Abstract
The management of severe recalcitrant atopic dermatitis (AD) is a challenging issue for clinicians and patients. We hypothesized that repeated intramuscular injections of autologous immunoglobulin (autologous immunoglobulin therapy: AIGT) might induce clinical improvements in patients with AD by stimulation of the active immune response to antigen-binding-site of pathogenic antibodies. We tried AIGT in 3 adult patients with severe recalcitrant AD whose clinical conditions could not be effectively controlled by medical treatments (including oral cyclosporine) for more than 2 years. Autologous immunoglobulin was purified from the autologous plasma by affinity chromatography using Protein A. The patients were treated by an intramuscular injection of 50 mg of autologous immunoglobulin twice a week for 4 weeks. A clinical severity score of AD (SCORAD value) showed a decrease greater than 30% at 8 weeks after the initiation of AIGT compared with the baseline before the initiation of AIGT in all 3 patients with severe recalcitrant AD. No significant side effects from treatment were observed. Further studies with larger numbers of patients are required to evaluate the clinical usefulness of AIGT for AD.
Keywords: Dermatitis, atopic, immunoglobulin, treatment
INTRODUCTION
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disease characterized by itching, dry skin, inflammation and exudation frequently associated with a personal or familial history of allergic diseases.1 Hypersensitivity reaction to environmental agents has been suggested as the pathogenetic mechanism responsible for the development and maintenance of chronic skin inflammation in patients with AD.2 However, the pathogenetic mechanism of AD seems to be more complex and associated with genetic abnormalities, environmental triggers, skin barrier dysfunction, and immunological abnormalities.2,3 The precise pathogenetic mechanism of AD is not completely understood.
Current standard medical therapies for AD (including topical corticosteroid and/or topical calcineurin inhibitor) are mainly focused on symptomatic relief; subsequently, their clinical efficacies are frequently disappointing to both patients and physicians.1 A significant number of patients with recalcitrant AD can be improved by systemic treatment with corticosteroid, cyclosporine, or mycophenolate mofetil; however, there is a possibility of toxicity from long-term treatment with these compounds.1 The further development of additional therapeutic modality for patients with recalcitrant AD is required.
We hypothesized that repeated intramuscular injections of autologous immunoglobulin (autologous immunoglobulin therapy: AIGT) might induce clinical improvements in patients with AD by stimulation of the active immune response to antigen-binding-site of pathogenic antibodies. In this preliminary report, we evaluated the clinical efficacy of AIGT in 3 adult patients with severe recalcitrant AD.
MATERIALS AND METHODS
Patients
Three adult patients with severe recalcitrant AD who fulfilled all of the criteria below were included in this report. Patients showed typical clinical features of AD compatible with the diagnostic criteria for AD suggested by Hanifin and Rajka.4 In this report, recalcitrant AD was defined when the clinical condition of a patient was not effectively controlled by current standard medical therapies (topical moisturizers, topical corticosteroids, topical calcineurin inhibitors, and oral antihistamines) for more than 2 months. All 3 patients in this report were treated with oral cyclosporine for more than 2 years before the initiation of AIGT. One patient (case 1) was also treated by subcutaneous allergen immunotherapy with house dust mite extract for more than 2 years without significant clinical improvement before the initiation of AIGT (Table 1). Medical therapies were maintained in all 3 patients with recalcitrant AD during the AIGT and follow-up after AIGT without changes in dose. This clinical trial was approved by the institutional review board. All patients provided written informed consent. This clinical trial was also registered in the Clinical Research Information Service of Korea (CRIS), one of the primary registries in the World Health Organization (WHO) international clinical trials registry platform (http://apps.who.int/trialsearch/trial.aspx?trialid=KCT0000549).
Table 1.
Clinical characteristics of 3 patients with severe recalcitrant atopic dermatitis at the initiation of autologous immunoglobulin therapy.
