Abstract
To facilitate accurate detection of estrogen receptor expression in breast tumors, the American Society of Clinical Oncology/College of American Pathologists recommends that cold ischemia time be kept under 1 h. However, data to address the upper threshold of cold ischemia time are limited. While it is our routine practice to keep cold ischemia time under 1 h for breast core biopsy specimens, this is difficult for surgical specimens because of the comprehensive intraoperative assessment performed at our institution. In this retrospective study, we compared estrogen receptor immunohistochemical staining results in paired breast tumor core biopsy specimens and resection specimens with cold ischemia times ranging from 64 to 357 min in 97 patients. The staining category (≥10%, positive; 1-9%, low positive; <1%, negative) between the core biopsy and resection specimens changed for 5 patients (5%). The weighted Kappa statistic for estrogen receptor staining category between the two specimen types was 0.86 (95% confidence interval, 0.74-0.99), indicating good concordance. The difference in the percentage of estrogen receptor staining between core biopsy and resection was not significantly associated with cold ischemia time (P = 0.81, Spearman correlation). Although we did not observe significant associations between the difference in estrogen receptor staining in the two specimen types and cold ischemia time after placing the patients in three groups of ‘increase’, ‘decrease’ and ‘no change’ using a difference of 25% in estrogen receptor staining percentage as the cutoff, a trend of decreased estrogen receptor staining with cold ischemia time > 2 h was detected. No statistically significant association was found between the change of estrogen receptor staining and the history of neoadjuvant chemotherapy. Our findings indicate that prolonged cold ischemia time up to 4 h (97% of our cohort) in the practice setting of our institution has minimal clinical impact on estrogen receptor immunohistochemical expression in breast tumors.
Keywords: estrogen receptor, breast, cold ischemia time, immunohistochemistry
Introduction
Estrogen receptor (ER) is a weak positive prognostic indicator and a strong predictor of response to hormonal therapies in patients with invasive breast cancer.(1) Accurate, standardized detection of ER expression in breast tumor cells is essential to appropriate clinical management. Immunohistochemistry has replaced ligand-binding assay since the 1990s and is now the standard method of detecting ER expression in tissue containing breast cancer.(2) Many preanalytical, analytical and postanalytical factors may affect ER immunohistochemistry results. The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) addressed issues related to these factors in the guideline recommendations published in 2010.(3)
Among the ASCO/CAP guidelines is the recommendation that cold ischemia time, defined as the time from the removal of the tissue from the patient to the initiation of tissue fixation, be shortened as much as possible, specifically, no more than 1h. In compliance with the ASCO/CAP guidelines, core biopsy specimens at our institution now routinely have cold ischemia time no more than 30 min. As a tertiary care center, we also see that this has become the general practice for core biopsy specimens nationwide based on cases from other hospitals sent to us for review. Because of widespread use of core biopsy to establish a diagnosis of breast cancer prior to surgery and because hormonal receptor assays performed on core biopsy specimens generally appear to be more reliable than those performed on surgical resection specimens,(4-6) ER/progesterone receptor (PR) expression is assessed on core biopsy specimens in the majority of breast cancer patients. However, ER immunohistochemical staining on the resected tumor is necessary in some patients. The scenarios requiring the use of resection specimens for ER immunohistochemistry include absence of staining of the tumor for ER with a lack of appropriate internal controls in the core biopsy, discordance between the results of staining and histomorphology in the core biopsy, and the presence of an invasive component identified only in the resection specimen. In addition, at our institution, we often repeat immunohistochemical staining on the surgical specimen if the tumor is triple-negative (negative for ER, PR and human epidermal growth factor receptor 2) in the core biopsy specimen.
Intraoperative assessment is performed for the vast majority of breast resection specimens at our institution. Gross examination, specimen radiography, and in some cases, frozen section analysis are helpful in ensuring excision of the lesion with adequate margins.(7,8) This process may take 30-60 min depending on the complexity of the case. With additional time needed for sampling tissue for microscopic evaluation,(9) limiting the cold ischemia time to less than 1h is difficult. However, the impression from our daily experience is that ER immunohistochemical staining results obtained from resection specimens are comparable with those from core biopsies. To date, data addressing the effect of cold ischemia time on ER immunohistochemical staining are very limited.(10-16) Therefore, in the present study, we compared ER immunohistochemical results in paired surgical resection and core biopsy specimens of breast tumors to determine the effect of prolonged cold ischemia time on ER detection.
