Abstract
Alterations in memory function due to alcohol exposure have been observed in both animal models and human populations. The human literature on neurocognitive consequences of binge alcohol use in emerging adults has not systematically investigated its potential negative impacts on visuospatial memory. For instance, these impacts have not yet been assessed using a human analogue of the Morris Water Maze Task (WMT), a key memory measure in the animal literature. Accordingly, this study compared performance between emerging adult binge drinkers (BD, n=22) and age- and sex-matched light drinkers (LD, n=29) using the Morris WMT, as well as verbal memory using the California Verbal Learning Test (CVLT). Emerging adult BD demonstrated worse performance on verbal learning and memory relative to LD. However, no significant group differences were observed on spatial learning and memory. Furthermore, no sex differences or interactions with drinking status were observed on either memory domain. These data suggest that in emerging adults who are at a heightened risk for alcohol abuse disorders, but who do not yet meet diagnostic criteria, verbal learning is uniquely impacted by the neurotoxic effects of binge drinking, whereas spatial learning is relatively spared between bouts of intoxication.
Keywords: emerging adulthood, binge drinking, spatial memory, verbal memory, WMT, CVLT
Introduction
Alcohol is currently one of the most widely used psychoactive substances and leading cause of serious public health problems [1]. The onset of alcohol use frequently occurs during adolescence, increasing in prevalence from approximately 3% of 12-year olds to 68% of 21-year olds reporting alcohol use [2]. Heavy episodic drinking (binge drinking) reaches a prevalence rate of approximately 39% between 18 to 24 years of age, peaking at 48% at age 21. It is therefore not surprising that the highest rate of alcohol use disorders (AUDs) also occurs within this age range.
The period from 18 to 24 years of age has been referred to as “emerging adulthood”, a time characterized by having greater independence than in adolescence, but less independence than during adulthood. Magnetic resonance (MR) techniques applied to characterize brain development have demonstrated that the rapid structural and functional brain changes occurring during adolescence do not begin to plateau until after age 20, with the majority of the fine-tuning continuing to occur primarily in the frontal and association cortices. Structural brain changes that last into the early 20s are associated with significant cognitive improvements [4,5], with some of the most dramatic improvements in executive functions occurring towards the end of the adolescent period and into emerging adulthood [6–8]. Significant developmental changes also occur in the hippocampus [9,10], suggesting that this region’s increased vulnerability to alcohol may extend into emerging adulthood.
Heavy alcohol consumption has been associated with deficits across several domains of cognition [11–14], with executive functioning and memory domains being the most vulnerable to disruptions by alcohol [15,16]. Binge drinking in young adults (approximately 21 years) has been associated with impairments in cognitive functions linked to the dorsolateral prefrontal cortex, as well as impairments in memory functions linked to the temporal lobe [17]. Adolescents with AUDs examined after three weeks of abstinence demonstrated poorer verbal learning on the California Verbal Learning Test (CVLT) and poorer visual reproduction on the Wechsler Memory Scale (WMS) Visual Reproduction subtest relative to adolescents without AUDs [18]. In 3-week abstinent adult alcoholics, deficits were observed on word recall [19]. In long-term abstinent alcoholics, deficits on spatial processing persisted beyond termination of alcohol use, whereas other domains of cognitive function recovered with extended abstinence [20].
Substantial animal data exist demonstrating that chronic alcohol exposure leads to hippocampal neurodegeneration via disruption in cell proliferation, cell survival and disruption of cell maturation [21]. Preclinical evidence also indicates that while adolescents are less impacted than adults by alcohol-induced sedation, motor-impairment, and hypothermia [22–28], hippocampal long-term potentiation and spatial memory are more impaired in adolescents than in adults [28,29]. Behavioral rodent studies using the classic Morris Water Maze Task (WMT) [30] show that chronic alcohol exposure significantly impairs acquisition and retention of spatial memory in adolescents but not in adults [31,32], and that impairments persist up to 25 days after exposure is terminated [32]. Intermittent binge alcohol exposure also has been shown to produce Morris WMT deficits in adolescent rodents that persist for two weeks after the last exposure [33]. Taken together, evidence from animal studies demonstrates that the hippocampus and memory-related functions are significantly more susceptible to alcohol exposure during the adolescent period than during adulthood.
