Abstract
Inactivation of TRPV1 receptors is one approach to analgesic drug development. However, TRPV1 receptors exert different effects on each modality of pain. Because muscle pain is clinically important, we compared the effect of TRPV1 ligands on musculoskeletal nociception to that on thermal and tactile nociception. Injected parenterally, capsaicin had no effect on von Frey fiber responses (tactile) but induced a transient hypothermia and hyperalgesia in both the tail flick (thermal) and grip force (musculoskeletal) assays, presumably by its agonistic action at TRPV1 sites. In contrast, RTX produced a chronic (>58 days) thermal antinociception, consistent with its reported ability to desensitize TRPV1 sites. In the same mice, RTX produced a transient hypothermia (7 h) and a protracted (28 day) musculoskeletal hyperalgesia in spite of a 35.5% reduction in TRPV1 receptor-immunoreactivity in muscle afferents. Once musculoskeletal hyperalgesia subsided, mice were tolerant to the hyperalgesic effects of either capsaicin or RTX while tolerance to hypothermia did not develop until after three injections. Musculoskeletal hyperalgesia was prevented but not reversed by SB-366791, a TRPV1 antagonist, indicating that TRPV1 receptors initiate but do not maintain hyperalgesia. Injected intrathecally, RTX produced only a brief musculoskeletal hyperalgesia (2 days) after which mice were tolerant to this effect.
Perspective
The effect of TRPV1 receptors varies depending on modality and tissue type such that RTX causes thermal antinociception, musculoskeletal hyperalgesia, and no effect on tactile nociception in healthy mice. Spinal TRPV1 receptors are a potential target for pain relief as they induce only a short musculoskeletal hyperalgesia followed by desensitization.
Keywords: TRPV1, musculoskeletal hyperalgesia, desensitization, RTX, capsaicin, thermal hyperalgesia, pain, nociception
Introduction
The transient receptor potential vanilloid-1 receptor (TRPV1), a non-selective cation channel, was first cloned by Caterina et al. and described as a heat sensor and transducer of thermal nociception.7 TRPV1 receptors are expressed all over the body including the brain, spinal cord and peripheral nervous system43,53,62 where they exert their nociceptive function through activation of primary afferent C-fibers22 and Aδ-fibers13 innervating the skin and muscles.22,23,40,61 TRPV1 is crucial to the development of many human conditions and animal models of hyperalgesia.12,16,54 In fact, TRPV1 receptor expression is increased in painful diseases like fibromyalgia,41 irritable bowel syndrome, 8 vulvodynia,65 mastalgia,20 and fibrosarcoma.36
Information about musculoskeletal pain is important because of its prevalence in the global population.5,70,72 Approximately 39% of men and 45% of women report chronic musculoskeletal pain,71 describing it as dull and aching rather than epicritic.44 A variety of modulators of primary afferent C-fiber activity (e.g. lactate and ATP), are poised to serve as endogenous mediators of musculoskeletal pain.40 Muscle pain is modulated by the same type of TRPV1 receptor-expressing C- and Aδ-primary afferent fibers that transmit thermal and mechanical nociceptive signals to the muscle as well as to the skin.13,30,31 In support of this, capsaicin injected into the masseter muscle of rats induces a tactile mechanical hyperalgesia (measured using von Frey fibers) that is prevented by the TRPV1 receptor antagonists capsazepine and AMG9810.52 Thus, while TRPV1 receptors play a crucial role in thermal hyperalgesia,28,29,45,49 their role in tactile mechanical hyperalgesia has been disputed with claims of no effect,3,4 an antinociceptive effect9,24 and additional claims of a hyperalgesic effect.28,64,68 With regard to muscle pain in particular, capsazepine also abolishes mechanical hyperalgesia produced by electrically-induced eccentric exercise of the gastrocnemius muscle of rats measured using the Randall-Selitto apparatus16 suggesting an association with muscle fatigue.
Although informative, previous studies concerning TRPV1 receptors and muscle pain only measured muscle sensitivity to pressure (tactile sensitivity) applied to the muscle. They do not address the deep dull muscle pain that accompanies muscle use. To address this, we hypothesized that TRPV1 receptor activity modulates musculoskeletal nociception in mice, as measured using the grip force assay. In this assay, the force generated when animals grip a wire grid is measured; decreases in their ability to hold onto the grid reflect either muscle pain or weakness. We examined the ability of the TRPV1 receptor agonist capsaicin,7 the receptor desensitizer RTX63 and the TRPV1 receptor antagonist SB-366791 [N-(3-methoxyphenyl)-4-chlorocinnamide]21,50,66 to influence grip force responses in mice. We differentiated pain from weakness by the ability of morphine to reverse decreases in grip force. Based on the presence of TRPV1 receptor-expressing interneurons in the spinal cord,26,38 we also examined their possible role in the transmission of nociception from muscles by assessing the effect of intrathecally (i.t.) injected RTX on musculoskeletal nociception.
Methods
Animals
Adult female Swiss Webster mice (Harlan Sprague Dawley Inc., Indianapolis IN) weighing 20–25 g were housed five per cage and allowed to acclimate for at least one week prior to use. Mice were allowed free access to food and water, and housed in a room with a constant temperature of 23°C on a 12-h light–dark cycle. Females were used to explore movement-evoked painful disorders and to reflect the higher incidence of musculoskeletal pain in most anatomic sites in females than in males when studied in humans.71 Because fluctuations in sex hormones were not the focus of our study, the estrus cycle of each mouse was not examined. All experimental procedures and measurements were done blinded to the treatment and performed according to the guidelines of the International Association for the Study of Pain, the University of Minnesota Animal Care and Use Committee, and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication NIH 78-23, revised 1995).
Drugs and chemicals
Capsaicin, a TRPV1 ligand that activates TRPV1 sites at low doses and desensitizes them at higher doses, was purchased from Sigma Chemical Company (St. Louis, MO). Capsaicin was dissolved in sesame oil prior to each use and delivered subcutaneously (s.c.) at a dose of 2 mg/kg.
Resiniferatoxin (RTX), a TRPV1 ligand that is reported to rapidly desensitize TRPV1 receptors with little or no agonistic activity, was obtained from LC Laboratories, Inc. (Woburn, MA) and delivered s.c. at doses of 0.1 mg/kg or 0.02 mg/kg in sesame oil or i.t. at a dose of 0.125 μg/animal dissolved in 5% Tween and 5% DMSO. SB-366791, a TRPV1 antagonist, was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY) and injected intraperitoneally (i.p.) at a dose of 0.5 mg/kg. This dose was based on one previously found to be effective by its ability to inhibit the number of eye wipe responses produced by application of capsaicin to the eye and to block capsaicin-induced hypothermia in rats.66 Due to its low solubility, SB-366791 was dissolved in 40% DMSO and injected 20 min before capsaicin or RTX. Injections made i.t. in mice were delivered at approximately the L5–L6 intravertebral space using a 30-gauge, 0.5 inch disposable needle on a 50 μL Luer tip Hamilton syringe in lightly restrained, unanaesthetized mice.25 Injections made s.c. were delivered in the medial lower back of the animal to avoid injection into muscle responsible for either grip force or von Frey fiber responses. Morphine sulfate, a μ opioid receptor agonist, was purchased from Mallinckrodt (St. Louis, MO). Morphine sulfate was dissolved in saline (pH 5.0) and injected i.p. at doses from 10 to 60 mg/kg, a range of doses that induce a potent antinociception in the tail flick assay in mice. For each drug treatment, control mice were injected with the same amount of vehicle.