Materials and Methods
Case selection
The pathology files at The University of Texas MD Anderson Cancer Center were retrospectively searched for all patients with invasive mammary carcinomas resected between February 1 and May 15, 2011. Cases in which the first specimen sent to pathology contained invasive carcinoma with ER staining performed in the corresponding core biopsy material were selected. The time from specimen accession to fixation for each case was recorded. The average time from specimen resection in the operating room to specimen accession was estimated to be 10 min, which was added as part of the cold ischemia time in each case (time in fixative – time of accession + 10 min). Of note, at our institution, all postresection specimen radiographs obtained after needle localization are taken in the pathology suite after the specimens are accessioned. Eight cases were excluded from our study because of cold ischemia times less than 1 h. The resulting 97 cases from 97 patients with cold ischemia times greater than 1 h were the subjects of the study. This project was approved by MD Anderson institutional review board.
Tissue processing and immunohistochemistry
For tissue processing, all of the resection specimens in our study were freshly sampled on the same day they were received. If immediate sampling is not expected after the tissue is sliced, it is common practice at our institution to cover the tissue at room temperature with damp paper towels soaked in tap water. Three- to four-millimeter tissue sections were fixed in 10% neutral buffered formalin and processed using automated tissue processors. The formalin fixation time was within the range from 6 to 72 h for 91 of the specimens, and from 72 to 78 h for the remaining 6 specimens. The fixation times for the latter 6 specimens was longer because formalin fixation was initiated late on a Thursday and they were therefore not processed until the evening of the following Sunday. There was no three-day holiday weekend during the period of time selected for the surgical resection specimens used in the study.
The polymeric biotin-free horseradish peroxidase method was used for ER immunohistochemical staining on a Leica Microsystems Bond Max stainer (Leica Microsystems, Buffalo Grove, IL). In each case, one whole slide unstained tissue section 4-μm thick that had been prepared from a representative paraffin block of the resected invasive breast carcinoma was incubated at 60°C for 20 min. Following heat-induced epitope retrieval with citrate buffer for 30 min at 100 °C, slides were incubated with mouse monoclonal antibody to estrogen receptor (clone 6F11, 1:35, Novocastra Laboratories, Leica Microsystems). The Refine Polymer Detection kit was used to detect bound antibody, with 3,3-diaminobenzidine serving as the chromogen (Leica Microsystems). Slides were counterstained with Mayer's hematoxylin. Results were evaluated with appropriate positive and negative tissue controls.
Pathologic review
Among the 97 cases, ER staining was performed retrospectively on the resection specimen in 80 for the purpose of this study. The stains were independently scored by three authors (XL, MG and LH) to estimate the percentage and intensity (strong, moderate, or weak) of the staining in the invasive carcinoma. The scores were reviewed by all three and significant differences reconciled, and then the average of the percentages given by the three authors was calculated as the final score. The remaining 17 cases had ER staining performed concurrently on the resection specimens as part of the routine diagnosis. The reason for repeating ER staining in the resection specimens appeared to be the presence of weak or low (less than 10%) staining in the core biopsy specimen in 11 patients, and was unclear in the remaining six. The ER staining slides were reviewed in 14 of these cases to confirm the results in the pathology reports. In the other three cases, the slides were not available for review, and the results were recorded from the pathology reports. The ER staining results on the corresponding core biopsy specimens were obtained from the pathology reports in each case. We routinely report the ER staining intensity and percentage and group the results into three categories: ≥10%, positive; 1-9%, low positive; <1%, negative.
Statistical analysis
All statistical tests were performed using SAS 9.2 by SAS Institute, Inc., Cary, NC, USA. The concordance of ER staining results between core biopsy and resection specimens was evaluated using weighted kappa statistics. To evaluate the difference in ER staining between the resection and biopsy specimens in association with cold ischemia time, the Kruskal-Wallis test was used for the analysis of categorical variables, and Spearman correlation was used for the analysis of continuous variables. The Fisher's exact test was used to examine the association between the difference in ER staining in the two specimen types and the cold ischemia time using 2 h as a cutoff, as well as the association between the change in ER staining and neoadjuvant chemotherapy. P-values of <0.05 indicated statistical significance except in pair-wise comparisons of three groups, in which P-values less than 0.016 were considered statistically significant.