The human literature on the neurocognitive consequences of alcohol use have not systematically investigated visuospatial memory, despite the large number of animal studies demonstrating alcohol-related hippocampal abnormalities on the Morris WMT [31,32]. Virtual versions of the WMT have been developed and used to assess spatial memory in humans [34–37], with limited data available examining the impact of alcohol on performance. In the single published study, children with fetal alcohol syndrome (FAS) demonstrate impaired spatial performance on the virtual Morris WMT, as measured by decreased time spent searching in the correct quadrant during the probe trial, despite similar motor control, attention and motivation [38].
The objective of the present study was to examine the effects of binge drinking on memory function in an emerging adult population relative to age-matched light alcohol drinkers, using a virtual analogue of the Morris WMT to measure spatial memory and the CVLT to measure verbal memory. Light drinkers were predicted to outperform binge drinkers on both memory tasks. These findings will fill a critical gap in the literature on the neurocognitive consequences of binge drinking on memory function in emerging adults, as well as provide a baseline for understanding the unique vulnerabilities associated with heavy episodic consumption during this age period.
METHOD
Participants
The study sample consisted of 22 binge drinkers (BD, 9 females) and 29 light drinkers (LD, 14 females). Ethnic breakdown of the sample was 62.7% Caucasian (29.4% BD; 33.3% LD). A trained psychologist conducted diagnostic clinical interviews using the Structured Clinical Interview for Diagnostic and Statistical Manual (DSM–IV) Non-Patient Edition (SCID-I/NP [39]). Inclusion criteria for the BD group required that subjects report at least 3 heavy-drinking episodes occurring per month for the past six months. Heavy episodic drinking was defined as at least 4 (women) or 5 (men) standard drinks per drinking occasion within a two-hour period. Inclusion criteria for the LD group required that subjects report drinking less than 3 drinks per occasion on no more than 5 days within a span of 30 days for the duration of the alcohol use history. Exclusion criteria for all participants included history of head injury, loss of consciousness, history of organic mental disorder, seizure disorder or central nervous system disease, or Axis I clinical pathologies (e.g., mood or anxiety disorders). Participant demographics are presented in Table 1.
Table 1.
Demographic, clinical, and alcohol use data
| Demographic | BD (n=22) | LD (n= 29) | p-value |
|---|---|---|---|
| Age | 22.1 ± 1.3 | 21.5 ± 1.7 | ns |
| Body Mass Index | 23.8 ± 2.6 | 22.4 ± 3.0 | ns |
| Years of Education | 15.1 ± 1.2 | 14.6 ± 1.5 | ns |
| Socioeconomic Status | 54.8 ± 9.3 | 49.9 ± 10.8 | ns |
| Family History of Alcoholism | FH + (n=7) FH − (n=15) |
FH + (n=8) FH − (n=21) |
|
| Clinical | |||
| Beck Depression Inventory Total | 2.3 ± 3.0 | 2.7 ± 4.9 | ns |
| Spielberger Trait Anxiety Inventory-State | 1.5 ± 0.4 | 1.5 ± 0.5 | ns |
| Spielberger Trait Anxiety Inventory-Trait | 1.6 ± 0.4 | 1.6 ± 0.4 | ns |
| BIS-Total | 57.2 ± 8.2 | 55.8 ± 10.3 | ns |
| YAACQ-Total | 7.5 ± 5.2 | 2.7 ± 3.9 | <.001 |
| Alcohol Use | |||
| Age of first onset | 17.1 ± 1.7 | 18.0 ± 2.0 | .15 |
| Days since last use | 6.0 ± 4.9 | 12.8 ± 14.6 | .03 |
| Recent use (# drinks) | 5.2 ± 2.2 | 2.3 ± 1.2 | <.001 |
| Avg. # of days of drinking/week, past 3 months | 1.8 ± 1.1 | 0.8 ± 0.7 | <.001 |
| Avg. # drinks consumed/week, past 3 months | 12.9 ± 14.5 | 1.7 ± 1.9 | <.001 |
| Peak drinking age | 19.8 ± 1.3 | 19.4 ± 1.7 | .08 |
| Estimated Blood Alcohol Level | 0.10 ± 0.05 | 0.01 ± 0.02 | <.001 |
| AUDIT | 8.5 ± 4.0 | 3.0 ± 1.9 | <.001 |
Data represent mean values ± SD. Abbreviations: BIS, Barratt Impulsivity Scale; YAACQ, Young Adult Alcohol Consequences Questionnaire
Procedure
Participants were recruited through local advertisement and screened by telephone interview to ensure they met criteria for inclusion in the study. All aspects of the clinical research protocol were reviewed and approved by the Institutional Review Board of McLean Hospital (Belmont, MA, USA). After a complete description of the study, all participants provided written informed consent and received monetary compensation for study completion.