Grip force assay
Forelimb grip force was measured using a grip force apparatus as described in rats33 and mice.34,39,67 The grip force apparatus consisted of a force transducer that is connected to a wire mesh grid (12 × 7 cm2 O.D. with an 0.5-cm square wire grid) and positioned on top of an aluminum frame approximately 30 cm above the bench top. During testing, each mouse was held by its tail and gently passed in a horizontal direction over the wire grid until it grasps the grid with its forepaws. The peak force that is exerted by the forelimbs of each mouse when pulling on the grid was recorded by the force transducer, to which the grid is attached. Two grip force measures were obtained at each time-point; the average of these measurements was used to represent each animal’s forelimb grip force at that particular time. Animals were familiarized with the grip force apparatus for three days by grip force testing prior to initiation of the experiment. On the third day, grip force measurements were obtained prior to each intervention to establish baseline values for each animal. Then the drug was administered and grip force measured at the times indicated. Grip force data are represented as raw data and expressed in terms of grams (g).
Tail flick assay
Animals were manually restrained and the tail submerged to a distance of 1 cm from the base of the tail in a water bath maintained at 49–51°C. The withdrawal latency was defined as the time for the animal to withdraw its tail from the water. To avoid tissue damage, cut-off times of 10–15 s were utilized.
Von Frey fiber assay
Mechanical sensitivity to a von Frey fiber (Semmes-Wein standard nylon filament #4.17 equivalent to 1.4 g) was measured in mice prior to and after s.c. injection of RTX or capsaicin. The responses to the von Frey fiber prior to injection did not differ amongst groups. After injection, mice were placed on a wire mesh under glass 6-ounce (177 ml) custard cups that prevent escape but allow movement of all four limbs and head. The fiber was applied to the plantar surface of each hind paw, to the point of bending, for a total of 10 applications/paw. A positive response was defined as a brisk shaking or licking of the paw. The number of positive responses elicited from both paws out of 20 was recorded as the mechanical sensitivity.
Body temperature measurement
Body temperature measurement took place in a room with an ambient temperature of 25°C. The animal was removed from it s cage, placed under a clean towel to create a barrier on three sides, and its colonic temperature measured using a rectal thermometer (ETI Microtherma 2K Thermometer connected to a RET-3 rectal probe).
Retrograde tracing and immunohistochemistry
Mice were deeply anesthetized with isoflurane and a small incision made in the calf to expose the gastrocnemius muscle. A total volume of 10 μl of 4% fluorogold (FG, Fluorochrome LLC, Denver CO) in distilled water was injected in the medial, proximal and distal regions of the muscle using a Hamilton syringe with a 31-gauge needle. Care was taken during these injections to prevent leakage of tracer into surrounding tissues by injecting slowly and wiping excess. The skin was then sutured and mice placed in a temperature-controlled cage. After recovery, mice were returned to their home cage and allowed food and water ad libitum. Some mice were injected first with FG and then 5 days later with RTX (0.1 mg/kg s.c.), and finally killed 2 days after RTX. Other mice, injected first with RTX and then 2 days later with FG, were killed 5 days after the FG injection.
Mice were deeply anesthetized with sodium pentobarbital (65 mg/kg i.p.) and perfused through the heart with phosphate buffered saline (PBS, 0.1 M, pH 7.4) followed by fixative (4% paraformaldehyde, 0.4% picric acid, pH 6.9) at room temperature. DRG from the lumbar region (L3–L5) were extracted and stored in 10% sucrose in PBS until sectioned. Each DRG was sectioned at a plane that is parallel to its widest axis on a cryostat (15μ thickness) and thaw mounted on gelatin-coated slides. From each DRG, every fourth section was taken for analysis, totaling 10–15 sections per DRG. From the sections taken, those with the highest number of fluorogold projections were used for the analyses, resulting in 5 sections counted for L3 and for L4 DRG and 3 sections were counted for L5 DRG such that equal numbers of sections were used from each DRG at each level (L3–L5) in all animals. Entire sections were counted and counting was done in a blinded fashion. Tissue was washed in blocking solution (0.1 M PBS, 0.3% Triton X-100, 10% normal donkey serum) for 1 h at room temperature then incubated overnight with the primary antibody (rabbit anti-TRPV1 C-terminus [1:1000 in blocking solution], Neuromics, Minneapolis MN). Following incubation with the primary antibody, the tissue was rinsed with PBS and incubated for 4 hr with the secondary antibody (Cy3-conjugated AffiniPure donkey anti-rabbit IgG [1:200 in PBS], Jackson Immunoresearch, West Grove PA). The tissue was then dehydrated in ascending concentrations of alcohol, cleared in xylene and coverslipped with DPX (Fluka, Milwaukee WI). Tissue sections were visualized under a fluorescence microscope, and images were captured with a cooled CCD camera system (Dage model CCD-300T-IFG, Michigan City IN).
Statisitical analyses
Mean values (± S.E.M.) are presented throughout the figures. Statistical analysis of the results was performed using Student’s unpaired t-test between two groups or ANOVA followed by post hoc Bonferroni’s or Newman-Keuls’ test for comparison between groups, as indicated. A difference was considered significant if the probability that it occurred because of chance alone was less than 5% (P<0.05).