Results
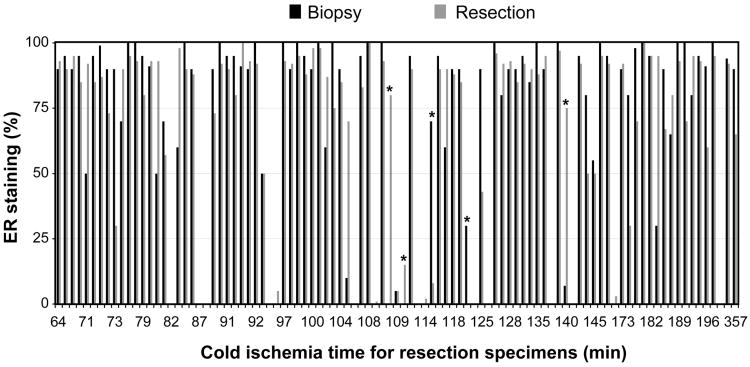
The percentages of ER immunohistochemical staining in the core biopsy and resection specimens were shown in Figure 1. Of the 97 patients, 87 had their resection specimens (90%) undergoing intra-operative evaluation requested by the surgeons. The cold ischemia times for the resection specimens ranged from 64 to 357 min (median, 109 min). The distribution of the cold ischemia times was as follows: 58 under 120 min, 25 between 121 and 180 min, 11 between 181 and 240 min, and 3 greater than 241 min. The ER staining results in the core biopsy specimens in the 97 patients in our study cohort were as follows: 78 were positive, 2 were low positive, and 17 were negative. To offset small differences in the percentage between the two specimen types that may have caused changes in the above categories, in evaluating the concordance in ER staining between the core biopsy and resection specimens, we considered any difference in ER staining greater than 5% that resulted in a change in category to be an event. According to such a definition, the staining category changed for 5 of the 97 cases (5%; Table 1). The weighted Kappa statistic for ER staining category between the core biopsy and resection specimens was 0.86 (95% confidence interval, 0.74-0.99), indicating good concordance. In addition to these 5 cases, 4 cases had 0% ER staining in the core biopsy specimens and ranged from 1% to 5% weak staining in the resection specimens. We did not consider the latter differences to be significant for the above analysis.
Figure 1.
ER staining results (%) for the core biopsy and resected breast tumor specimens according to cold ischemia time. *The five cases with changes in ER staining category.
Table 1. Summary of the five cases of breast cancer with differences in estrogen receptor staining category between the core biopsy and resection specimens.
| Case | Cold ischemia time (min) | Staining results | Diagnosis/histologic grade | Pre-operative chemotherapy | |
|---|---|---|---|---|---|
| Biopsy | Resection | ||||
| 1 | 115 | 70%, moderate | 8%, weak | Invasive ductal carcinoma/3 | Yes |
| 2 | 122 | 30%, weak | 0% | Invasive ductal carcinoma/3 | Yes |
| 3 | 109 | 0% | 80%, moderate | Invasive ductal carcinoma/3 | Yes |
| 4 | 110 | 0% | 15%, weak | Invasive ductal carcinoma/3 | No |
| 5 | 140 | 7%, weak | 75%, strong | Invasive lobular carcinoma/1 | No |
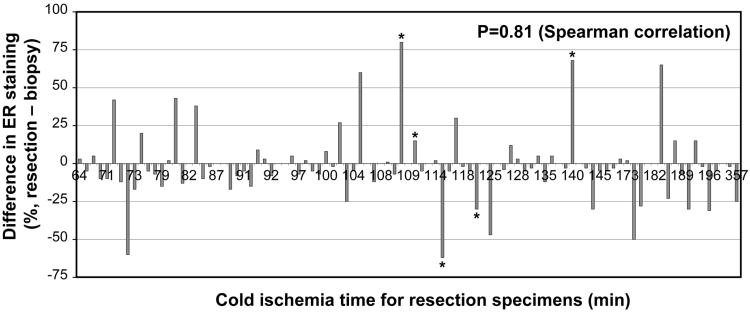
The difference in the percentage of ER staining between core biopsy and resection was not significantly associated with cold ischemia time (P = 0.81, Spearman correlation; Figure 2). To further evaluate the association between the difference in ER staining in the two specimen types and cold ischemia time, the cohort was divided into three groups using a difference in ER staining between the two specimen types of 25% as the cutoff: 1) increased ER staining, defined as an increase in ER staining in the resection specimen resulting in classification in another staining category as described above or an increase in ER staining by more than 25%; 2) decreased ER staining, defined as a decrease in ER staining in the resection specimen resulting in classification in another category as described above or a decrease in ER staining by more than 25%; and 3) no change in ER staining. We observed no significant associations between difference in ER staining and cold ischemia time after placing the patients in these three groups or combining the patients in groups 1 and 2 and then comparing them with the patients in group 3 (Table 2).
