mice (6 wk old, 5 mice/group) were sensitized by 2 intraperitoneal (i.p.) injections (on Days 0 and 7) of ovalbumin (OVA, 20 µg; chicken egg albumin grade V, Sigma Chemical Co., St. Louis, USA) dissolved in 100 µL saline and adsorbed with an equal volume of alum solution (Alumimject, Pierce, IL, USA). On Days 14-16, all mice inhaled for 30 min (once per day) with an aerosol containing 1% OVA in saline. Control mice were sensitized and challenged with saline.
Preparation of sMA
The sMA (Neopharm, Daejeon, Korea) was synthesized and kindly donated as described previously.11 Briefly, MA was synthesized in 3 steps: 1) dimerization of octanoyl chloride into a ketene dimer (E)-4-heptylidene-3-hexyl-oxetan-2-one; 2) selective reduction of heptylidene-3-hexyl-oxetan-2-one by hydrogenation to produce 2-hexyldecanoic β-lactone; and 3) β-lactone ring cleavage under alkaline conditions. This reaction was then acidified with 6 mol/L HCl and the product purified using a column packed with silica gel. Then, the sMA (15 mg) was mixed with 20 mg phosphatidylcholine at 180 mg/mL in chloroform. The organic solvent was evaporated, and the lipids were recovered in sterile PBS (15 mL). Control liposome suspensions were prepared without sMA.
Animal treatment of sMA
In preliminary study, we analyzed using multiple-dose protocols (100 µg, 200 µg, and 500 µg) and found optimal data at 100 µg (data not shown). In addition, the efficacy was estimated by the time of administration (before or after sensitization) and it was determined as 5 days after last sensitization for treatment protocol (on day 12). So, sMA was intraperitoneally injected on 5 days after sensitization at 100 µg. Liposome as a control was injected without sMA.
In vivo treatment with an anti-CD25 monoclonal antibody (mAb)
To examine the involvement of Tregs in the mechanism underlying allergy suppression by sMA, OVA-sensitized mice were injected i.p. with 250 µg of anti-CD25 mAb (clone PC61, eBioscience, San Diego, USA) 1 day before the first intra-nasal challenge with 1% OVA. Control mice were treated with 250 µg of rat IgG (Bioxcell, West Lebanon, NH, USA).
Measurement of bronchial hyperresponsiveness (BHR)
BHR was measured in conscious unrestrained mice using a barometric whole-body plethysmography (All Medicus, Anyang, Korea) as described previously.12 Briefly, 24 h after the final OVA challenge, enhanced pause (Penh) was measured at 10-sec intervals for 3 min after inhalation of saline and each concentration of methacholine (MeCh).
Bronchoalveolar lavage (BAL) and determination of the cellular composition of the BAL fluid
After measurement of BHR, mice were anaesthetized by i.p. administration of zoletil and BAL was performed as described previously.12 Briefly, the trachea was immediately exposed after anesthesia. Then, BAL was performed through a catheter inserted into the exposed trachea following instillation of normal saline (2 mL) at 37℃. The BAL fluid was centrifuged at 2,000 rpm for 5 min at 4℃. After discarding the supernatant, the pellet was resuspended in 100 µL PBS. Total BAL cell counts were performed using a haemacytometer. To count the different types of cells in the BAL fluid, cytospin slides were prepared and stained with Wright stain. The different cell types were identified on the basis of their standard morphology under a light microscope (Nikon, Tokyo, Japan). Five sections per slide were evaluated. The analyses were random, double blinded, and performed by 2 independent investigators.
Flow cytometry
Mouse Treg cells were collected from spleen. Mouse regulatory T cell staining kits with FITC-labeled anti-CD4, PE-labeled anti-CD25, APC-labeled anti-Foxp3, and each isotype control (eBioscience, San Diego, USA) were used for analysis of CD4, CD25 and Foxp3 expression according to the manufacturer's directions. Staining was analyzed by flow cytometry on a FACS Calibur with CellQuest software (BD Biosciences, Mountain View, CA, USA).