Participants completed urine screening to rule out current psychoactive substance use (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, Biosite, San Diego, CA) and a breath alcohol screen (Alco Sensor II, Intoxicometers Inc.) on study day. Negative results were required for all tests for study inclusion. Alcohol use was assessed using the Time Line Follow-Back (TLFB) procedure [40] and the Alcohol Use Disorders Identification Test (AUDIT [41]). Blood alcohol levels (BALs) were estimated for each day of drinking over the past 30 days using the procedure established by Hustad and colleagues [42] and alcohol-related consequences were assessed using the Young Adult Alcohol Consequences Questionnaire (YAACQ [43]).
Clinical measures
The Barratt Simplified Measure of Social Status (BSMSS) was used to measure socioeconomic status (SES [44]). Clinical measures included Beck Depression Inventory (BDI [45]), the Spielberger Trait Anxiety Inventory (STAI [46]), and the Barratt Impulsivity Scale (BIS [47]).
Neurocognitive measures
A measure of general intellectual ability (IQ) was derived using two of the four subtests (vocabulary and matrix reasoning) from the Wechsler Abbreviated Scale of Intelligence (WASI [48]). Visuospatial ability and spatial perception were assessed using the WASI Block Design subtest [48] and Mental Rotation Task [49].
Virtual Morris Water Maze Task
A PC-compatible laptop was used to administer the virtual WMT (NeuroInvestigations, Inc., Lethbridge, AB, Canada). The virtual environment consisted of a circular pool located in the center of a square room, with four large abstract pictures located on the walls, which served as landmarks. Details of the task have been published previously [36,50]. The platform was always located in the northeast (NE) quadrant for all trials.
Participants completed a training phase that consisted of four visible platform trials in a virtual environment distinct from the experimental virtual environment. Subsequently, participants completed three experimental conditions: Learning (Hidden trials), Retention (Probe trial), and Motor Control (Visible trials). The Learning condition included hidden platform trials (1 trial starting in each quadrant (NW, NE, SW, SE), 4 trials per 4 blocks, 16 learning trials total). The Probe trial lasted for 30 seconds, during which time the platform was removed from the pool, unbeknownst to participants. The Motor Control condition included visible platform trials (1 trial starting in each quadrant (NW, NE, SW, SE), 4 trials per 2 blocks, 8 trials total).
Dependent measures included: swim latency (sec) and percent distance traveled in the target quadrant (NE), each measure was averaged across trials. For the Probe trial, dependent measures included percent distance traveled in the target quadrant (NE), reflecting an assessment of memory retention. Heading error towards the platform was calculated as the angular deviation from a straight path to the center of the platform from the starting position, and was measured at the first occurrence that participant distance was greater than 25% of the pool diameter from the starting position.
Independent raters blind to participant group rated navigation strategies used by binge and light drinkers during the Probe trial: 1) direct strategy, where participants navigated directly to the platform location, or 2) a non-direct strategy, where participants navigated in a circuitous or random route that was not in the direction of the platform quadrant (NE). Pearson’s correlation coefficient (two-tailed) demonstrated significantly high inter-rater reliability for strategy coding, r=.74, p<.001.
California Verbal Learning Test
Participants were administrated the CVLT-II [51], which consisted of a list of 16 items from four different semantic categories (List A) presented on 5 sequential Learning trials, followed by presentation of an interference list, List B. Measures of free recall and semantically-cued recall of List A items were obtained immediately after each Learning trial, after recall of List B (short delay), and after a 20-minute delay (long delay). Participants also completed a Recognition trial by giving a ‘yes’ or ‘no’ response in discriminating List A items from List B and distractor (non-list) items. Semantic clusters were also calculated during free recall trials, based on grouping response order based on semantic categorical properties (e.g., “all items on the list that are things to wear”). The semantic cluster ratio was adjusted for chance and was calculated across Learning trials for List A. The slope of the learning curve was calculated based on number of correctly recalled words on Trial 1 and on Trial 5.