Figure 2.
Association of the difference in ER staining between core biopsy and resection specimens with cold ischemia time. The difference was calculated as the percentage of staining in the resection specimens subtracted by that in the core biopsy specimens. *The five cases with changes in ER staining category.
Table 2. Association of the difference in estrogen receptor staining between the core biopsy and resection specimens with cold ischemia time using a difference of 25% (resection-biopsy) as the cutoff or with a change in estrogen receptor staining category.
| Group | Cold ischemia time (min) | P-value (Kruskal-Wallis test) | ||
|---|---|---|---|---|
| Median | Minimum | Maximum | ||
| No change (n = 78) | 108 | 64 | 357 | 0.32 |
| Change (n = 19) | 115 | 71 | 196 | |
| No change (n = 78, 82%) | 108 | 64 | 357 | 0.10 |
| Increase (n = 10,10%) | 107 | 71 | 184 | |
| Decrease (n = 9, 9%) | 144 | 73 | 196 | |
Because two previous studies suggested that ER staining start to decrease at 2 h of cold ischemia,(13,16) we separated our cohort into two groups by cold ischemia time: 1) up to 2 h and 2) more than 2 h. Using the 25% cutoff as described above, we found that change in ER staining was not significantly associated with cold ischemia time up to 2 h versus more than 2 h (Table 3). Although not statistically significant, a marginal difference was found between the ‘no change’ and the ‘decrease’ groups in association with cold ischemia time in pair-wise comparison of the three groups (P = 0.03), suggesting a trend of decreased ER staining with cold ischemia times greater than 2 h (Table 3).
Table 3. Association of the difference in estrogen receptor staining between the core biopsy and resection specimens with cold ischemia time using 2 h as the cutoff for cold ischemia time.
| Groups | Cold ischemia time | P-value (Fisher's exact test) | |
|---|---|---|---|
| Less than 2 h (n=58) | Greater than 2 h (n=39) | ||
| No change | 48 | 30 | 0.60 |
| Change | 10 | 9 | |
| No change | 48 | 30 | 0.03a |
| Increase | 8 | 2 | |
| Decrease | 2 | 7 | |
In pair-wise comparison of the three groups, a P value between 0.016 and 0.05 was considered a trend.
The results on the intensity of ER staining in the core biopsy and resection specimens were compared. Since differences in the ER staining intensity from strong to moderate and vice versa are frequent owing to interobserver variability, and the impact of these differences on clinical management is minimal, we assessed cases with weak staining in the core biopsy and/or the resection specimens. As shown in Table 4, we identified 12 such patients. In addition to the 4 patients whose staining results were included in Table 1 because of a change in ER staining category, it was noted that 2 patients had a drastic difference in ER staining between the core biopsy and the resection specimens. One of them had a low grade invasive carcinoma with ductal and lobular features with 10% weak ER staining in the core biopsy and 70% strong staining in the resected tumor (No. 8 in Table 4), and the other had a pleomorphic invasive lobular carcinoma with 80% strong staining in the core biopsy and 30% weak staining in the resected tumor (No. 12 in Table 4).
Table 4. Estrogen receptor staining results and cold ischemia time of patients whose core biopsy and/or resection specimens showed weak estrogen receptor staining.
| Case | 1 | 2 | 3 | 4 | 5a | 6 | 7a | 8 | 9a | 10 | 11a | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staining resultsb | Core biopsy | 0 | 0 | 0 | 0 | 0 | 5w | 7w | 10w | 30w | 50w | 70m | 80s |
| Resection | 5w | 1w | 2w | 3w | 15w | 5w | 75s | 70s | 0 | 50w | 8w | 30w | |
| Cold ischemia time (min) | 95 | 108 | 114 | 171 | 110 | 109 | 140 | 105 | 122 | 93 | 115 | 174 | |
The information on these patients was included in Table 1.