Histopathological analysis of the lungs
Lung tissue samples were prepared for histological analysis as described previously.13 Inflammation was scored by 2 independent blinded investigators. The degree of peribronchial and perivascular inflammation was evaluated on a subjective scale of 0-3. Briefly, a value of 0 meant that no inflammation was detectable, a value of 1, occasional cuffing with inflammatory cells, a value of 2, most bronchi or vessels were surrounded by thin layer (1 to 5 cells thick) of inflammatory cells, and a value of 3, most bronchi or vessels were surrounded by a thick layer (more than 5 cells thick) of inflammatory cells.
Cellular infiltration was assessed in 5 randomly-selected fields under a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, USA) at 100× magnification. The analyses were random, double blinded and performed by 2 independent investigators.
Statistical analysis
Results were expressed as means±SEM values, and groups were compared using the Mann-Whitney U test. The statistical significance was calculated using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). A P value that was less than 0.05 was considered significant.
RESULTS
sMA inhibits BHR and airway inflammation in a mouse model of asthma and anti-CD25mAb abrogates the inhibitory effects
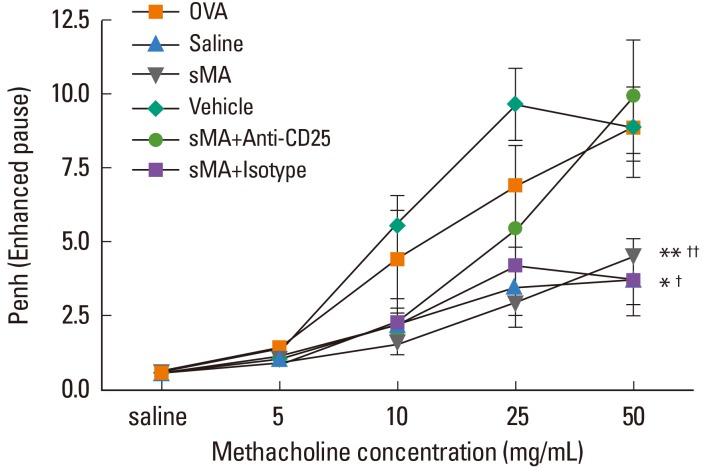
Mice sensitized and challenged with OVA alone developed MeCh-BHR, and showed high total cell and eosinophil numbers in the BAL fluid. Mean Penh value of sMA-treated mice was attenuated to approximately half compared to OVA mice at maximum MeCh dose (50 mg/mL) (4.45±0.64 and 8.85±1.10, P<0.01; Fig. 1). By contrast, the mean Penh value in sMA-treated mice that received Anti-CD25 (9.90±2.46) was high compared to mice that received sMA alone (4.45±0.64) at maximum MeCh dose (50 mg/mL) (P<0.01, Fig. 1).
Fig. 1.
Treatment of ovalbumin (OVA)-induced allergic asthmatic mice with synthetic mycolic acid (sMA) and effects of anti-CD25 monoclonal antibody (mAb) treatment on the BHR. OVA: mice sensitized with intraperitoneal injections of OVA on Days 0 and 7 and then challenged with aerosolized OVA (1%) on Days 14-16 (a single 30-min challenge per day); Saline: mice sensitized and challenged with saline following the same time schedule described for OVA group; sMA: mice that were injected intraperitoneally with the sMA on 5 days after sensitization and challenge with OVA; Vehicle: mice were injected intraperitoneally with liposome as a control for sMA on 5 days after sensitization and challenge with OVA; sMA + Anti-CD25: mice received sMA plus the anti-CD25 mAb; sMA + Isotype: mice received sMA and rat IgG (isotype) as a control for anti-CD25 mAb. Bronchial hyperresponsiveness (BHR). *P<0.05; **P<0.01 compared to OVA; †P<0.05; ††P<0.01 compared to sMA + Anti-CD25. The values represent the mean±SEM of the results from 5 mice per group.