Statistical analyses
SPSS 18.0 (SPSS, Chicago, IL) was used for all statistical analyses (α=.05). All analyses included sex as a covariate, due to the reported sex differences in verbal and spatial learning [52]. Two-way (Group x Trial) repeated measures analyses of variance (ANOVAs) were conducted for swim latency and percent distance traveled in the target quadrant on WMT Learning and Motor Control trials. One-way (Group) univariate ANOVAs were conducted for percent distance in the target quadrant and heading error on the Probe trial. Chi-square non-parametric analyses were conducted to compare navigation strategies employed (i.e., direct strategy versus the non-direct strategy) by each group. Two-way (Group x Trial) repeated measures ANOVAs were conducted for percent correct on CVLT Learning Trials 1–5, and one-way (Group) ANOVAs were conducted for number correct on List A Trial 1 and 2, List B, short and long delay free and cued recall, recognition hits, false alarms, misses and discriminability. A two-way (Group x Trial) repeated measures ANOVA was conducted for chance adjusted semantic cluster ratios (recall strategy) across Trials 1–5 and a one-way (Group) univariate ANOVA was conducted for total semantic cluster ratios for Trials 1–5.
RESULTS
Demographic, Clinical and Cognitive Variables
Clinical and cognitive measures are presented in Table 1. No significant differences in demographic variables were observed between BD and LD. No significant group differences were evident for clinical measures, with the exception of the YAACQ, in which BD had significantly higher scores than LD. Furthermore, BD demonstrated greater scores on all alcohol-related measures except for age of first alcohol use (p=.15) and peak drinking age (p=.08) as compared to the LD group (Table 1). Cognitive measures are presented in Table 2. There were no significant group differences in IQ (BD: 123.3 ± 11.5, LD: 118.1 ± 12.2, not significant (ns)), or on the two measures of visuospatial perception, WASI block design (BD: 57.6 ± 8.5, LD: 57.8 ± 10.0, ns) or mental rotation (BD: 16.8 ± 5.4, 14.6 ± 5.22, ns).
Table 2.
CVLT Performance
| CVLT Measure | BD (n=22) | LD (n= 29) | p-value |
|---|---|---|---|
| Trial 1 | 7.7 ± 1.6 | 9.0 ± 2.5 | .04 |
| Trial 2 | 10.6 ± 2.8 | 12.0 ± 2.2 | .05 |
| Learning Slope | 1.7 ± 0.6 | 1.2 ± 0.4 | <.005 |
| List B | 8.0 ± 2.5 | 8.2 ± 2.7 | ns |
| Short Delay | |||
| Free Recall | 12.9 ± 3.0 | 13.4 ± 2.6 | ns |
| Cued Recall | 13.4 ± 2.9 | 13.9 ± 2.0 | ns |
| Long Delay | |||
| Free Recall | 13.7 ± 2.4 | 14.0 ± 2.2 | ns |
| Cued Recall | 13.7 ± 2.5 | 13.9 ± 2.3 | ns |
| Recognition | |||
| Hits | 15.2 ± 1.2 | 15.8 ± 0.4 | .02 |
| False alarms | 0.81 ± 1.5 | 0.75 ± 1.5 | ns |
| Misses | 0.81 ± 1.2 | 0.21 ± 0.41 | .02 |
| Semantic Clusters (chance adjusted) | |||
| Trial 1 | 1.3 ± 0.3 | 1.6 ± 0.5 | .04 |
| Trial 2 | 10.6 ± 2.8 | 12.0 ± 2.2 | .05 |
| Total Trials 1–5 | 2.7 ± 3.4 | 3.0 ± 4.0 | ns |
Data represent mean values ± SD. CVLT, California Verbal Learning Task.