The staining results were shown in percentage followed by intensity. S, strong; m, moderate; w, weak.
Thirty-eight patients in the study received neoadjuvant chemotherapy: 31 with no change in ER staining, 6 with decreased ER staining, and 1 with increased ER staining using the 25% cutoff described above. Of the remaining 59 patients who did not receive chemotherapy, 47 had no change in ER staining, 9 had increased ER staining and 3 had decreased ER staining. Neither change in ER staining (decreased and increased ER staining combined) nor decreased ER staining was associated with neoadjuvant chemotherapy (P = 1.00 and P = 0.16, respectively).
Discussion
Our study showed no association between the difference in ER expression in core biopsy and resection specimens and cold ischemia time between 64 min and 357 min. Formalin is known to fix tissue specimens by chemical reactions that cross-link proteins and large molecules necessary for immunohistochemical analysis. (17,18) Prolonged tissue ischemia may cause proteolytic degradation and therefore decreased antigenicity in immunohistochemistry. One study showed a significant downward trend in ER positivity in breast from the tumor periphery toward the center in large tissue sections, indicating that delayed fixation may decrease ER staining.(4) Conceivably, optimal ER staining results are obtained when tissue is thinly sectioned and placed in formalin immediately after procurement. However, a practical cold ischemia time that can be allowed for results of ER immunohistochemical staining acceptable for clinical practice is largely unknown from the limited data on this subject in the literature. An early study showed that delayed fixation of breast tumor tissue after storage of tissue at 4°C for 12 h resulted in a nearly 40% decrease in the percentage of ER staining in tumor cells compared with control tissue that was not subjected to delayed fixation.(10) Another study demonstrated decreased ER immunoreactivity in Allred score in breast tumor sections with delayed fixation for 18-24 h.(11) A third study, presented only in abstract form, showed that delayed fixation of small portions of breast tumor samples in 4% Baker's formal calcium (a modified formalin fixative) for up to 120 min did not have a significant negative effect on ER staining compared with immediately fixed controls.(12) These results suggested that the upper limit of cold ischemia time not significantly affecting ER immunohistochemical staining may be between 2 and 12 h.
In two recent studies, the authors investigated 10 excised breast tumor samples.(13,14) After receiving tumors from the operating room, they divided tumor tissue into portions and fixed them in formalin at between 0 min and 8hrs as well as after storage at 4°C overnight. The processed tissue sections were constructed into tissue microarrays and immunohistochemical staining results recorded using the Q-score method.(19,20) The authors observed a decrease in ER staining starting at 2 h of delayed fixation for clones 1D5 and 6F11, and at 1h for clone SP1, with the lowest Q-scores recorded at 8h; these results were statistically insignificant, however. In keeping with the above observations, a newly published study concluded that significant reduction in ER immunohistochemical staining generally does not occur until 4 h of cold ischemia for refrigerated samples and 2 h for non-refrigerated samples.(16) In that study, portions of 25 freshly collected breast tumor samples were subjected to variable cold ischemia times from 0.5-48 h within the refrigerator and at room temperature. After processing, ER immunohistochemical staining was performed using clone SP1, and the results were semi-quantitatively scored with the H-score method (21) and compared with those previously obtained in core biopsy specimens. The difference in the mean H-score for ER in the two specimen types did not reach statistical significance for refrigerated samples but became statistically significant at 3 h of cold ischemia time for non-refrigerated samples. In contrast, another recent study found no effect of cold ischemia time of 4 days on ER staining.(15) In that study, using a patient's excised sample of an invasive lobular carcinoma after 4 days of storage at 4°C, core-sized pieces of the tumor were fixed in various fixatives for different times. The tumor exhibited no degradation, and the authors found no differences in ER staining with clone 6F11 in samples fixed in 10% formalin. A tumor section processed according to the ASCO/CAP guidelines served as the control.