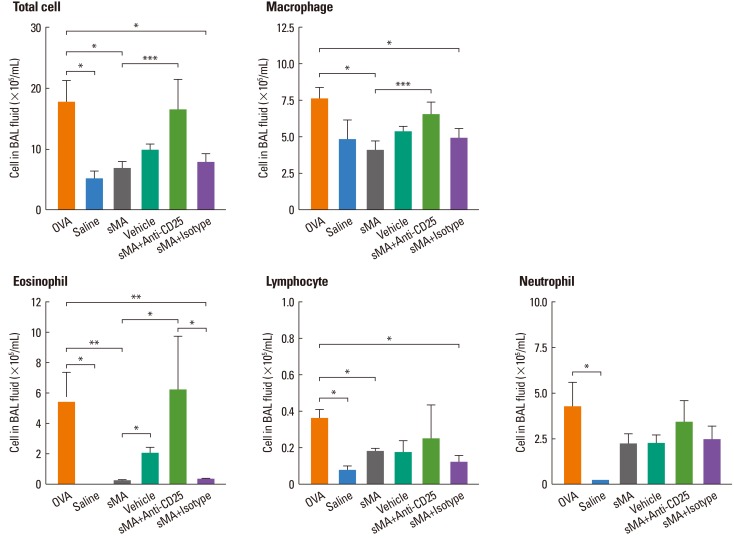
As shown, sMA-treated mice showed significantly lower cell number in terms of the total number of inflammatory cells (6.68±1.26×105/mL vs. 17.56±3.70×105/mL, P<0.05; Fig. 2) and the number of eosinophils (0.23±0.06×105/mL vs. 5.38±2.04×105/mL, P<0.01; Fig. 2) than OVA-treated mice in BAL fluid. However, the administration of Anti-CD25 mAb reversed the total cells and the eosinophils compared to sMA-treated mice in the BAL fluid (6.68±1.26×105/mL vs. 16.35±5.10×105/mL, P=0.05 and 0.23±0.06×105/mL vs. 6.20±3.60×105/mL, P<0.05; Fig. 2).
Fig. 2.
Treatment of OVA-induced allergic asthmatic mice with sMA and effects of anti-CD25 mAb treatment on the differential cell numbers in BAL fluid. The plot legends are as described in Fig. 1. sMA treatment inhibited the number of total cells, macrophages, eosinophils, lymphocytes, and neutrophils in BAL fluid. However, it did not show in vehicle and anti-CD25 mAb treatment. Values represent the mean±SEM of the results from five mice per group. *P<0.05; **P<0.01; ***P=0.05.
sMA inhibits pulmonary inflammation and anti-CD25 mAb abrogates the inhibitory effects
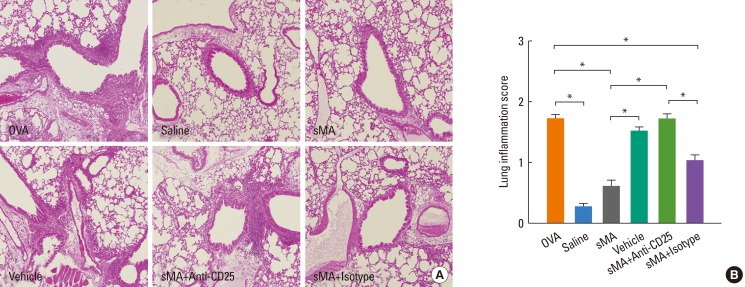
Histological analysis of the lungs from the mice described above revealed that fewer peribronchial and perivascular inflammatory cells were recruited to the lungs in sMA-treated mice than in OVA-treated mice. However, the inflammatory cells were observed in vehicle- and anti-CD25 mAb-treated mice (Fig. 3A). The inflammation scores were significantly lower in sMA-treated mice than in OVA-treated mice (0.60±0.07 vs. 1.72±0.07, P<0.01; Fig. 3B). The mean values of the inflammation score in vehicle- and anti-CD25 mAb-treated mice are similar to OVA-treated mice (Fig. 3B).
Fig. 3.