Results
TRPV1 agonists decreased grip force responses
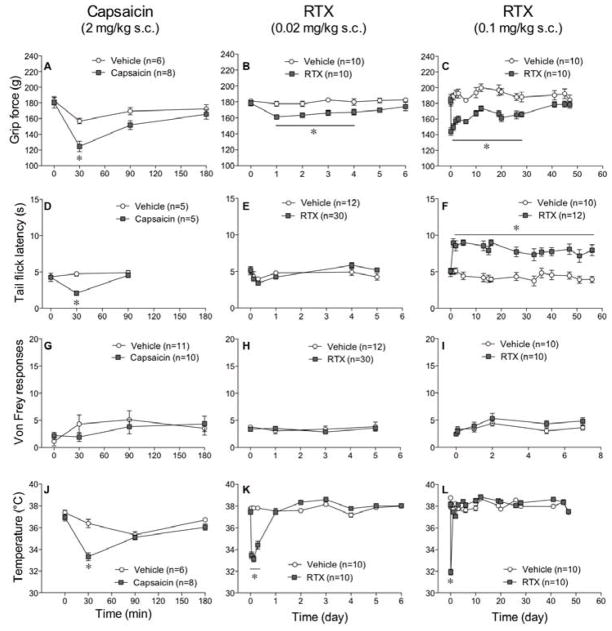
A single injection of capsaicin (2 mg/kg s.c.) at a dose in the range reported to induce hypothermia,48,51 decreased grip force responses (Fig 1A), tail flick latencies (Fig 1D), and body temperatures (Fig 1J) when compared to vehicle-injected control mice. All changes induced by capsaicin were maximal 30 min after injection and faded between 90 to180 min later (Fig 1A, 1D, 1J). In spite of the decrease in grip force responses, there was no effect of capsaicin on von Frey fiber responses (Fig 1G), indicating that musculoskeletal nociception, measured using grip force, differs from tactile mechanical nociception, measured using von Frey fiber fibers, in their sensitivity to TRPV1 ligands. These data indicate that the decrease in grip force does not result from increased tactile sensitivity of the surface of their paws, which would have been reflected in an increased sensitivity to von Frey fibers.
Figure 1.
A single injection of capsaicin or RTX decreases grip force but causes variable responses in tail flick latencies, von Frey fiber responses and body temperature. Mice were injected s.c. with 2 mg/kg of capsaicin (A, D, G, J), 0.02 mg/kg of RTX (B, E, H, K), or 0.1 mg/kg of RTX (C, F, I, L). Grip force (A, B, C), tail flick (D, E, F), von Frey (G, H, I) measurements, and body temperature (J, K, L) were recorded. Statistical analyses were performed using a two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis where the asterisk indicates P<0.05 when compared to vehicle.
Responses to a low dose of RTX (0.02 mg/kg s.c.) differed from those produced by capsaicin in that the decrease in grip force (Fig 1B) persisted longer (4 days) in spite of its lack of effect on responses in the tail flick assay (Fig 1E) or on von Frey fiber sensitivity (Fig 1H). Biologic activity at this dose of RTX was confirmed by its ability to induce a transient decrease in body temperature that lasted for 7 hr (Fig 1K). A higher dose of RTX (0.1 mg/kg s.c.) induced hyperalgesia that persisted for 28 days (Fig 1C), had no effect on von Frey fiber sensitivity (Fig 1I), and produced a persistent (>56 days) increase in tail flick responses (Fig 1F), consistent with desensitization of TRPV1 sites along thermal nociceptive pathways. The decrease in body temperature after injection of this higher dose of RTX (31.91±0.26°C, n=10) was only sl ightly greater than that following the lower dose (33.14±0.28°C, n=10) and l asted for the same amount of time (7 hr) as that following the lower dose (Fig 1L). The effect of RTX on grip force (28 days) was in marked contrast to its much shorter duration of action on body temperature (<24h) and its much longer duration on thermal nociception (>56 days) when measured in the same groups of mice.
Tolerance developed to TRPV1 agonist activity
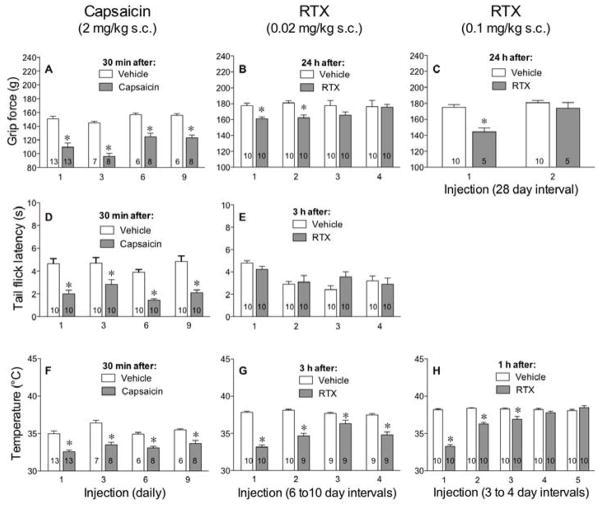
When delivered daily at a dose of 2 mg/kg s.c., tolerance to the effects of capsaicin, including the decrease in body temperature and musculoskeletal hyperalgesia, were not observed (Fig 2A, 2D, 2F). This may be due to the short duration of capsaicin’s effect and the long interval (24 h) between challenges.
Figure 2.
Unlike capsaicin, multiple injections of RTX cause tolerance to its effect on grip force but variable effects on tail flick and body temperature. Mice were injected daily with 2 mg/kg of capsaicin (A, D, F), a low dose (0.02 mg/kg) of RTX every 6–10 days (B, E, G), or high dose (0.1 mg/kg) of RTX (C, H). Grip force (A, B, C) and tail flick (D, E) and body temperature (F, G, H) measurements were recorded after each injection, as indicated. Statistical analyses were performed using the unpaired Student’s t-test where the asterisk indicates P<0.05 when compared to vehicle on the same day.
We then tested the ability of a lower dose of RTX (0.02 mg/kg s.c.) to desensitize TRPV1 receptors along musculoskeletal pathways and thus relieve muscle pain. The dose was chosen for its lack of thermal antinociception and shorter musculoskeletal hyperalgesia compared to the high dose of RTX (0.1 mg/kg s.c.). RTX at the lower dose had no effect on tail flick latencies, even when delivered at 6–10 day intervals. However, the low dose of RTX produced musculoskeletal hyperalgesia and tolerance developed to this hyperalgesic effect by the third injection when administered at 6- to 10-day intervals (Fig 2B). This variable interval was required to allow mice to recover from hyperalgesia produced by the previous injection before delivering subsequent injections. In the same mice, the low dose of RTX decreased body temperature but no tolerance developed to this effect when injected 4 times at 6- to 10-day intervals (Fig 2G).
After the musculoskeletal hyperalgesia dissipated following injection of the higher dose of RTX, a second injection failed to produce hyperalgesia. This indicates that tolerance developed to the hyperalgesic effect of the higher dose of RTX after a single injection (Fig 2C). In contrast, tolerance to the decrease in body temperature produced by the higher dose was not apparent until the fourth injection (Fig 2H). At that time, the high dose of RTX injected s.c. increased body temperature (38.6±0.22°C, n=10) 24 h after its 4th injection when compared to vehicle-injected control mice (37.8±0.21°C, n=10, Student’s t-test, P=0.012). Together these data indicate that TRPV1 receptors supporting musculoskeletal hyperalgesia (grip force) do not desensitize as fast as those supporting thermal nociceptive fibers (tail flick), however, they desensitize much faster than those supporting thermoregulatory sensory fibers (body temperature).
