Abstract
Aims
We sought to prospectively assess limb volume change (LVC) and associated symptoms in patients with melanoma undergoing sentinel lymph node (SLN) biopsy and/or therapeutic lymph node dissection (TLND).
Methods
Limb volume was measured pre-operatively and post-operatively at 6 and 12 months using a perometer (JUZO 1000M). LVC calculated and used to define 3 groups: <5%, 5-10%, and >10%. A 19-item lymphedema symptom questionnaire was administered at baseline, 6-month and 12-months.
Results
One hundred eighty-two patients were enrolled. Twelve months after axillary surgery, 9% had LVC 5-10%, and 13% had LVC >10%. Twelve months after inguino-femoral surgery, 10% had LVC 5-10%, and 13% had LVC >10%. There was a significant 7- to 9-fold increase in symptoms for patients with LVC greater than >10% compared to those with LVC <5% (P<.05). By multivariate analysis, TLND versus SLN biopsy (odds ratio [OR] = 3.18 P<0.01) and borderline significance for lower-versus upper-extremity procedures (OR=1.72; P=0.07) were associated with LVC >5%.
Conclusion
LVC greater than 5% is common at 12 months following nodal surgery for melanoma and is associated with symptoms. Informed consent for melanoma patients undergoing lymph node surgery should include a discussion of the risks of post-operative lymphedema.
Keywords: melanoma, lymphedema, perometry, symptom assessment
INTRODUCTION
Secondary lymphedema is a complication affecting many patients after surgical resection of primary or regionally-metastatic solid tumors, including melanoma [1]. The clinical impact of lymphedema has been difficult to accurately assess in patients with melanoma of variance in definition and reported incidence [1]. All published studies to date on lymphedema after surgery for melanoma have been retrospective [2-14], and many of those studies have used subjective criteria to define lymphedema [4, 5]. Because of the lack of prospective assessment with objective measurement tools and the lack of consensus about the definition of lymphedema, there remains little understanding about the true incidence of lymphedema in patients who have undergone surgery for melanoma. A delay in diagnosis of lymphedema prevents early treatment of this progressive, morbid condition, and the cascade of associated physical and psychosocial impairments can disrupt recovery and cancer-directed therapies, as has been described in the breast cancer literature [15]. The clinical impact of varying degrees of change in limb volume has only recently been addressed in breast cancer patients [16] and the relationship between the degree of volume change and symptoms in patients with melanoma has not been reported.
The most frequently utilized objective measurement method for assessing lymphedema remains serial limb circumference measurement [3, 6-8, 11, 17]. The limitations of this method are primarily related to the specific anatomic locations measured and the total number of interval measures, as well as inter-observer variability [2, 3, 10, 12]. More recently, an objective measurement method for assessing lymphedema using a perometer, an optoelectronic device that uses infrared light to calculate limb volume, has been applied in clinical and research settings with promising results [16].
The objective of our study was to estimate the incidence of post-operative lymphedema and associated symptoms in patients with stage I to III melanoma at 6 and 12 months after sentinel lymph node (SLN) biopsy and/or therapeutic lymph node dissection (TLND). We assessed limb volume using objective perometry and assessed symptoms using a validated 19-item instrument. We hypothesized that a number of patient-specific and treatment-specific risk factors would be associated with increased risk of lymphedema, including elevated body mass index (BMI), lower-extremity (versus upper-extremity) procedure, extensive tumor nodal burden, and receipt of radiation therapy. We also hypothesized that even patients undergoing SLN biopsy alone would experience lymphedema and associated symptoms, as has been described in the breast cancer literature [18].