Preparation of autologous plasma using a double blood bag system
A total of 400 mL of venous blood was collected into a double blood bag system (Green Cross PBM, Seoul, Korea) that contained 56 mL of citrate phosphate dextrose as anticoagulant. The venous blood in the double blood bag was separated into packed red blood cells and plasma by centrifuging the double blood bag at 3,500 rpm and 4℃ for 10 minutes. The separated autologous plasma (approximately 200 mL) was stored at -20℃.
Preparation of autologous immunoglobulin
Autologous immunoglobulin was purified from autologous plasma by affinity chromatography using Protein A with an aseptic technique in a sterile environment using sterilized devices as found in previous reports.5,6 The purified autologous immunoglobulin solution was tested for bacterial contamination using an endotoxin assay kit (Associates of Cape Cod Inc., MA, USA). The autologous immunoglobulin solution was aliquoted in sterile glass vials and stored at -20℃.
Analysis of protein concentrations in plasma and autologous immunoglobulin solution
The concentration of albumin was measured by the biuret method, using ADVIA 2400 analyzer (Siemens Healthcare Diagnostics Ltd, Camberley, UK). Concentrations of IgA, IgG, and IgM were measured by turbidimetric immunoassay using a Cobas Integra analyzer (F. Hoffmann-La Roche, Basel, Switzerland). The concentration of IgE was measured by ImmunoCAP system (Thermo Scientific Inc., MI, USA).
Treatment procedure of AIGT
AIGT was started 4 weeks after a venous blood sampling using a double blood bag system. The frozen autologous immunoglobulin solution was thawed in a glass vial at room temperature at each time for injection. The patients received intramuscular injections of 50 mg of autologous immunoglobulin twice a week for 4 weeks (total 8 injections).
Clinical assessment
The primary efficacy outcome was the change in the clinical severity score of AD, measured using a standardized clinical severity scoring system for AD (SCORAD).7 SCORAD value was assessed at the baseline, at every week during the 4 weeks of AIGT, and then every 4 weeks.
RESULTS
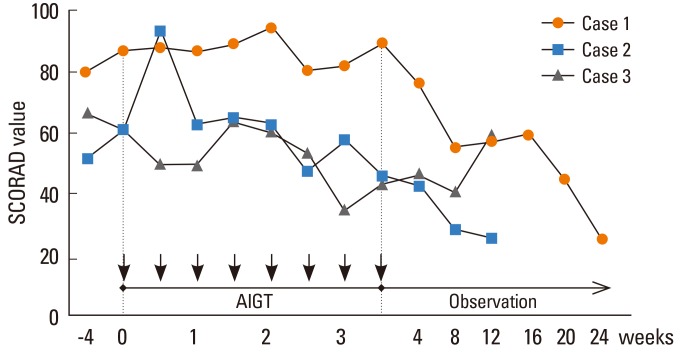
The analysis of protein concentrations in the autologous immunoglobulin solutions from 3 patients with severe recalcitrant AD showed that IgG was a major protein component with small amounts of IgA, IgM, and IgE (Table 2). Endotoxin concentrations in autologous immunoglobulin solutions for all 3 patients were below the detection limit (<0.25 EU/mL). A clinical severity score of AD (SCORAD value) showed a decrease greater than 30% at 8 weeks after the initiation of AIGT compared to the baseline before the initiation of AIGT in all 3 patients with severe recalcitrant AD (Figure). No significant side effects from the treatment were observed in the 3 patients. The detailed clinical courses of 3 patients with severe recalcitrant AD before and after AIGT are as follows.
Table 2.
Analysis of protein concentrations in plasma sample, autologous plasma prepared by a double blood bag system, and autologous immunoglobulin solution in 3 patients with recalcitrant atopic dermatitis who received autologous immunoglobulin therapy.