Treatment of OVA-induced allergic asthmatic mice with sMA and effects of anti-CD25 mAb treatment on the lungs. The lungs were were stained with hematoxylin and eosin. The plot legends are as described in Fig. 1. (A) Lung pathology. (B) Peribronchial and perivascular lung inflammation score. The values represent the mean±SEM of the results from 5 mice per group. *P<0.01.
sMA induces the production of Foxp3+ Treg cells
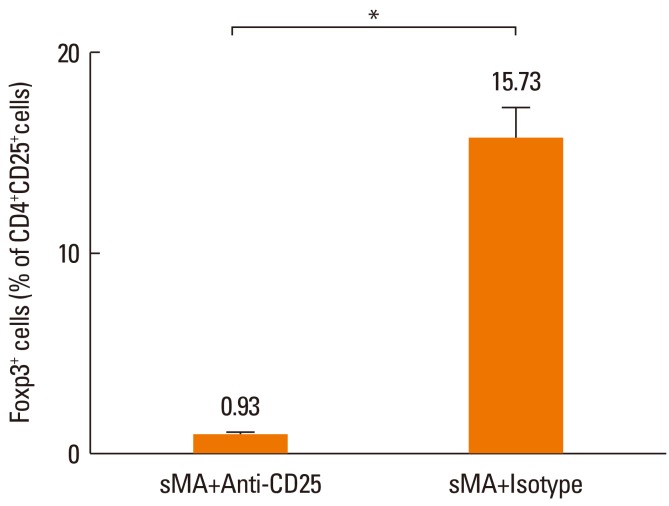
Treg cells were totally depleted from mice treated with anti-CD25 mAb in sMA treated-mice (0.93%±0.09%) compared to mice treated with IgG isotype with sMA (15.73%±1.55%, P=0.05, respectively, Fig. 4). However, Treg cell numbers were slightly higher in mice treated with sMA (15.05%±0.73%) than in mice treated with OVA (13.57%±0.27%), although the difference was not significant statistically (P=0.121, data not shown).
Fig. 4.
Effect of sMA on the percentage of Foxp3+ Treg cells on the splenocytes. The Treg cell population was totally depleted by the anti-CD25 mAb. The values represent the mean±SEM of the results from 5 mice. *P=0.05.
DISCUSSION
A number of studies have examined the role of mycobacterial cell components, such as peptidoglycan, LAM, and LPS in the development of allergic inflammatory disorders in both humans and experimental animal models.14,15 MA is a novel cell wall component derived from Mycobacterium tuberculosis and the role of MA in asthma development remains unknown. Thus, the aim of the present study was to examine the role of sMA in an OVA-induced asthmatic mouse model.
Treatment with sMA inhibited allergic inflammatory responses, including the development of BHR and infiltration of inflammatory cells into the airways and lungs. In addition, sMA showed tendency to increase the number of CD4+CD25+Foxp3+ T cells. These effects were abrogated by an anti-CD25mAb. These findings suggest that new sMA might have a novel function as an immunomodulator to control allergic inflammation. This is the first report to the best of our knowledge to identify sMA as a novel modulator of the immune responses that cause asthma. In previous study, this sMA used for identifying the effect on the biogradation.11 but it was not studied on the allergic diseases, such as asthma. Thus far, a study has been performed on experimental mouse model of asthma for identifying effects and mechanisms of MA using natural MA derived from M. tuberculosis.10 Unlikely, we used artificially synthesized MA based on basic MA-motif. This implies that it is easy to produce in large quantities and can be newly contributing factor for the development of novel preventive and therapeutic approaches on the allergic diseases including asthma.
Our data showed tendency to increase in the expression of Foxp3+ Treg cells after the treatment of sMA. In addition, the suppressive effects of BHR and allergic inflammation were abrogated by an anti-CD25mAb. These suggest that the mechanism of sMA may be associated with CD4+CD25+Foxp3+ Treg cells possibly through not only the number but also the function.