MATERIALS AND METHODS
Patients
Patients were eligible for this prospective, longitudinal study if they had a confirmed diagnosis of invasive cutaneous melanoma, stage I to III, without a prior operation on the regional nodal basin at the time of enrollment. In order to participate in the study, patients were required to be fluent in English, be at least 18 years old, and give informed consent. Patients with distant metastatic disease, implanted medical devices, or a concurrent malignancy that required active treatment prior to enrollment in the study were not eligible. Patients with previous limb trauma resulting in disfiguring or insertion of screws or other metal joints were not eligible. All patients were evaluated and followed at The University of Texas MD Anderson Cancer Center. Baseline demographic and clinical data were obtained from each patient at the time of the initial interview. All patients enrolled in the study subsequently underwent SLN biopsy and/or TLND based on standard clinical protocols. Patients returned for follow-up measurements and questionnaire completion at 3-6 months and 9-12 months. Patient identification information was coded to ensure confidentiality. This study was approved by the Institutional Review Board.
Limb Volume Assessment
Limb volume was measured using an onsite Pero-System® 1000M perometer (JUZO, Cuyahoga Falls, OH), an opto-electronic volumetry device that uses infrared light to calculate limb volume [19]. For each patient, limb volume was measured pre-operatively (baseline) and then 6 and 12 months later; both the limb on the affected side and the contralateral limb were measured. Patient measurements were completed of the entire limb (e.g. the lower extremity from the foot to the hip joint and the upper extremity from the hand to the shoulder) at each visit. At the time of limb volume assessment, patients with signs and symptoms of limb swelling and patients with a greater than 10% adjusted increase in the volume of the affected limb from baseline (according to the formula below) were referred for a consultation with a lymphedema healthcare provider while continuing clinical follow-up. Limb volume change (LVC) on the affected side between baseline and follow-up was expressed as a percentage of the baseline limb volume and calculated according to the following formula: LVC = [(I (follow – up) – I (baseline)) – (C (follow – up) – C (baseline))/ I(baseline)] × 100 [18].
In this formula, I indicates the volume of the ipsilateral (affected) limb, and C indicates the volume of the contralateral limb. The change in volume of the contralateral limb was subtracted from the change in volume of the affected limb to account for changes in patient body weight between assessments and then divided by I(baseline) and multiplied by 100%. The adjustment for changes in weight over time based on differences between the ipsilateral (affected) and the contralateral limb is likely underestimated in patients undergoing bilateral procedures. Three LVC groups were defined: less than 5% (no or latent lymphedema[20]), 5% to 10% (mild lymphedema), and greater than 10% (moderate lymphedema)[21]. Although arbitrary, these LVC categories have been shown to be associated with symptom assessment changes in previous longitudinal studies in breast cancer patients [16].
Symptom Assessment
In order to assess for lymphedema-associated symptoms, we used a previously-validated questionnaire, the Lymphedema and Breast Cancer Questionnaire [22], which has been modified to include assessment of lower extremity symptoms. The questionnaire inquires about symptoms including tenderness, swelling, redness, blistering, numbness, stiffness, and tightness, among others. Patients completed the 19-item symptom assessment tool at the pre-operative (baseline) visit and 6 and 12 months later. Scores reported reflect the number of affirmative responses. For each patient and for each follow-up assessment (6-month and 12-month), the change in symptom score between baseline and follow-up was calculated by subtracting the total number of affirmative responses at baseline from the total number of affirmative responses at follow-up.
Statistical Analysis
A mixed longitudinal logistic regression model was used to examine whether the variables age, BMI (kg/m2), upper- versus lower-extremity surgery, and SLN biopsy versus TLND were associated with the magnitude of LVC for patients with LVC at least 5%. Covariates adjusted in the regression model were based on their significance in univariate analyses. Although tumor burden and radiation therapy were included in univariate analysis, they were not found to be statistically significant with LVC >5% and therefore there were not included in the multivariate model. All the reported P values were 2-sided, and values <.05 were considered statistically significant. The statistical analyses were performed using SAS® software, version 9.2 (SAS, Inc., Cary, NC).