Virtual Water Maze
Learning – Hidden Platform Trials
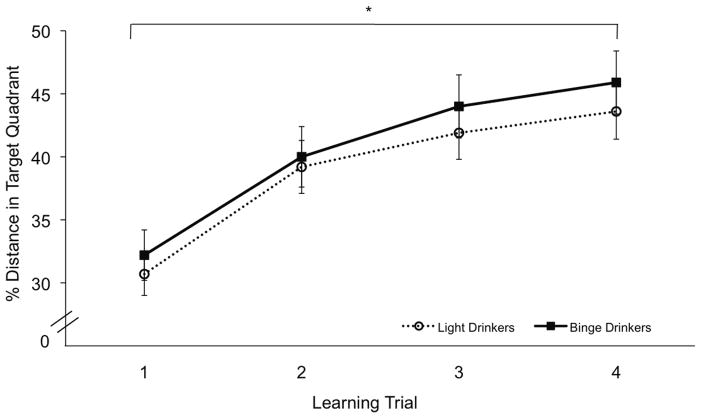
Significant learning was observed, as evidenced by significant main effect of Trial for percent distance traveled in the target quadrant (NE) (F(3,144)=7.5, p<.001). All participants displayed greater percent distances in the target quadrant by Trial 4 (Figure 1). There was no significant main effect or Trial x Group interaction observed for swim latency on learning trials (Trial 1: 17.6 ± 10.1; Trial 2: 12.6 ± 12.5; Trial 3: 11.5 ± 11.1; Trial 4: 11.6 ± 12.0).
Figure 1.
Average percent distance in target quadrant during learning (hidden) trials on the WMT, in LD (open circles, dashed lines) and BD (closed squares, solid line). * p< .05.
Retention – Probe Trial
There was no significant difference between BD and LD for percent distance traveled in the target quadrant (NE) on the Probe trial (BD: 47.9 ± 11.4, LD 45.7 ± 12.6, ns). There was a trend for a difference between group for Heading Error, with BD exhibiting a greater heading error (30.8 degrees (°) ± 25.5) than LD (19.3 ± 19.0), (F(1,47)=3.8, p=.06).
Motor Control – Visible Platform Trials
There was no significant main effect of Trial or Trial x Group interaction observed for swim latency (Trial 1: 4.0 ± 0.9; Trial 2: 3.8 ± 0.6) on Motor Control trials.
Navigation Strategy
LD demonstrated a significant Strategy preference, with a greater percentage of LD subjects utilizing a direct strategy (72%, n=21) than those utilizing a non-direct strategy (28%, n=8) (χ2(1,29)=5.8, p<.05). In contrast, 55% (n=12) of BD subjects utilized a direct strategy and 45% (n=10) of BD subjects utilized a non-direct strategy to reach the platform; these percentages were not significantly different in the BD group (χ2(1,22)=.18, ns).
California Verbal Learning Test
Learning – Recall on Trials 1–5 and List B
Significant learning was observed, as evidenced by a significant main effect of Trial for number of words correctly recalled from Trial 1 to Trial 5 (F(4,188)=22.2, p<.001; Figure 2) across participant groups. There was a significant Group x Trial interaction, with LD recalling a greater number of words than BD (F(4,188)=5.2, p<.005). Post hoc analyses revealed that LD had superior performance on the first two learning trials, Trial 1 (F(1,47)=4.5, p<.05) and Trial 2 (F(1,47)=3.9, p=.05), but that group differences were no longer evident by Trial 3. In addition, learning slope was significantly steeper in BD (1.69 ± 0.58) than LD (1.23 ± 0.45) (F(1,47)=10.2, p<.005). No significant group differences were observed for recall of List B (Table 2).
Figure 2.
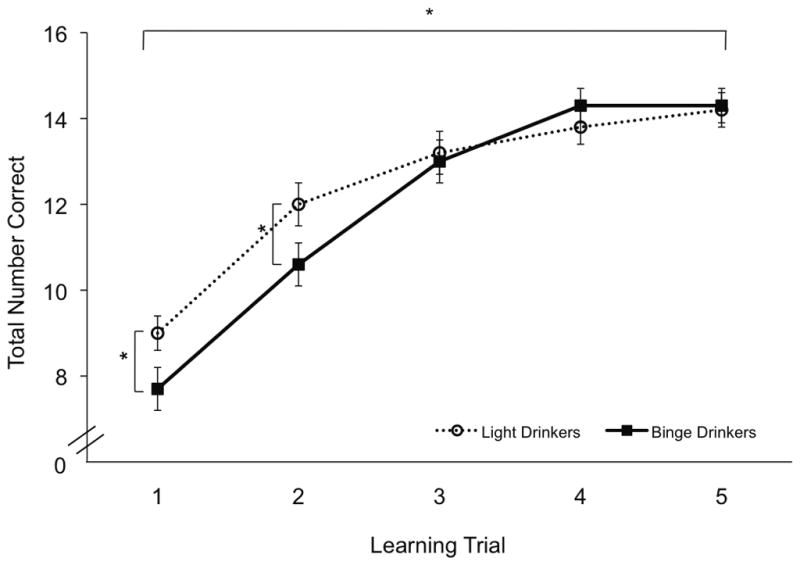
Average total number of words correctly recalled during learning (1–5) trials on CVLT, in LD (open circles, dashed line) and BD (closed squares, solid line). * p< .05.