In the present study, our aim was to determine the effect of cold ischemia time on ER staining of breast tumor specimens under conditions as close as possible to those in routine clinical practice. Several factors were considered while the study was designed. 1) Although there is no one gold standard assay available for ER detection in breast carcinoma, because ER staining is routinely performed on core biopsy specimens in compliance with the ASCO/CAP guidelines (3) and the results of these stains are used to guide clinical management in the majority of cases, we considered the results from the core biopsy specimens as the “true” standard for each case. 2) Unlike experimental designs using small pieces of tumor,(13-15) we used whole slide sections cut from archived pathology tissue blocks of surgical specimens to mimic routine practice. This design may also have an advantage over using tissue microarrays in cases with patchy distribution of ER expression in that it decreases the likelihood of false results caused by intratumoral heterogeneity. 3) We selected a relatively large set of tumors received from the operating room over 3 months, so that subtle differences in tissue processing and immunohistochemical staining in individual cases would not significantly affect the results. 4) Three observers evaluated the stains on the resected tumor sections, and we used the average score for the ER staining percentage to minimize the impact of interobserver variability on the results. 5) Additionally, one factor known to affect ER staining is fixation time. Although the ASCO/CAP guidelines included a fixation time of 6-72 h in 10% neutral buffered formalin, studies have shown that although the minimum formalin fixation time is most likely critical for accurate ER immunohistochemical results, overfixation, unless it is particularly extended, does not have a significant negative impact on ER staining.(11,15,22-24) Therefore, the few cases with fixation times slightly longer than 72 h in our study cohort should not be concerning. Despite these efforts, our study had intrinsic limitations owing to a lack of control for the interplay among many preanalytical, analytical, and postanalytical variables included in the ASCO/CAP guidelines.
In our study, we compared no change, increase and decrease groups. Authors have previously described intratumoral heterogeneity in ER expression in breast owing to biological heterogeneity.(4,25) Limited tissue sampling using core biopsy may result in underestimation or overestimation of ER expression when immunohistochemistry is performed on breast tumors with such heterogeneity. On the other hand, homogeneous ER expression within a tumor may appear heterogeneous due to the effects of preanalytical, analytical, and postanalytical factors, especially when the staining is performed on two separately processed specimens. When we considered the ER results obtained from core biopsy specimens as the true standards in our study, the assumption was that preanalytical, analytical, and postanalytical factors had minimal adverse impact on these ER results, and therefore, an increase in ER staining in resection specimens would be a reflection of biological heterogeneity in ER expression. Thus, the increase group in our study served as controls for biological heterogeneity. We chose the cutoff of 25% because it was unlikely to observe such a difference because of interobserver variability (less than 10% of the cases in this study had a scored percentage different by greater than 25% among the three observers) and also because in previous studies of preanalytical factors for ER expression, the percentage component of the Q-score method changed every 25%. (13,14,22) Pair-wise comparison of the no change, increase and decrease groups demonstrated no significant association with cold ischemia time. However, a few cases with drastically decreased ER staining were alarming, including the two cases in Table 1, and the case of pleomorphic lobular carcinoma with 80% strong staining in the core biopsy specimen and 30% weak staining in the resection specimen at a cold ischemia time of 174 min. It is difficult to determine whether variation in sampling had played a role in generating discrepancies in ER staining in such cases, but a negative effect of prolonged cold ischemia time on ER expression in these cases cannot be excluded. Although authors have reported that the use of neoadjuvant chemotherapy is associated with altered ER expression in breast tumors, its effect on ER expression remains controversial.(26-30) Because repeat ER staining is necessary for some resected breast tumors after therapy, we included such cases in our study. We observed no statistically significant differences in the change of ER expression between the patients who did and did not receive neoadjuvant chemotherapy.