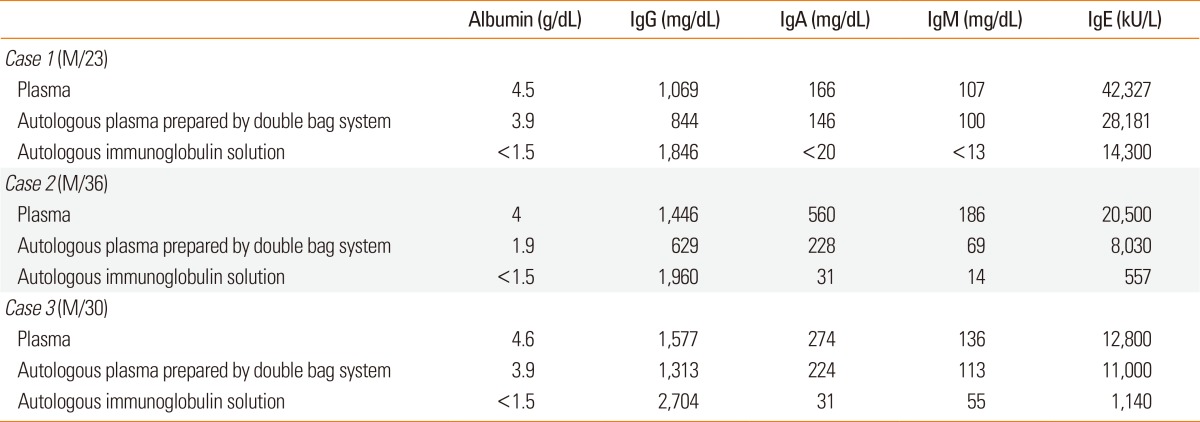
Figure.
Changes in the clinical severity score of atopic dermatitis (SCORAD value) in 3 patients with severe recalcitrant atopic dermatitis before and after autologous immunoglobulin therapy (AIGT) that consisted of intramuscular injections of 50 mg of autologous immunoglobulin twice a week for 4 weeks. The arrows indicate the timing of the injection of autologous immunoglobulin.
Case 1
A 21-year-old male patient with severe AD visited the emergency room of our university hospital with complaints of intractable pruritus and difficulty in sleep due to pruritus on August 30, 2010. The patient was diagnosed with AD at 5 months of age and reported a progressive worsening of his eczema over the past 5 years before the initial visit to our hospital. He had also allergic rhinitis; however, there was a no family history of allergic diseases. On physical examination, eczematous skin lesions on the whole body surface area were noted. The clinical severity score of AD in this patient (SCORAD value) at the initial visit was 81.5. Serum house dust mite-specific IgE assay (ImmunoCAP, Thermo Scientific Inc.) showed a strong positive result (Table 1). After admission, he was treated with a topical corticosteroid, systemic corticosteroid, and oral cyclosporine (200 mg/day). During the admission, he received subcutaneous allergen immunotherapy with house dust mite extract with ultra-rush schedule as previously reported.8 He was discharged from the hospital with clinical improvement. After discharge, he was treated with topical corticosteroid, topical calcineurin inhibitor, emollients, oral antihistamine, and oral cyclosporine (200 mg/day) as well as a monthly maintenance dose of subcutaneous allergen immunotherapy at the out-patient clinic of our university hospital. However, his clinical condition was not completely controlled even after treatment. At 11 months after the initial admission to our hospital, the patient was hospitalized again due to a severe acute exacerbation of AD with secondary bacterial skin infection. After admission, the acute flare-up was treated by a systemic corticosteroid with oral antibiotics. After discharge, he was again treated with oral cyclosporine (125 mg/day) and a monthly maintenance dose of subcutaneous allergen immunotherapy; however, these treatments did not effectively control AD symptoms and only the intermittent use of oral corticosteroids were effective for the control of acute exacerbations of AD.
On November 1, 2012 (at age 23), AIGT was started in this patient. The baseline SCORAD value of the patient measured at the day of initiation of AIGT was 88.0 (Table 1). The clinical severity of AD did not significantly improve in the patient during the first 4 weeks after the initiation of AIGT; however, the clinical condition started to improve 5 weeks after the initiation of AIGT. The SCORAD value was 55.0 at 8 weeks after the initiation of AIGT (37.5% decrease in the SCORAD value compared with the baseline value) (Figure). Moreover, his AD symptoms progressively improved every 4 weeks during the follow-up for 24 weeks after the initiation of AIGT (Figure). At 24 weeks after the initiation of AIGT, the SCORAD value was measured at 24.7 in the patient (a 71.9% decrease in SCORAD value compared with the baseline value). This clinical condition at 24 weeks after the initiation of AIGT was the best condition during the clinical observation of this patient during the follow-up over 3 years after the initial visit to our hospital. No side effects were observed during and after AIGT.