Retention – Short and Long Delay Recall
There were no significant main effects of Group for short delay free (F(1,47)=0.4, ns) or cued recall (F(1,47)=0.5, ns), and for long delay free (F(1,47)=0.1, ns) or cued recall (F(1,47)=0.04, ns) (Table 2).
Recognition
A main effect of Group was evident for Recognition (F(1,47)=5.8, p<.05), with LD correctly identifying more target words than BD. There was a significant main effect for Misses, with more misses observed in BD (0.8 ± 1.2) than in LD (0.2 ± 0.4), (F(1,47)=5.8, p<.05). No significant effects were observed for False alarms (F(1,47)=0.01, ns) or Discriminability (BD: 96.4 ± 5.3, LD: 97.9 ± 3.6, ns) (Table 2).
Recall Strategy
A significant main effect of Trial was observed for semantic cluster ratios across Trials 1–5 (F(4,188)=17.9, p<.001), with increasing ratio scores across trials. While the Trial x Group interaction did not reach significance p=.33), post hoc comparisons demonstrated that BD had significantly lower semantic cluster ratio scores on Trial 1 (F(1,47)=4.5, p<.05) and on Trial 2 (F(1,47)=3.9, p=.05) relative to LD. Significant group differences were no longer evident by Trial 3.
DISCUSSION
The results of the present study demonstrate a differential impairment of memory performance, as evidenced by worse performance on verbal memory, but unimpaired spatial memory, in emerging adult BD compared to LD. These study findings are consistent with previous work by Parada and colleagues [53], in which binge drinking was associated with verbal but not spatial memory deficits in a similarly aged cohort of emerging adults. In the current study, BD demonstrated inferior learning and recognition, relative to light drinking counterparts. Comparable total learning of word lists in the CVLT was observed across groups for learning Trials 1–5. However, there were subtle learning differences observed between groups, whereby BD recalled less words than LD on the first two learning trials, but significant group differences were no longer evident by Trial 3 or for total number of words recalled across all learning trials. The BD group also demonstrated worse recognition than the LD group, with lower recognition scores being due to omission of target words (misses), as opposed commission errors (false alarms). Given that the use of semantic clustering strategies is associated with superior verbal memory performance, it was not surprising that LD exhibited higher semantic cluster scores on the first two learning trials than BD. However, this group difference was no longer evident after the second trial and was not apparent for total cluster score.
In contrast, no performance differences were evident on the spatial memory task in BD compared to LD, which is also consistent with previous findings [53]. Both groups demonstrated similar performance on learning trials (percent distance traveled in the target quadrant and swim latency), as well as retention of the platform location on the Probe trial. This finding is somewhat surprising given that the Morris WMT has been a classic tool for examining the disruptive effects of alcohol on spatial learning and memory in rodents [54]. Differences in spatial memory in this population may manifest primarily as differences in the strategy employed to solve the task [55], as significantly more LD employed a direct navigation route, which relies on a spatial strategy that is the most efficient means to solve the task. For BD, however, approximately half used a direct approach and the other half used a non-direct approach to complete the task. BD performed at the same level as LD, however, despite using a less efficient strategy. Similar findings have been reported in marijuana users on the same virtual Morris WMT [36], in which behavioral performance was similar between groups, but marijuana users demonstrated parahippocampal hypoactivation, suggestive of differences in neuronal resources utilized during memory retrieval. It is possible that in order for BD to perform at the same level as LD, neural compensation is necessary, warranting future investigation of functional activation during spatial memory performance in binge drinkers.