Macrophages and dendritic cells are antigen presenting cell and a major cell population that resides in tissues throughout the body, where they play a crucial role as a first-line of defense against invading pathogens. When MA-treated macrophages were instilled into the airways of OVA-sensitized mice, total inflammatory cell counts and eosinophil counts in the BAL fluid decreased.10 Also, alveolar macrophage-depletion after sensitization significantly increased the number of eosinophils and the production of IL-4 and IL-5.16 In addition, a mixture of cell wall components derived from BCG, including peptidoglycans, arabinogalactans, and MA, induced the maturation of DCs via TNF-α secreted from themselves.17 Therefore, APCs contribute to MA-related mechanism.
Further studies are needed to refine several aspects of this study. We found that the number of Foxp3+ Tregs in sMA-treated mice was not significantly high compared with that in OVA-treated mice. Therefore, it was not completely confirmed that sMA has affected the development of Tregs. In addition, the anti-CD25mAb experiments only implicate Treg cells in the underlying mechanisms indirectly. Anti-CD25 mAb is not really sufficient to fully deplete murine Treg cells, and it also depletes activated T cells.18 Further studies are needed to identifiy the mechanism related Treg of sMA, especially focused on the expression of Treg-related cytokine, IL-10 or TGF-β, and Treg cells from regional immune organ in more detail.
MA is expressed in different forms, which may influence its regulatory function and pathogenic behavior.19 Therefore, the novel structure of the sMA used in the present study may play a role in its effects against allergic inflammation.
In conclusion, we have developed a novel sMA molecule that appears to modulate allergic inflammatory responses, which might be related with CD4+CD25+Foxp3+ T cells. This novel compound may provide a new strategy for treating or controlling asthma.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084144).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008;51:343–350. [Google Scholar]
- 4.Lee SI. Prevalence of childhood asthma in Korea: international study of asthma and allergies in childhood. Allergy Asthma Immunol Res. 2010;2:61–64. doi: 10.4168/aair.2010.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E ALEX Study Team. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 7.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 8.Korf J, Stoltz A, Verschoor J, De Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005;35:890–900. doi: 10.1002/eji.200425332. [DOI] [PubMed] [Google Scholar]
- 9.Obihara CC, Beyers N, Gie RP, Potter PC, Marais BJ, Lombard CJ, Enarson DA, Kimpen JL. Inverse association between Mycobacterium tuberculosis infection and atopic rhinitis in children. Allergy. 2005;60:1121–1125. doi: 10.1111/j.1398-9995.2005.00834.x. [DOI] [PubMed] [Google Scholar]
- 10.Korf JE, Pynaert G, Tournoy K, Boonefaes T, Van Oosterhout A, Ginneberge D, Haegeman A, Verschoor JA, De Baetselier P, Grooten J. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med. 2006;174:152–160. doi: 10.1164/rccm.200507-1175OC. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Kim MK, Kwon MJ, Park BD, Kim MH, Goodfellow M, Lee ST. Effect of the synthesized mycolic acid on the biodegradation of diesel oil by Gordonia nitida strain LE31. J Biosci Bioeng. 2005;100:429–436. doi: 10.1263/jbb.100.429. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res. 2010;2:199–205. doi: 10.4168/aair.2010.2.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 14.Sayers I, Severn W, Scanga CB, Hudson J, Le Gros G, Harper JL. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004;114:302–309. doi: 10.1016/j.jaci.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Hasegawa A, Hosokawa H, Yamashita M, Motohashi S, Naka T, Okamoto Y, Fujita Y, Ishii Y, Taniguchi M, Yano I, Nakayama T. Human Th1 differentiation induced by lipoarabinomannan/lipomannan from Mycobacterium bovis BCG Tokyo-172. Int Immunol. 2008;20:849–860. doi: 10.1093/intimm/dxn043. [DOI] [PubMed] [Google Scholar]
- 16.Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp Mol Med. 2011;43:275–280. doi: 10.3858/emm.2011.43.5.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehérvari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Vander Beken S, Al Dulayymi JR, Naessens T, Koza G, Maza-Iglesias M, Rowles R, Theunissen C, De Medts J, Lanckacker E, Baird MS, Grooten J. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur J Immunol. 2011;41:450–460. doi: 10.1002/eji.201040719. [DOI] [PubMed] [Google Scholar]