As in two previous studies showing that ER expression starts to decline at 2 h of cold ischemia with clone 6F11,(13,14) when we grouped our cohort by cold ischemia time (less and greater than 2 h), we also observed a trend of more frequent decreased ER staining in those with the longer ischemia time, consistent with previous observations that prolonged cold ischemia time may have a negative impact on ER staining. Therefore, we agree with the ASCO/CAP recommendation to keep the cold ischemia time as short as possible. However, although keeping the cold ischemia time under 1 h raises no practical issues for core biopsy specimens, for resection specimens it may be difficult for reasons such as transporting specimens in routine clinical practice and those in our institution. The benefit of altering established practices for the purpose of reducing the cold ischemia time should be evaluated carefully. Because the hormonal status of breast tumors is determined using core biopsy specimens in most patients, ER staining is performed on resection specimens in a minority of patients (17 of 97 in our cohort [18%]). As suggested in the present study and previous studies performed by others, with rare exceptions, prolonged cold ischemia time is unlikely to significantly impact breast tumors with high ER expression, whereas occasional tumors with weak or borderline ER expression may be affected the most.(13-15) If this small population can be identified from the results in the core biopsy specimens before surgery, an alternative to reducing the cold ischemia time for all tissue sections is to take a section of the tumor and fix it in formalin before the rest of the tissue is evaluated and sampled. The drawback of this approach is that the section may not have the tumor material most appropriate for ER staining or may lack adequate internal controls. In addition, it may not be applicable when an obvious tumor mass is not identified grossly. Fixing sliced tissue specimen within 1 h of cold ischemia time is not the same as keeping cold ischemia time within 1 h for tissue sections. Because of the mixed fatty and fibrotic nature of breast specimens, our experience demonstrates that a segmental mastectomy specimen can be sliced at 7-mm intervals and a total mastectomy specimen can be sliced at 1.5-cm intervals by an experienced grossing pathologist or physician assistant. Penetration of tissue by formalin takes approximately 1 h per mm, and actual fixation takes much longer.(31,32) Thus, even when submerged in formalin, the center of a tissue slice would stay in an ischemic state without fixation for hours. For the same reason, the thickness of tissue sections placed in cassettes should be limited to 3-4 mm in thickness for less ischemia time and good fixation. However, fixing sliced specimens would apparently have an advantage over fixing whole specimens in terms of shortening cold ischemia time if tissue sections cannot be taken immediately, especially if specimen transportation to a different location is necessary as in some practice settings.
In summary, our study showed good concordance in ER immunohistochemical staining using core biopsy and corresponding resection specimens with a cold ischemia time between 64 min and 357 min mimicking routine clinical practice. We identified a decrease in ER expression resulting in a change in ER staining category in 2 of the 97 patients and a decrease greater than 25% in 9 of the patients. This difference in ER staining was not significantly associated with cold ischemia time. However, we observed a trend of more frequent decrease in ER staining in the subgroup with a cold ischemia time longer than 2 h than in the subgroup with the shorter ischemia time. While we support the ASCO/CAP recommendation to keep the cold ischemia time as short as possible, our findings indicate that prolonged cold ischemia time up to at least 4 h (97% of our cohort) in the practice setting of our institution has minimal clinical impact on ER immunohistochemical expression in breast tumors.
Acknowledgments
We thank Ariana Trevino for her excellent clerical assistance and Kim-Anh Vu for her assistance with the figures. We also thank Donald Norwood for his thorough editing of the manuscript. This project was supported in part by MD Anderson institutional Start-Up Funds (to L.H.) and in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
References
- 1.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. Review. [DOI] [PubMed] [Google Scholar]
- 2.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 3.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–22. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas-Jones AG, Collett N, Morgan JM, Jasani B. Comparison of core oestrogen receptor (ER) assay with excised tumour: intratumoral distribution of Erin breast carcinoma. J Clin Pathol. 2001;54:951–5. doi: 10.1136/jcp.54.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann GB, Fahey VD, Feleppa F, et al. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005;23:5148–54. doi: 10.1200/JCO.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, Tolles J, Cheng H, et al. Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Invest. 2011;91:1253–61. doi: 10.1038/labinvest.2011.75. Epub 2011 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chagpar A, Yen T, Sahin A, et al. Intraoperative margin assessment reduces reexcision rates in patients with ductal carcinoma in situ treated with breast-conserving surgery. Am J Surg. 2003;186:371–7. doi: 10.1016/s0002-9610(03)00264-2. [DOI] [PubMed] [Google Scholar]