There are limitations that should be considered when interpreting the current study results. Although joystick use or video game experience was not assessed in LD and BD, groups were age-matched and performed similarly on the motor control condition. Nonetheless, future studies of spatial memory on the WMT should account for joystick experience. Second, differences associated with alcohol-related withdrawal could have also affected study findings. Rodent studies have shown that acute withdrawal behavior is observed 9 to 12 hours after ethanol administration, when BAL reaches zero, and lasting up to 18 hours, more than 3 hours after reaching an undetectable BAL [56,57]. Given that a minimum of 48 hours was required between last drink and study participation, and on average days since last alcohol use was 6 days in BD, withdrawal-related effects in the present study were unlikely, but should be accounted for in future work. Finally, while no Sex x BD interactions were observed for hippocampal-based memory performance in the present study, or in previous investigations [17,53], fluctuations in hormone levels associated with menstrual cycle phase could have contributed variability to the study findings, since better spatial ability is evident during the follicular phase of the menstrual cycle in adult women [58]. This is also unlikely, however, given that the majority of women in the current study were tested during the follicular phase (LD: follicular 79%, luteal 14%, unknown cycle status 7%; BD follicular 100%), and that sex was included in the statistical models as a covariate.
In conclusion, this study confirms evidence for impaired verbal learning performance in the absence of spatial memory deficits, measured using the Morris Water Maze task in emerging adults who meet the criteria for heavy episodic consumption. To the extent that rodent studies have demonstrated impaired spatial memory on the Morris WMT after an acute ethanol challenge [59,60] or after chronic intermittent ethanol exposure [61], it is possible that binge-related impairments in spatial memory would have been observed had the current study participants been intoxicated. For instance, Acheson and colleagues [62] found that an acute alcohol challenge impaired semantic (verbal) and figural (spatial) memory, with impairment depending on the age of the subjects; young adults (21–24 years) were more impaired relative to an older group (25–29 years). Given that IQ, vocabulary and visuospatial perception did not differ between groups, these data suggest that pre-existing differences in cognitive functioning were unlikely, and that verbal learning is uniquely sensitive to the neurotoxic effects of binge drinking, whereas spatial learning is relatively spared between bouts of intoxication. It will be important for future studies to determine if verbal memory decrements are persistent by longitudinally investigating if continued binge drinking further impacts memory, if memory impairments recover with prolonged abstinence and if verbal memory impairments are predictive of risk for the manifestation of later alcohol use disorders.
Acknowledgments
This work was supported by NIAAA grants K01 AA014651 and R01 AA018153 (MMS).
Abbreviations
- ANOVAs
analyses of variance
- AUD
alcohol use disorders
- AUDIT
Alcohol Use Disorders Identification Test
- BALs
Blood alcohol levels
- BD
binge drinker
- BDI
Beck Depression Inventory
- BIS
Barratt Impulsivity Scale
- BSMSS
Barratt Simplified Measure of Social Status
- CVLT
California Verbal Learning Task
- DSM–IV
Diagnostic and Statistical Manual
- FAS
fetal alcohol syndrome
- IQ
intellectual ability
- LD
light drinker
- MR
magnetic resonance
- NE
northeast
- ns
not significant
- NW
northwest
- SCID-I/NP
Structured Clinical Interview for Diagnostic and Statistical Manual Non-Patient Edition
- SD
standard deviation
- SE
southeast
- SES
socioeconomic status
- STAI
Spielberger Trait Anxiety Inventory
- SW
southwest
- TLFB
Time Line Follow-Back
- WASI
Wechsler Abbreviated Scale of Intelligence
- WMS
Wechsler Memory Scale
- WMT
Water Maze Task
- YAACQ
Young Adult Alcohol Consequences Questionnaire
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. Institute for Social Research, The University of Michigan; Ann Arbor: 2011. Monitoring the Future national survey results on drug use, 1975–2010: Volume I. [Google Scholar]
- 2.SAMHSA; Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The OAS Report: A Day in the Life of American Adolescents: Substance Use Facts Update. Rockville, MD: 2010. [PubMed] [Google Scholar]
- 3.SAMHSA. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Office of Applied Studies; Rockville, MD: 2010. NSDUH Series H-38A, HHS Publication No. SMA 10-4856 Findings. [Google Scholar]
- 4.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Anderson V. Assessing executive functions in children: biological, psychological, and developmental considerationst. Pediatr Rehabil. 2001;4:119–136. doi: 10.1080/13638490110091347. [DOI] [PubMed] [Google Scholar]
- 7.Rosso IM, Young AD, Femia LA, Yurgelun-Todd DA. Cognitive and emotional components of frontal lobe functioning in childhood and adolescence. Ann N Y Acad Sci. 2004;1021:355–362. doi: 10.1196/annals.1308.045. [DOI] [PubMed] [Google Scholar]
- 8.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Chin VS, Van Skike CE, Matthews DB. Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol. 2010;44:3–14. doi: 10.1016/j.alcohol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- 12.Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institutes of Health; Bethesda, MD: 2000. pp. 437–472. [Google Scholar]