Case 2
A 33-year-old male patient with severe AD presented to the out-patient clinic of our university hospital with complaints of severe pruritus on July 20, 2010. The patient was diagnosed with AD shortly after birth. He also had asthma, allergic rhinitis, and allergic conjunctivitis. He had a family history of allergic rhinitis (elder brother). He was diagnosed with iatrogenic Cushing syndrome at age 11 on the basis of a moon face, obesity, and history of long-term oral corticosteroid treatment at the dermatology clinic of another university hospital. He was treated with a topical corticosteroid and oral antihistamine in the out-patient clinic of another university hospital for 22 years from the age of 11; however, the clinical condition of the patient was not effectively controlled by treatment. He had also received phototherapy (ultra-violet ray therapy) at the age of 22 for 31 months but discontinued due to concerns over the development of side effects from long-term phototherapy. He had received surgery for cataract and glaucoma. On physical examination, eczematous skin lesions on the whole body surface area were noted. The clinical severity score of AD in this patient (SCORAD value) at the initial visit was 60.4. Serum house dust mite-specific IgE assay (ImmunoCAP, Thermo Scientific Inc.) showed a strong positive result (Table 1). After the initial visit to our hospital, he was treated with oral cyclosporine (200 mg/day) for 1 year and then with a combination of oral cyclosporine (100 mg/day) and oral methotrexate (12.5 mg/week) for an additional 1 year in the out-patient clinic of our hospital. However, his clinical symptom of AD had not been effectively controlled.
On February 19, 2013 (at age 36), AIGT was started in this patient. The baseline SCORAD value of the patient measured at the day of initiation of AIGT was 60.7 (Table 1). The patient reported a subjective improvement in clinical symptoms of pruritus from 1 week after the initiation of AIGT and he reported a 100% improvement in the quality of sleep scores (scores from 10 to 0) at 3 weeks after the initiation of AIGT injection. The SCORAD value decreased to 43.1 at 4 weeks after the initiation of AIGT (29.0% decrease in SCORAD value compared with the baseline) and 28.2 at 8 weeks after the initiation of the AIGT (53.5% decrease in the SCORAD value compared with the baseline) in this patient (Figure). The SCORAD value further decreased to 25.7 at 12 weeks after the initiation of AIGT (57.7% decrease in the SCORAD value compared with the baseline) in this patient. No side effects were observed during and after AIGT.
Case 3
A 27-year-old male patient with severe AD presented to the out-patient clinic of our university hospital with a complaint of pruritus on March 4, 2010. The patient was diagnosed with AD at the age of 14. He also had a past history of allergic rhinitis and asthma and a family history of atopic dermatitis (grandfather and aunt). On physical examination, severe eczematous skin lesions involving the face, neck, and anterior chest surface area were noted. He had been treated by a topical corticosteroid, oral antihistamine, and intermittent oral corticosteroid at the out-patient dermatology clinics of 2 different university hospitals for 12 years before the initial visit to our hospital. Additionally, he was also treated with oral cyclosporine (100 mg/day) for the last 6 months. However, his clinical condition was not effectively controlled. He had received surgery for cataracts of the right eye at the age 20. The clinical severity score of AD in this patient (SCORAD value) at the initial visit was 45.2. Serum house dust mite-specific IgE assay (ImmunoCAP, Thermo Scientific Inc.) showed a strong positive result (Table 1). He was treated with oral cyclosporine (100 mg/day), topical calcineurin inhibitor, and oral anatihistamine for 3 years at our clinic; however, his clinical condition of AD was not effectively controlled.