After 4 injections of 0.02 mg/kg of RTX at 6- to 10-day intervals, mice were cross-tolerant to the hyperalgesic effect of capsaicin (Fig 3A) as well as to the high dose RTX (Fig 3B). When tested in the tail flick assay for thermal nociception, four injections of the low dose of RTX prevented the thermal hyperalgesic effect of capsaicin (Fig 3C) and attenuated but did not prevent the thermal antinociceptive effect of a high dose RTX (Fig 3D). These data demonstrate that a low dose of RTX can desensitize TRPV1 receptors along musculoskeletal and thermal nociceptive pathways while sparing thermoregulatory pathways from complete TRPV1 receptor desensitization that leads to a tonic increase in body temperature (Fig 2G).
Figure 3.
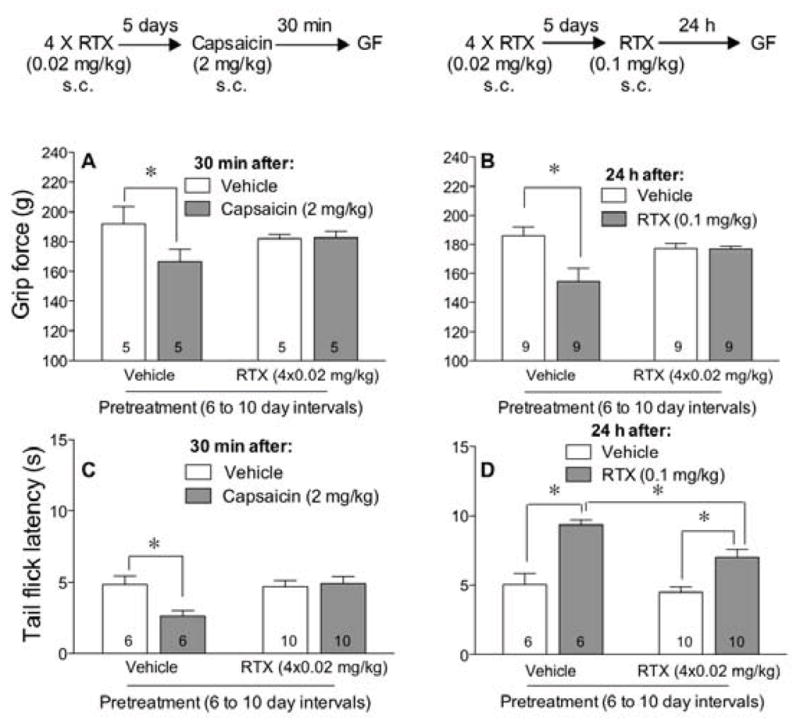
Repeated injections of a low dose of RTX decreases sensitivity of TRPV1 receptors to capsaicin and to RTX. Animals were injected s.c. 4 times with 0.02 mg/kg of RTX at 6- to 10-day intervals and 5 days later challenged with either 2 mg/kg of capsaicin (A, C) or 0.1 mg/kg of RTX (B, D). Grip force (A,B) and tail flick (C, D) measurements were taken 30 min after capsaicin or 24 h after RTX. Statistical analyses were performed using a two-way ANOVA followed by Bonferroni’s post hoc analysis where the asterisk reflects P<0.05 when compared to the values indicated.
Morphine-attenuated hyperalgesia
To confirm that the decrease in grip force responses resulting from injection of capsaicin was due to hyperalgesia and not weakness, mice were injected with various doses of morphine 15 min prior to injection of capsaicin or vehicle and grip force was measured 30 min later (Fig 4A). Morphine prevented the capsaicin-induced decrease in grip force responses in a dose-dependent manner (Fig 4A).
Figure 4.
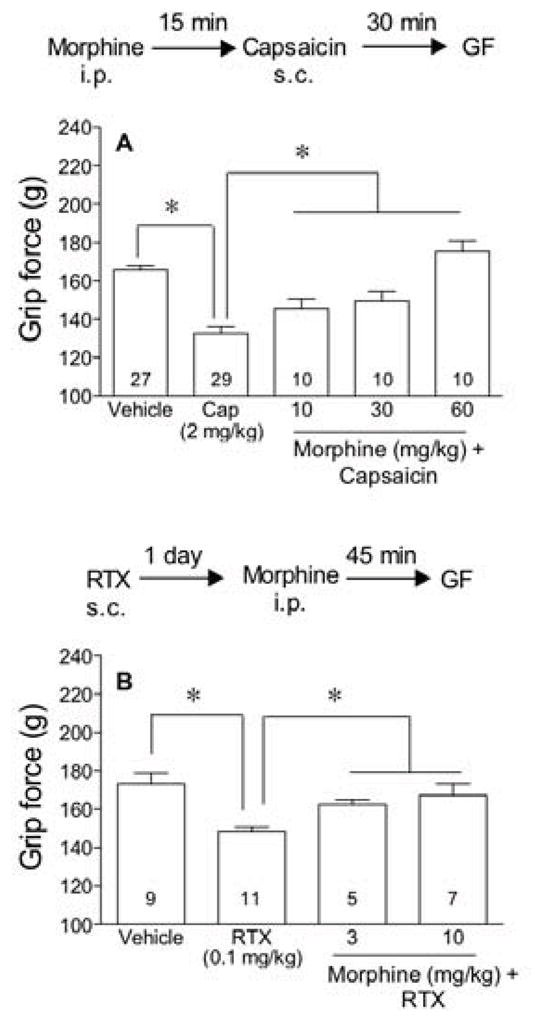
Morphine prevents and reverses the decrease in grip force produced by capsaicin (A) or RTX (B). Grip force measurements were taken 45 min after the i.p injection of morphine and 30 min after the s.c. injection of either capsaicin (2 mg/kg) or 24 h after the injection of RTX (0.1 mg/kg). Statistical analyses were performed using a one-way ANOVA followed by Newman-Keuls’ post hoc analysis where the asterisk represents P<0.05, when compared to the values indicated.
To test the effect of morphine on RTX-induced hyperalgesia, we initially injected mice with RTX (0.1 mg/kg s.c.) and 24 h later, after hyperalgesia was present, mice were injected intraperitoneally with increasing doses of morphine or vehicle. Grip force measurements were recorded 45 min after morphine or vehicle. Morphine reversed the RTX-induced decrease in grip force (Fig 4B).
Musculoskeletal hyperalgesia is inhibited by a TRPV1 receptor antagonist
To verify that RTX produces its hyperalgesic effect via the capsaicin-sensitive receptor (TRPV1), mice were pretreated with SB-366791, a TRPV1 antagonist21 (0.5 mg/kg i.p.) and 20 min later injected with capsaicin (2 mg/kg s.c.) or RTX (0.1 mg/kg s.c.) or their vehicle. SB-366791 attenuated capsaicin- and RTX-induced musculoskeletal hyperalgesia in the grip force assay (Fig 5A, 5B). When the same dose of SB-366791 was injected 8 days after RTX (0.1 mg/kg s.c.), it failed to reverse the hyperalgesic effect of RTX (Fig 5C).