- 13.Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Res Health. 2003;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- 14.Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr. 1990;101:136–155. [PubMed] [Google Scholar]
- 15.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oscar-Berman M, Ellis RJ. Cognitive deficits related to memory impairments in alcoholism. Recent Dev Alcohol. 1987;5:59–80. doi: 10.1007/978-1-4899-1684-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Scaife JC, Duka T. Behavioural measures of frontal lobe function in a population of young social drinkers with binge drinking pattern. Pharmacol Biochem Behav. 2009;93:354–362. doi: 10.1016/j.pbb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- 19.Schottenbauer MA, Hommer D, Weingartner H. Memory deficits among alcoholics: performance on a selective reminding task. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:505–516. doi: 10.1080/13825580600681305. [DOI] [PubMed] [Google Scholar]
- 20.Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- 22.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 23.Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- 24.Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- 25.Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 26.Little P, Kuhn C, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat: II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- 28.Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;4:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- 29.Swartzwelder H, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19(6):1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 30.Morris RG. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- 32.Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- 33.Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J Addiction. doi: 10.1155/2013/461029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sneider JT, Sava S, Rogowska J, Yurgelun-Todd DA. A preliminary study of sex differences in brain activation during a spatial navigation task in healthy adults. Percept Mot Skills. 2011;113:461–480. doi: 10.2466/04.22.24.27.PMS.113.5.461-480. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (Clinical Version) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 40.Sobell LM, Sobell MB. Alcohol Timeline Followback User’s Manual. Addiction Research Foundation; Toronto: 1995. [Google Scholar]
- 41.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 42.Hustad JT, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. J Stud Alcohol. 2005;66(1):130–138. doi: 10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 43.Read JP, Kahler CW, Strong DR, Colder CR. Development and preliminary validation of the young adult alcohol consequences questionnaire. J Stud Alcohol. 2006;67:169–177. doi: 10.15288/jsa.2006.67.169. [DOI] [PubMed] [Google Scholar]
- 44.Barratt W. Barratt simplified measure of social status (BSMSS) Indiana State University; 2006. [Google Scholar]
- 45.Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 46.Spielberger CD. STAI: manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- 47.Patton JM, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. The Psychological Corporation, Hartcourt Brace and Company; San Antonio, TX: 1999. [Google Scholar]
- 49.Vanderberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Percept Mot Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- 50.Sneider JT, Cohen-Gilbert JE, Acharya D, Crowley DJ, Covell MJ, et al. Age and sex differences on spatial and verbal memory performance: Evidence from the Morris Water Maze and California Verbal Learning Tasks. Journal of Adolesc Health under review. [Google Scholar]
- 51.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Adult Version. 2. Psychological Corporation; San Antonio: 2000. [Google Scholar]
- 52.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 53.Parada M, Corral M, Caamano-Isorna F, Mota N, Crego A, et al. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res. 2011;35:1475–1484. doi: 10.1111/j.1530-0277.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 54.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 55.Rodgers MK, Sindone JA, Moffat SD. Effects of age on navigation strategy. Neurobiol Aging. 2012;33:215–202. doi: 10.1016/j.neurobiolaging.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCormick CM, Teillon SM. Menstrual cycle variation in spatial ability: relation to salivary cortisol levels. Horm Behav. 2001;39:29–38. doi: 10.1006/hbeh.2000.1636. [DOI] [PubMed] [Google Scholar]
- 59.Chin VS, Van Skike CE, Berry RB, Kirk RE, Diaz-Granados J, et al. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–483. doi: 10.1016/j.alcohol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Matthews DB, Morrow AL. Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus. 2000;10:122–130. doi: 10.1002/(SICI)1098-1063(2000)10:1<122::AID-HIPO13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 61.Van Skike CE, Novier A, Diaz-Granados JL, Matthews DB. The effect of chronic intermittent ethanol exposure on spatial memory in adolescent rats: the dissociation of metabolic and cognitive tolerances. Brain Res. 2012;1453:34–39. doi: 10.1016/j.brainres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]