RESULTS
Patient Characteristics and Treatment Details
A total of 182 patients had enrolled in the study at the time of this analysis. Characteristics of the patients at baseline are summarized in Table 1. There were slightly more men (52.7%) than women (47.3%), and the majority of the patients (61.5%) were over the age of 50 years. The median weight for the cohort was 85.3 kg, and 75% of the patients were either overweight (38% with BMI ≥25) or obese (37.4% with BMI ≥30). 46.2% of the patients underwent SLN biopsy alone, and 53.8% of the patients underwent TLND with or without a prior SLN biopsy. Most patients (78%), regardless of procedure, had either microscopic or no lymph node disease. Of the 91 patients undergoing TLND, 44 (44.9) had an axillary dissection, while 29 (29.6%) had a superficial inguinofemoral node dissection alone while 25 (25.5%) underwent a deep iliac/obturator node dissection in addition to a superficial node dissection. The majority of the patients (62.6%) were diagnosed with a primary melanoma of the extremity. Only 11% of the patients received radiation therapy as part of their therapy.
Table 1.
Patient and treatment characteristics: overall and stratified by limb volume change
| Overall Patient characteristics (n=182) |
Limb Volume Change at 6 Months (n=154) |
Limb Volume Change at 12 Months (n=117) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| <5% | 5%-10% | >10% | <5% | 5%-10% | >10% | |||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||||
| ≤ 50 | 70 | 38.8 | 37 | 64.9 | 13 | 22.8 | 7 | 12.3 | 27 | 58.7 | 10 | 21.7 | 9 | 19.6 |
| >50 | 112 | 61.5 | 57 | 58.8 | 17 | 17.5 | 23 | 23.7 | 45 | 63.4 | 9 | 12.7 | 17 | 23.9 |
|
| ||||||||||||||
| Gender | ||||||||||||||
| Male | 96 | 52.7 | 52 | 64.2 | 12 | 14.8 | 17 | 21.0 | 41 | 61.2 | 12 | 17.9 | 14 | 20.9 |
| Female | 86 | 47.3 | 42 | 57.5 | 18 | 24.7 | 13 | 17.8 | 31 | 62.0 | 7 | 40.0 | 12 | 24.0 |
|
| ||||||||||||||
| BMI, kg/m2 | ||||||||||||||
| ≤ 24.9 | 44 | 24.6 | 24 | 68.6 | 6 | 17.1 | 5 | 14.3 | 19 | 67.9 | 2 | 7.0 | 7 | 25.0 |
| 25-29.9 | 68 | 38.0 | 35 | 60.3 | 15 | 25.9 | 8 | 13.8 | 26 | 60.5 | 10 | 23.3 | 7 | 16.3 |
| ≥ 30 | 67 | 37.4 | 33 | 56.9 | 8 | 13.8 | 17 | 29.3 | 25 | 56.8 | 7 | 15.9 | 12 | 27.3 |
|
| ||||||||||||||
| Extremity at risk | ||||||||||||||
| Upper extremity | 92 | 50.5 | 55 | 67.1 | 15 | 18.3 | 12 | 14.6 | 47 | 67.1 | 9 | 13.0 | 13 | 18.8 |
| Lower extremity | 90 | 49.5 | 39 | 54.2 | 15 | 20.8 | 18 | 25.0 | 25 | 52.1 | 10 | 20.8 | 13 | 27.1 |
|
| ||||||||||||||
| Surgery | ||||||||||||||
| SLN biopsy | 84 | 46.2 | 54 | 71.1 | 12 | 15.8 | 10 | 13.2 | 43 | 70.5 | 9 | 14.8 | 9 | 14.8 |
| TLND | 98 | 53.8 | 40 | 51.3 | 18 | 23.1 | 20 | 25.6 | 29 | 51.8 | 10 | 17.9 | 17 | 30.4 |