On January 13, 2013 (at age 30), AIGT was started in this patient. The baseline SCORAD value of the patient measured at the day of initiation of AIGT was 60.4 (Table 1). The patient reported a subjective improvement in the clinical symptoms of pruritus at 3 weeks after the initiation of AIGT. The SCORAD value decreased to 45.5 at 4 weeks after the initiation of AIGT (24.7% decrease in the SCORAD value compared with the baseline) and 39.3 at 8 weeks after the initiation of AIGT (34.9% decrease in the SCORAD value compared with the baseline) in this patient (Figure). The SCORAD value increased to 59.2 at 12 weeks after the initiation of AIGT in this patient. No side effects were observed during and after AIGT.
DISCUSSION
In this report, AIGT resulted in marked clinical improvements in 3 patients with severe recalcitrant AD. In addition, AIGT was well-tolerated and produced no significant side effects.
The justification for this study originated from our hypothesis on the possible therapeutic mechanism of autologous blood therapy (ABT).9 Survey of physicians in Germany have shown ABT as the most frequently used complementary alternative medicine for AD.10 ABT has been used for about 100 years in many countries for the treatment of various chronic diseases.11 ABT is a treatment of repeatedly administering small amounts of autologous blood (1-5 mL) by intramuscular injection immediately after the sampling of venous blood from a patient.9 Clinical efficacy of ABT has been demonstrated by randomized placebo-controlled studies in patients with AD and chronic urticaria.12,13 Autologous serum therapy (repeatedly administering a small amount of serum separated from the clotted autologous venous blood of patients by intramuscular injection) also has been reported to be effective in patients with chronic urticaria.14 The induction of an anti-idiotype immune response (immune response to the antigen-binding portion of immunoglobulin) has been commonly suggested as the main mechanism for the development of immune tolerance in allergic diseases and autoimmune diseases.15,16 Based on these studies, we hypothesized that repeated intramuscular injections of autologous immunoglobulin might induce clinical improvements in patients with AD by the stimulation of an active immune response to the antigen-binding-site of pathogenic antibodies. In this report, we observed marked clinical improvements in 3 adult patients with severe recalcitrant AD after AIGT; however, further clinical studies with larger numbers of patients are required to evaluate the clinical usefulness of AIGT for AD.
The clinical efficacy of intravenous administration of polyclonal immunoglobulins purified from multiple healthy blood donors has been reported in patients with severe recalcitrant atopic dermatitis.17 Clinical efficacy of intravenous immunoglobulin therapy was observed quickly after the start of treatment (usually within 1-2 weeks).17 However, the clinical efficacy of intravenous immunoglobulin therapy persisted for a short term (usually less than 1 month) and required monthly administrations of intravenous immunoglobulin to maintain clinical improvement.17 However, we observed that the clinical efficacy of AIGT was maintained for more than 1 month in all 3 patients of this report after the completion of treatment. In the first case of this report, the positive clinical efficacy of AIGT was only observed at 4 weeks after the completion of AIGT and continued for more than 20 weeks after the completion of AIGT. The duration and onset time of clinical efficacy of AIGT seems various among the 3 patients with recalcitrant AD in this report. The difference in duration and onset of clinical efficacy between intravenous immunoglobulin therapy and AIGT could be the result of differences in the therapeutic mechanism of the 2 treatment methods; however, further studies are required to evaluate the therapeutic mechanism of AIGT.