- 8.Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14:1458–71. doi: 10.1245/s10434-006-9236-0. Epub 2007 Jan 28. [DOI] [PubMed] [Google Scholar]
- 9.Huo L. A practical approach to grossing breast specimens. Ann Diagn Pathol. 2011;15:291–301. doi: 10.1016/j.anndiagpath.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Von Wasielewski R, Mengel M, Nolte M, Werner M. Influence of fixation, antibody clones, and signal amplification on steroid receptor analysis. Breast J. 1998;4:33–40. [Google Scholar]
- 11.Oyama T, Ishikawa Y, Hayashi M, Arihiro K, Horiguchi J. The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer. 2007;14:182–8. doi: 10.2325/jbcs.976. [DOI] [PubMed] [Google Scholar]
- 12.Brearley N, Kumah P, Bell JA, Wencyk PM, Ellis IO, Pinder SE. Delay in fixation of invasive breast carcinoma: effect on mitotic count, MIB 1, ER and p53 expression. J Pathol. 2001;195:A6(Suppl) [Google Scholar]
- 13.Khoury T, Sait S, Hwang H, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457–67. doi: 10.1038/modpathol.2009.117. [DOI] [PubMed] [Google Scholar]
- 14.Qiu J, Kulkarni S, Chandrasekhar R, et al. Effect of delayed formalin fixation on estrogen and progesterone receptors in breast cancer: a study of three different clones. Am J Clin Pathol. 2010;134:813–9. doi: 10.1309/AJCPVCX83JWMSBNO. [DOI] [PubMed] [Google Scholar]
- 15.Apple S, Pucci R, Lowe AC, Shintaku I, Shapourifar-Tehrani S, Moatamed N. The effect of delay in fixation, different fixatives, and duration of fixation in estrogen and progesterone receptor results in breast carcinoma. Am J Clin Pathol. 2011;135:592–8. doi: 10.1309/AJCPB1RIT5YXMRIS. [DOI] [PubMed] [Google Scholar]
- 16.Yildiz-Aktas IZ, Dabbs DJ, Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol. 2012 Mar 30; doi: 10.1038/modpathol.2012.59. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Puchtler H, Meloan SN. On the chemistry of formaldehyde fixation and its effects on immunohistochemical reactions. Histochemistry. 1985;82:201–4. doi: 10.1007/BF00501395. [DOI] [PubMed] [Google Scholar]
- 18.Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71:224–30. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- 19.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74:1445–51. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–61. [PubMed] [Google Scholar]
- 21.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–21. [PubMed] [Google Scholar]
- 22.Goldstein NS, Ferkowicz M, Odish E, et al. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 2003;120:86–92. doi: 10.1309/QPHD-RB00-QXGM-UQ9N. [DOI] [PubMed] [Google Scholar]
- 23.Tong LC, Nelson N, Tsourigiannis J, Mulligan AM. The effect of prolonged fixation on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast cancer: a prospective study. Am J Surg Pathol. 2011;35:545–52. doi: 10.1097/PAS.0b013e31820e6237. [DOI] [PubMed] [Google Scholar]
- 24.Arber DA. Effect of prolonged formalin fixation on the immunohistochemical reactivity of breast markers. Appl Immunohistochem Mol Morphol. 2002;10:183–6. doi: 10.1097/00129039-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Chung GG, Zerkowski MP, Ghosh S, et al. Quantitative analysis of estrogen receptor heterogeneity in breast cancer. Lab Invest. 2007;87:662–9. doi: 10.1038/labinvest.3700543. [DOI] [PubMed] [Google Scholar]
- 26.Piper GL, Patel NA, Patel JA, Malay MB, Julian TB. Neoadjuvant chemotherapy for locally advanced breast cancer results in alterations in preoperative tumor marker status. Am Surg. 2004;70:1103–6. [PubMed] [Google Scholar]
- 27.Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST) Anticancer Res. 2008;28:1797–804. [PubMed] [Google Scholar]
- 28.Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005;446:489–96. doi: 10.1007/s00428-005-1244-0. Epub 2005 Apr 19. [DOI] [PubMed] [Google Scholar]
- 29.Adams AL, Eltoum I, Krontiras H, Wang W, Chhieng DC. The effect of neoadjuvant chemotherapy on histologic grade, hormone receptor status, and HER2/neu status in breast carcinoma. Breast J. 2008;14:141–6. doi: 10.1111/j.1524-4741.2007.00544.x. Epub 2008 Jan 31. [DOI] [PubMed] [Google Scholar]
- 30.Gong Y, Han EY, Guo M, Pusztai L, Sneige N. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117:705–13. doi: 10.1002/cncr.25506. Epub 2010 Oct 11. [DOI] [PubMed] [Google Scholar]
- 31.Fox CH, Johnson FB, Whiting J, et al. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 32.Helander KG. Kinetic studies of formaldehyde binding in tissue. Biotech Histochem. 1994;69:177–9. doi: 10.3109/10520299409106282. [DOI] [PubMed] [Google Scholar]