| axillary dissection | 44 | 44.9 | 25 | 67.6 | 7 | 18.9 | 5 | 13.5 | 20 | 64.5 | 5 | 16.1 | 6 | 19.4 |
| Superficial | ||||||||||||||
| inguinofemoral | 29 | 29.6 | 9 | 42.8 | 6 | 28.6 | 6 | 28.6 | ||||||
| dissection | 5 | 38.5 | 2 | 15.4 | 6 | 46.1 | ||||||||
| superficial and deep iliac/obturator dissection |
25 | 25.5 | 6 | 30.0 | 5 | 25.0 | 9 | 45.0 | 4 | 33.3 | 3 | 25.0 | 5 | 41.7 |
|
| ||||||||||||||
| Surgery side | ||||||||||||||
| Right | 88 | 48.6 | 48 | 60.8 | 14 | 17.7 | 17 | 21.5 | 36 | 56.4 | 12 | 19.1 | 15 | 23.8 |
| Left | 85 | 47.0 | 44 | 65.7 | 11 | 16.4 | 12 | 17.9 | 32 | 65.3 | 7 | 14.3 | 10 | 20.4 |
| Bilateral | 8 | 4.4 | 2 | 25.0 | 5 | 62.5 | 1 | 12.5 | 4 | 80.0 | 0 | 0.0 | 1 | 20.0 |
|
| ||||||||||||||
| Radiation therapy | ||||||||||||||
| Yes | 20 | 11.0 | 10 | 52.6 | 6 | 31.6 | 3 | 15.8 | 6 | 40.0 | 4 | 26.7 | 5 | 33.3 |
| No | 162 | 89.0 | 84 | 62.2 | 24 | 17.8 | 27 | 20.0 | 66 | 64.7 | 15 | 14.7 | 21 | 20.6 |
|
| ||||||||||||||
| Disease Stage | ||||||||||||||
| Stage I | 62 | 34.1 | 33 | 35.1 | 8 | 26.7 | 11 | 36.7 | 27 | 37.5 | 9 | 47.4 | 4 | 15.4 |
| Stage II | 29 | 15.9 | 11 | 11.7 | 5 | 16.7 | 2 | 6.7 | 9 | 12.5 | 3 | 15.8 | 1 | 3.9 |
| Stage III | 91 | 50.0 | 38 | 40.4 | 14 | 46.6 | 14 | 46.6 | 29 | 40.3 | 6 | 31.6 | 17 | 65.4 |
| Stage IV | 0 | 0.0 | 12 | 12.8 | 3 | 10.0 | 3 | 10.0 | 7 | 9.7 | 1 | 5.3 | 4 | 15.4 |
BMI, body mass index; SLN, sentinel lymph node; TLND, therapeutic lymph node dissection
Limb Volume Change
Follow-up information was available for 154 patients at 6 months and 117 patients at 12 months (Table 1). At both 6 and 12 months, obese patients had a higher incidence of LVC greater than 10% than did patients with lower BMI. Similarly, at both 6 and 12 months, patients with melanomas of the lower extremity had a higher incidence of LVC greater than 10% than did patients with melanomas of the upper extremity, and patients who underwent TLND had a higher incidence of LVC greater than 10% than did patients who underwent SLN biopsy alone. For patients that underwent TLND, there were a higher percentage of patients who had a combined superficial and deep iliac/obturator dissection who met the criteria for mild and moderate lymphedema at 12 months compared to the superficial inguino-femoral node dissection alone. In patients who underwent SLN biopsy alone, 15.8% met the criteria for mild lymphedema (LVC 5%-10%) and 13.2% met the criteria for moderate lymphedema (LVC >10%) at the 6-month follow-up assessment; at the 12-month assessment, 14.8% met the criteria for mild and 14.8% for moderate lymphedema. Radiation therapy was associated with an increased risk of LVC greater than 10% compared to no radiation therapy at 12 months but not at 6 months.