There is evidence on the possible involvement of an anti-idiotypic immunoregulatory mechanism in allergic diseases and autoimmune diseases based on in vitro studies and in vivo animal models; however, there is limited clinical data to support the concept of anti-idiotypic therapy in human subjects with allergic diseases or autoimmune diseases.15,16 Recently, the clinical efficacy of recombinant idiotype vaccine (prolongation of disease-free survival) has been demonstrated in patients with B cell lymphoma by a randomized placebo-controlled clinical trial.18 The idiotype could be used as a tumor-specific antigen for the induction of an active immune response to tumor cells because tumor cells in patients with B cell lymphoma express monoclonal immunoglobulin on the surface.18 The preliminary results of our report suggest a possibility that AIGT could be developed as a new immunomodulatory therapy for allergic diseases and autoimmune diseases. Further studies on the clinical efficacy with larger numbers of patients are required to evaluate the clinical usefulness of AIGT in allergic diseases.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A102065) and a Yonsei-Ajou Research Grant (2008-0001). The idea for autologous immunoglobulin therapy was produced during a discussion on the therapeutic mechanism of autologous blood therapy between the two authors of this report (Dr. Dong-Ho Nahm and Dr. Sook-Yeong Jeon).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A, Leung DY, Lipozencic J, Luger TA, Muraro A, Novak N, Platts-Mills TA, Rosenwasser L, Scheynius A, Simons FE, Spergel J, Turjanmaa K, Wahn U, Weidinger S, Werfel T, Zuberbier T European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006;118:152–169. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Novak N, Bieber T, Leung DY. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112(6 Suppl):S128–S139. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 4.Hanifin JM, Rajka G. Diagnostic features of atopic eczema. Acta Derm Venereol Suppl (Stockh) 1980;92(Suppl):S44–S47. [Google Scholar]
- 5.Buchacher A, Iberer G. Purification of intravenous immunoglobulin G from human plasma--aspects of yield and virus safety. Biotechnol J. 2006;1:148–163. doi: 10.1002/biot.200500037. [DOI] [PubMed] [Google Scholar]
- 6.Machiels JJ, Somville MA, Lebrun PM, Lebecque SJ, Jacquemin MG, Saint-Remy JM. Allergic bronchial asthma due to Dermatophagoides pteronyssinus hypersensitivity can be efficiently treated by inoculation of allergen-antibody complexes. J Clin Invest. 1990;85:1024–1035. doi: 10.1172/JCI114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 8.Kim ME, Kim JE, Sung JM, Lee JW, Choi GS, Nahm DH. Safety of accelerated schedules of subcutaneous allergen immunotherapy with house dust mite extract in patients with atopic dermatitis. J Korean Med Sci. 2011;26:1159–1164. doi: 10.3346/jkms.2011.26.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffen C. Why a historical approach has clinical benefits: Staphylococcus toxoid and autohemotherapy. Skinmed. 2005;4:316–319. doi: 10.1111/j.1540-9740.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004;93(2 Suppl 1):S5–S10. doi: 10.1016/s1081-1206(10)61481-0. [DOI] [PubMed] [Google Scholar]
- 11.Asefi M, Augustin M. Regulative therapy: treatment with nonspecific stimulants in dermatology in traditional and modern perspectives. Forsch Komplementarmed. 1999;6(Suppl 2):9–13. doi: 10.1159/000057140. [DOI] [PubMed] [Google Scholar]
- 12.Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, double-blind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol. 2003;148:307–313. doi: 10.1046/j.1365-2133.2003.04921.x. [DOI] [PubMed] [Google Scholar]
- 13.Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, Opper B, Magerl M, Lüdtke R, Kromminga A, Maurer M. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006;212:150–159. doi: 10.1159/000090656. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008;74:109–113. doi: 10.4103/0378-6323.39691. [DOI] [PubMed] [Google Scholar]
- 15.Wallmann J, Pali-Schöll I, Jensen-Jarolim E. Anti-Ids in allergy: timeliness of a classic concept. World Allergy Organ J. 2010;3:195–201. doi: 10.1097/WOX.0b013e3181e61ebf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoenfeld Y. The idiotypic network in autoimmunity: antibodies that bind antibodies that bind antibodies. Nat Med. 2004;10:17–18. doi: 10.1038/nm0104-17. [DOI] [PubMed] [Google Scholar]
- 17.Lamb SR, Rademaker M. Intravenous immunoglobulin therapy for the treatment of severe atopic dermatitis. Expert Opin Pharmacother. 2001;2:67–74. doi: 10.1517/14656566.2.1.67. [DOI] [PubMed] [Google Scholar]
- 18.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, McGaughey DS, Jaffe ES, Chong EA, Reynolds CW, Berry DA, Santos CF, Popa MA, McCord AM, Kwak LW. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]