Figure 5.
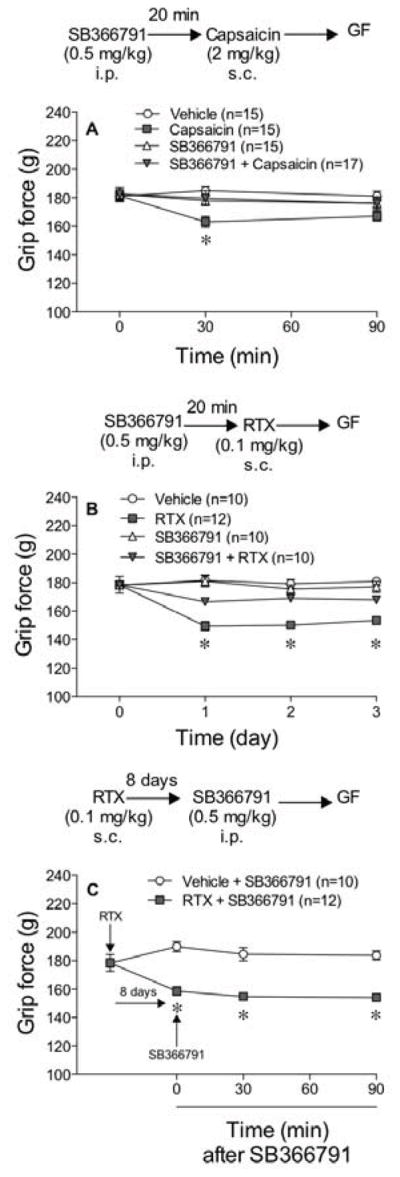
TRPV1 antagonist (SB-366791) prevents the musculoskeletal hyperalgesia produced by capsaicin and by RTX. Animals were injected with SB-366791 (0.5 mg/kg i.p.) and 20 min later they were injected s.c. with either 2 mg/kg of capsaicin (A) or 0.1 mg/kg of RTX (B). Grip force was measured 30 min after capsaicin and 24 hr after RTX. In panel C, mice were injected with SB-366791 at this same dose 8 days after the injection of RTX and grip force was measured at the times indicated. Statistical analyses were performed using a two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis where the asterisk indicates P<0.05 when compared to all the other values at that time.
Intrathecal RTX
To assess the importance of centrally located TRPV1 receptors in musculoskeletal hyperalgesia, mice were injected intrathecally with RTX (0.125 μg/animal i.t.). Grip force values, von Frey fiber sensitivity, and tail flick latencies were measured at the times indicated in figure 6. Intrathecal RTX decreased grip force values for 2 days (Fig 6A) suggesting musculoskeletal hyperalgesia. Von Frey fiber responses in RTX-pretreated mice did not differ from those in vehicle-injected control mice (Fig 6B) suggesting a lack of tactile hyperalgesia. Tail flick latencies in RTX-pretreated mice were increased (Fig 6C) reflecting thermal antinociception that persisted for the 14 days measured. In addition, RTX injected i.t. increased body temperature (38.33±0.13°C, n=19) 24 h after its injection when compared to vehicle-injected control mice (37.50±0.13°C, n=21, Student’s t-test, P=8.5 e−5).
Figure 6.
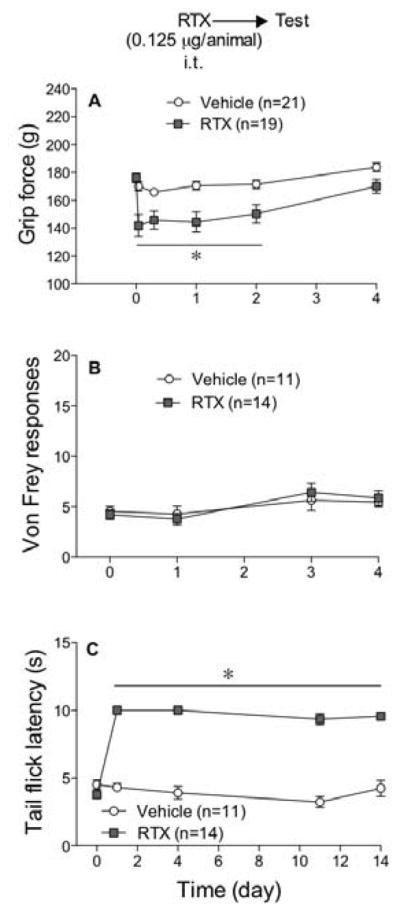
Intrathecal (i.t.) injection of RTX produced a transient (2 day) musculoskeletal hyperalgesia. Mice were injected i.t. with 0.125 μg/animal of RTX or vehicle and grip force (A), von Frey fiber (B) and tail flick (C) responses were measured at the times indicated. Statistical analyses were performed using a two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis where the asterisk indicates P<0.05 when compared to vehicle controls measured at the same time.
When musculoskeletal hyperalgesia subsided after the intrathecal injection of RTX, mice were challenged with RTX. Mice were not only tolerant to the hyperalgesic effect produced by a second injection of RTX injected i.t. (Fig 7A), they were also tolerant to the hyperalgesic effect of a systemic dose of RTX (0.1 mg/kg) injected s.c. (Fig 7B).
Figure 7.
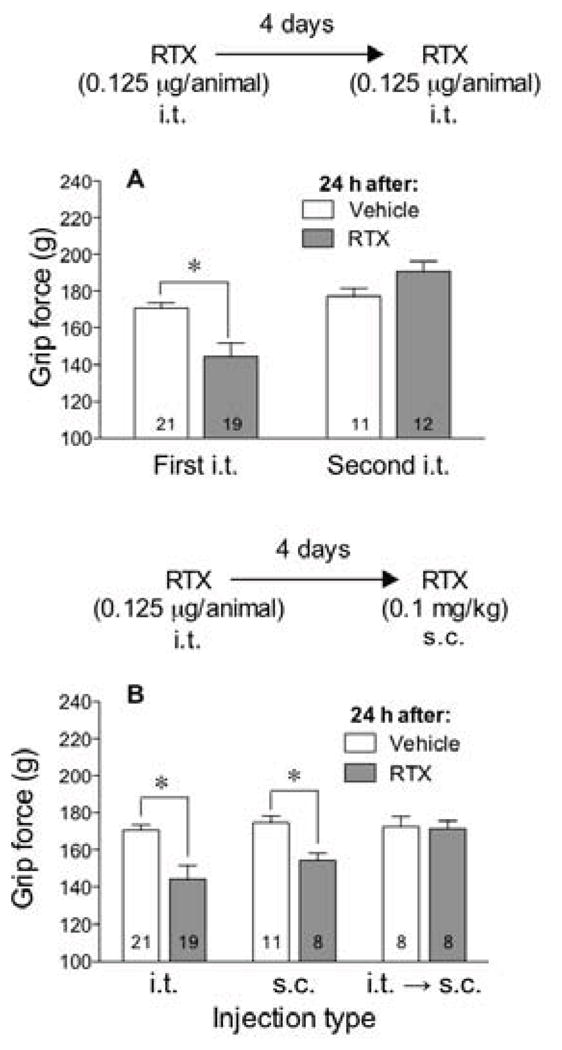
Intrathecal (i.t.) injection of RTX resulted in tolerance to the hyperalgesic effect of RTX injected i.t. or s.c. In panel A, mice were injected i.t. with 0.125 μg/animal of RTX or vehicle 4 days prior to a second challenge with the same i.t. dose of RTX or vehicle and grip force measured 24 h after each injection. In panel B, mice were injected with 0.125 μg/animal of RTX i.t. and 4 days later challenged with 0.1 mg/kg of RTX or vehicle injected s.c. and grip force measured 24 h later. Statistical analyses were performed using an unpaired Student’s t-test where the asterisk indicates P<0.05 when compared to vehicle-injected controls.
Effect of RTX on skeletal muscle afferents expressing TRPV1 receptor immunoreactivity
To determine the effect of RTX on muscle afferents, mice were injected with RTX (0.1 mg/kg s.c.) prior to or after injections of FG into the gastrocnemius muscle. In one group of animals, FG was injected 5 days before RTX (FG→TRX) to assess numbers of DRG (L3–L5) cells innervating muscle without the influence of RTX. In a second group of mice, FG was injected after RTX (RTX→FG) to test whether prior exposure to RTX affected the ability to detect DRG cells retrogradely labeled from muscle. When compared to vehicle-treated mice, the FG→RTX and the RTX→FG mice did not differ from each other in the total number of FG-labeled neurons, indicating that RTX did not produce its musculoskeletal hyperalgesia through degeneration of TRPV1-expressing neurons (Fig 8D). However, RTX down-regulated TRPV1 receptor-immunoreactivity in neurons innervating the gastrocnemius muscle by 35.5% as documented by the decrease in number of cells stained with both FG plus TRPV1 receptor-immunoreactivity in the group injected with RTX compared to those in vehicle-injected controls (Fig 8E).
Figure 8.
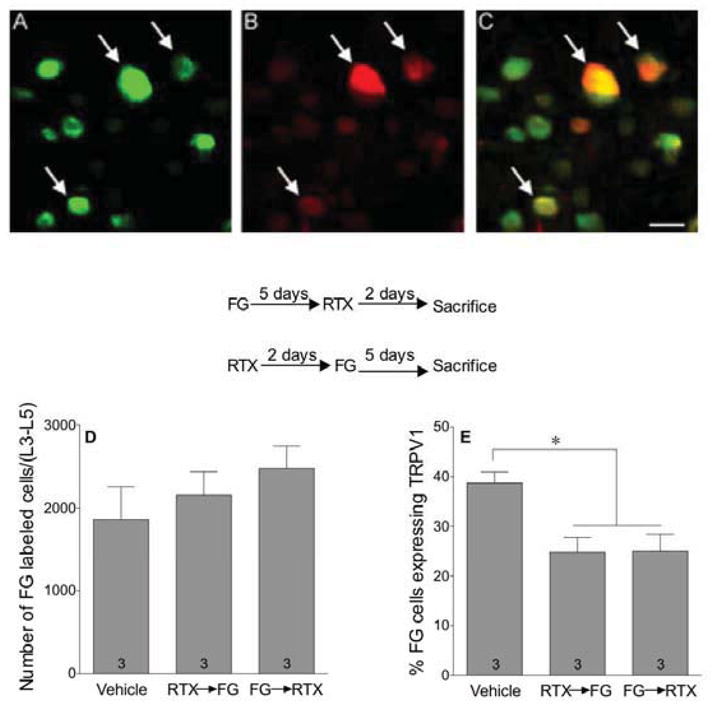
RTX decreased TRPV1 receptor-immunoreactivity in afferents projecting to skeletal muscle without causing their degeneration. Mice were injected with fluorogold (FG 4%, 10 μL in the gastrocnemius muscle) either 5 days before (FG→RTX) or 2 days after (RTX→FG) injection of RTX (0.1 mg/kg s.c.). Representative pictures from an L4 DRG of a vehicle-injected mouse showing cells that are (A) retrogradely labeled with fluorogold (green), (B) immunoreactive for TRPV1 receptors (red), (C) and double-labeled for both fluorogold and TRPV1 receptors (Yellow). Arrows indicate fluorogold-labeled cells that are also immunopositive for TRPV1 receptors. Scale bar=20 μ. The total number of FG-labeled projections in the DRG (L3–L5) was counted (D), as well as the percentage of FG projections that were TRPV1 receptor-immunopositive (E). Statistical analyses were performed using a one-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc analysis where the asterisk indicates P<0.05 when compared to vehicle.
Discussion
In healthy animals, TRPV1 receptors play a crucial role in thermal hyperalgesia28,29,45,49 and in thermoregulation.17,18 Following inflammation or nerve injury, they also mediate tactile mechanical hyperalgesia (von Frey fiber) 28,38,64,68,69 suggesting that these receptors are upregulated on injury.2,37,73,74 Our results confirm the hypothermic effect of TRPV1 ligands, their antinociceptive effect in the tail flick assay, and their lack of effect on tactile mechanical sensitivity in healthy normal mice. In addition, we found a unique role for TRPV1 receptors in modulating musculoskeletal nociception as measured using the grip force assay in healthy normal mice. Specifically, administration of RTX induced a prolonged musculoskeletal hyperalgesia, even at a low dose that produced no thermal antinociception. These data document an important distinction between the modulation of mechanical nociception when measured using the von Frey fiber assay (tactile) compared to that when measured using the grip force assay (musculoskeletal). They also suggest that muscle pain may be a side effect of some TRPV1 receptor ligands developed for their analgesic activity for cancer and inflammatory pain.
TRPV1 receptors are usually activated by low doses of capsaicin resulting in thermal hyperalgesia and hypothermia18,57 whereas higher doses desensitize these sites.35 In contrast, RTX may briefly activate TRPV1 receptors but then desensitize them for much longer periods of time.60 Sometimes RTX desensitization is so rapid that little to no receptor activation is observed in models of thermal nociception.59 Our data indicate that the rate of TRPV1 receptor desensitization depends on its anatomical location and function. For example, TRPV1 sites on thermoregulatory afferents were extremely resistant to desensitization requiring 3 to 4 parenteral injections of even high doses of RTX. In contrast, TRPV1 sites along thermal nociceptive pathways were very sensitive to desensitization requiring just one injection of RTX to produce a protracted (>56 days) thermal antinociception. TRPV1 receptors along musculoskeletal pathways are also desensitized after a single injection of RTX, however, the initial activation of the receptor appears to be responsible for the protracted hyperalgesia.
In our hands, musculoskeletal hyperalgesia produced by RTX was prevented by prior desensitization or pharmacologic antagonism of TRPV1 receptors. However, once established, hyperalgesia was no longer sensitive to TRPV1 receptor antagonists suggesting that the hyperalgesic effect is maintained by a mechanism that no longer requires TRPV1 receptor activity. When initiated by TRPV1 receptor activity, activation of ERK is believed to participate in the transition from acute hyperalgesia to chronic hyperalgesia.56,75 ERK is generally activated in cases of peripheral inflammation and nerve injury.10,56,75 For example, the ability of TRPV1 receptors to initiate but not maintain chronic hyperalgesia is characteristic of a rodent model of pancreatitis56 in which caerulein-induced pancreatic hyperalgesia is attenuated by a TRPV1 antagonist only within 3 weeks of caerulein treatment. The failure of the antagonist to reverse hyperalgesia thereafter was attributed to increased ERK phosphorylation leading to enhancing expression of other pronoiceptive modulators like BDNF,46,47 prodynorphin, and NK-1 receptors27 rather than TRPV1 receptors.56 A similar mechanism may be responsible for TRPV1 receptor-induced hyperalgesia in muscle.
Muscle pain differs from cutaneous pain in that muscle pain is deep, dull and poorly localized while cutaneous pain is more localized.44 This difference could be attributed to the variation in their innervation by large and small fibers. For instance, about 47% of afferents innervating muscles have myelinated axons compared to 24% of those innervating the skin.11 Approximately 40% of afferents from muscle are TRPV1 receptor immuno-positive compared to an estimated 16% of afferents projecting to skin.11 This may account for the fact that muscle afferents are twice as responsive to TRPV1 receptor activation than those from skin.42 Furthermore, TRPV1 receptors innervating muscle are expressed by sensory neurons with larger soma compared to those innervating skin.11 One might speculate that these differences in innervation may have contributed to the concurrent appearance of musculoskeletal hyperalgesia and thermal antinociception after administration of RTX. The uniqueness of afferents projecting to muscle and their associated TRPV1 populations, may explain the involvement of TRPV1 receptors in some types of musculoskeletal hyperalgesia, including cancer19,29,32 and nerve injury38,64,68,69, but their lack of involvement in others, including carregeenan26 and stress-induced hyperalgesia.1
The rate of desensitization of TRPV1 receptor populations may depend on the strategic survival advantage of maintaining their activity. For example, mammals tend to operate optimally at temperatures dangerously close to lethality.55 For this reason, it is important to maintain normal thermoregulatory function, including sensitivity to increased ambient temperatures that may be deleterious to survival. Because the total absence of TRPV1 receptor activity leads to hyperthermia,17,18 the most advantageous survival strategy is to suppress the development of tolerance to these ligands. Consistent with this, desensitization to the hypothermic effect of RTX required three parenteral injections of 0.1 mg/kg of RTX compared to the single injection that was sufficient to desensitize thermal nociceptors. One might speculate that the slow development of tolerance to RTX by TRPV1 sites in muscle may similarly reflect the importance of detecting muscle pain, helping avoid damage by overuse. The previously reported ability of capsazepine to abolish mechanical hyperalgesia produced by eccentric exercise16 is in agreement with a role for TRPV1 receptor activity in muscle fatigue.
Histologically, RTX is known to reduce TRPV1 receptor-immunoreactivity in lumbar DRG.3,9,24 However, it is unclear whether RTX destroys TRPV1 receptor-expressing afferents or simply downregulates these receptors in muscle. To address this, we traced muscle afferents innervating the gastrocnemius muscle using FG and then stained their cell bodies for TRPV1 receptors. We found that RTX did not affect the total number of afferent fibers projecting to muscles, however, RTX reduced the percentage of muscle afferent cells in DRG expressing the TRPV1 receptor by 35.5% compared to vehicle-treated mice. This argues that RTX did not produce its musculoskeletal hyperalgesia via the degeneration of muscle afferents. Instead, the decrease in percent of cells that were double stained suggests downregulation of TRPV1 receptors on these afferents. Still, it is unclear whether musculoskeletal hyperalgesia results from that desensitization or a persistent activation of the remaining TRPV1 receptors on these fibers. The lack of hyperalgesia after injection of a TRPV1 antagonist and the temporal overlap between RTX-induced thermal antinociception and musculoskeletal hyperalgesia suggest that musculoskeletal hyperalgesia is due to a persistent activation of muscle nociceptors on which TRPV1 sites reside rather by their desensitization.
Central TRPV1 receptors are important for pain transmission.15,38 When RTX is injected intrathecally, it causes a long lasting thermal antinociception in models of inflammatory3,4,26 and cancer pain.6,29 Central RTX also decreases TRPV1 receptor, CGRP, and substance P expression on nerve terminals in the spinal cord without affecting their expression in DRG.3,26 RTX also prevents activation of the ERK pathway in spinal cord-injured mice.14 Because the receptor population appears to differ in the cord than in the periphery, we studied the effect of intrathecal RTX on musculoskeletal hyperalgesia to see if spinal receptors respond differently to RTX than those in the periphery. Initially, RTX injected i.t. produced an acute (2 days) musculoskeletal hyperalgesia, however when that subsided, mice were fully tolerant to the hyperalgesia produced by either central or systemic injections of RTX, indicating receptor desensitization. As a result of this shorter hyperalgesic episode, central targeting of TRPV1 receptors may provide a better method for pain therapy than parenteral injections, consistent with previous proposals.3,6,15,26,28,38,58
In conclusion, activation and desensitization of TRPV1 receptor populations on afferent fibers lead to different effects depending on their location and the type of tissue they innervate. In healthy normal control mice, tactile mechanical nociception is not mediated by TRPV1 activity whereas musculoskeletal nociception is enhanced by peripherally administered RTX, inducing a surprisingly protracted hyperalgesia. TRPV1 receptors in the spinal cord desensitize more rapidly than those in the periphery, minimizing the musculoskeletal hyperalgesia and making this a better route for clinical pain relief for these drugs.
Acknowledgments
Funding source:
This work was funded by NIH Grant AT056092 from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdelhamid RE, Kovacs KJ, Pasley JD, Nunez MG, Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology. 2013;72:29–37. doi: 10.1016/j.neuropharm.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, Robinson PP. Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. Eur J Pain. 2007;11:192–201. doi: 10.1016/j.ejpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Bishnoi M, Bosgraaf CA, Premkumar LS. Preservation of acute pain and efferent functions following intrathecal resiniferatoxin-induced analgesia in rats. J Pain. 2011;12:991–1003. doi: 10.1016/j.jpain.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishnoi M, Bosgraaf CA, Abooj M, Zhong L, Premkumar LS. Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: role of transient receptor potential vanilloid 1(TRPV1) and inflammatory mediators. Mol Pain. 2011;7:52. doi: 10.1186/1744-8069-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks PM. The burden of musculoskeletal disease--a global perspective. Clin Rheumatol. 2006;25:778–781. doi: 10.1007/s10067-006-0240-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 8.Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006;95:3086–3096. doi: 10.1152/jn.01343.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Willcockson HH, Valtschanoff JG. Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthritis Cartilage. 2009;17:244–251. doi: 10.1016/j.joca.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 13.Churyukanov M, Plaghki L, Legrain V, Mouraux A. Thermal detection thresholds of Adelta- and C-fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PLoS One. 2012;7:e35817. doi: 10.1371/journal.pone.0035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz CD, Charrua A, Vieira E, Valente J, Avelino A, Cruz F. Intrathecal delivery of resiniferatoxin (RTX) reduces detrusor overactivity and spinal expression of TRPV1 in spinal cord injured animals. Exp Neurol. 2008;214:301–308. doi: 10.1016/j.expneurol.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh YL, Chiang H, Lue JH, Hsieh ST. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced neuropathy. Exp Neurol. 2012;235:316–325. doi: 10.1016/j.expneurol.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 26.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanai Y, Hara T, Imai A, Sakakibara A. Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia. J Pharm Pharmacol. 2007;59:733–738. doi: 10.1211/jpp.59.5.0015. [DOI] [PubMed] [Google Scholar]
- 29.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman MP, Hayes SG. Receptor synergy from thin fiber muscle afferents. Focus on “Dorsal Root Ganglion Neurons Innervating Skeletal Muscle Respond to Physiological Combinations of Protons, ATP, and Lactate Mediated by ASIC, P2X, and TRPV1”. J Neurophysiol. 2008;100:1169–1170. doi: 10.1152/jn.90693.2008. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 32.Kawamata T, Niiyama Y, Yamamoto J, Furuse S. Reduction of bone cancer pain by CB1 activation and TRPV1 inhibition. J Anesth. 2010;24:328–332. doi: 10.1007/s00540-010-0919-0. [DOI] [PubMed] [Google Scholar]
- 33.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 34.Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy WR, Vanhove GF, Lu SP, Tobias J, Bley KR, Walk D, Wendelschafer-Crabb G, Simone DA, Selim MM. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain. 2010;11:579–587. doi: 10.1016/j.jpain.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, Hanson AE, Simone DA, Seybold VS. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;27:10289–10298. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB. Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;9:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs KJ, Papic JC, Larson AA. Movement-evoked hyperalgesia induced by lipopolysaccharides is not suppressed by glucocorticoids. Pain. 2008;136:75–84. doi: 10.1016/j.pain.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med. 2011 doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menigoz A, Boudes M. The Expression Pattern of TRPV1 in Brain. J Neurosci. 2011;31:13025–13027. doi: 10.1523/JNEUROSCI.2589-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105:214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- 47.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rami HK, Thompson M, Wyman P, Jerman JC, Egerton J, Brough S, Stevens AJ, Randall AD, Smart D, Gunthorpe MJ, Davis JB. Discovery of small molecule antagonists of TRPV1. Bioorg Med Chem Lett. 2004;14:3631–3634. doi: 10.1016/j.bmcl.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 51.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ro JY, Lee JS, Zhang YP. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Roberts K, Shenoy R, Anand P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J Clin Neurosci. 2011;18:926–932. doi: 10.1016/j.jocn.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 55.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 Antagonists Prevent the Transition of Acute to Chronic Inflammation and Pain in Chronic Pancreatitis. J Neurosci. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 58.Szabo T, Olah Z, Iadarola MJ, Blumberg PM. Epidural resiniferatoxin induced prolonged regional analgesia to pain. Brain Res. 1999;840:92–98. doi: 10.1016/s0006-8993(99)01763-1. [DOI] [PubMed] [Google Scholar]
- 59.Szallasi A, Blumberg PM. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990;47:1399–1408. doi: 10.1016/0024-3205(90)90518-v. [DOI] [PubMed] [Google Scholar]
- 60.Szallasi A, Joo F, Blumberg PM. Duration of desensitization and ultrastructural changes in dorsal root ganglia in rats treated with resiniferatoxin, an ultrapotent capsaicin analog. Brain Res. 1989;503:68–72. doi: 10.1016/0006-8993(89)91705-8. [DOI] [PubMed] [Google Scholar]
- 61.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 62.Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- 63.Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- 64.Tender GC, Li YY, Cui JG. Vanilloid receptor 1-positive neurons mediate thermal hyperalgesia and tactile allodynia. Spine J. 2008;8:351–358. doi: 10.1016/j.spinee.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8:129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 66.Varga A, Nemeth J, Szabo A, McDougall JJ, Zhang C, Elekes K, Pinter E, Szolcsanyi J, Helyes Z. Effects of the novel TRPV1 receptor antagonist SB366791 in vitro and in vivo in the rat. Neurosci Lett. 2005;385:137–142. doi: 10.1016/j.neulet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Wacnik PW, Kehl LJ, Trempe TM, Ramnaraine ML, Beitz AJ, Wilcox GL. Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. Pain. 2003;101:175–186. doi: 10.1016/s0304-3959(02)00312-3. [DOI] [PubMed] [Google Scholar]
- 68.Watabiki T, Kiso T, Tsukamoto M, Aoki T, Matsuoka N. Intrathecal administration of AS1928370, a transient receptor potential vanilloid 1 antagonist, attenuates mechanical allodynia in a mouse model of neuropathic pain. Biol Pharm Bull. 2011;34:1105–1108. doi: 10.1248/bpb.34.1105. [DOI] [PubMed] [Google Scholar]
- 69.Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, Kakimoto S, Aoki T, Matsuoka N. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336:743–750. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- 70.WHO. Global burden of musculoskeletal disease revealed in new WHO report. Bull World Health Organ. 2003;81:853–854. [PMC free article] [PubMed] [Google Scholar]
- 71.Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006;22:717–724. doi: 10.1097/01.ajp.0000210912.95664.53. [DOI] [PubMed] [Google Scholar]
- 72.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BMJ. 2001;322:1079–1080. doi: 10.1136/bmj.322.7294.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zakir HM, Mostafeezur RM, Suzuki A, Hitomi S, Suzuki I, Maeda T, Seo K, Yamada Y, Yamamura K, Lev S, Binshtok AM, Iwata K, Kitagawa J. Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One. 2012;7:e44023. doi: 10.1371/journal.pone.0044023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]