Symptoms and Correlation with LVC
At baseline, the most frequently reported symptoms included tenderness (15.6%), swelling (12.9%), and aching (17.6%). The median baseline symptom score was 0, and 19 out of the 182 patients reported at least one symptom at baseline. The most commonly reported new symptoms at the 6-month follow-up assessment were numbness (42% of patients), firmness/tightness (37%), swelling (31%), and tenderness (25%). At 12 months, the most commonly reported symptoms were the same: numbness (32.4% of patients), swelling (29.8%), firmness/tightness (25.8%), and tenderness (13.5%). In a subset analysis, there were no associations with symptoms at baseline assessment and subsequent LVC or change in symptom scores at 12 months. At both the 6-month and 12-month follow-ups, patients with LVC at least 5% had more lymphedema-associated symptoms than patients with LVC less than 5%, and patients with LVC greater than 10% had the most symptoms (Figure 1a). For all patients the median change in symptom scores between baseline and 6 and 12 months was higher for patients who underwent TLND than those who underwent SLNB(Figure 2). In addition, lower extremity median total symptom scores increased for TLND patients, whereas upper extremity total symptoms and LE SLNB symptoms decreased between 6 and 12 months (Figure 2). Regardless of the site of operation (upper or lower extremity), the median change in symptom score at both 6 and 12 months was higher in patients with greater LVC (Table 2). The median change in symptom score was higher for patients who underwent TLND than for patients who underwent SLN biopsy for both lower- and upper-extremity operations and at both 6-month and 12-month follow-up (Figure 2).
Figure 1.
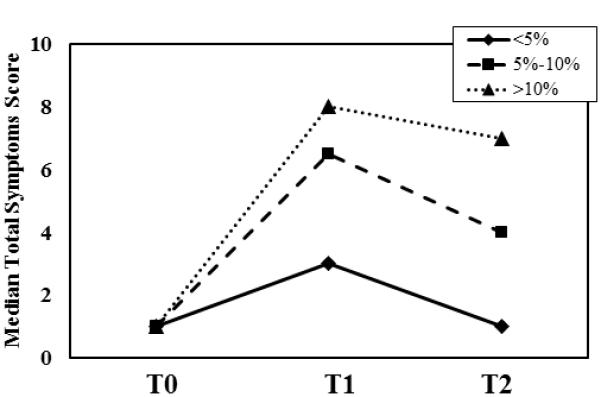
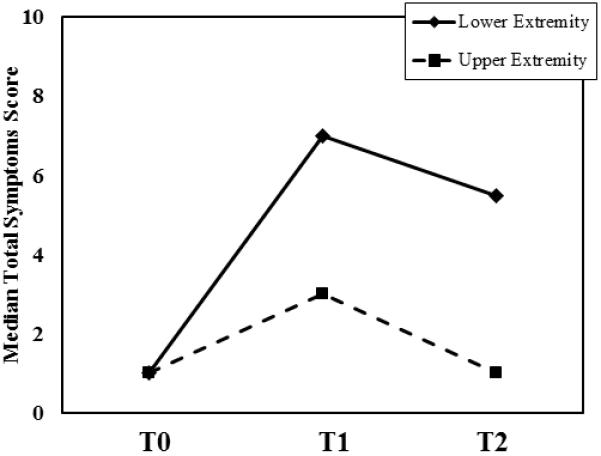
Median symptom assessment scores at baseline (T0), 6 months (T1), and 12 months (T2) stratified by limb volume change expressed as a percentage of the limb volume at baseline (a) and by site of surgery (b).
Figure 2.
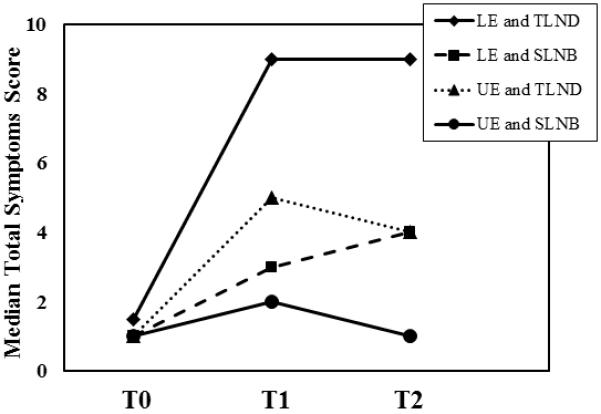
Median symptom assessment scores at baseline (T0), 6 months (T1), and 12 months (T2) stratified by site of surgery and type of operation. LE, lower extremity; UE, upper extremity; SLNB, sentinel lymph node biopsy; TLND, therapeutic lymph node dissection
Table 2.
Change in symptom score stratified by limb volume change
| Limb Volume Change at 6 Months (n=154) | Limb Volume Change at 12 Months (n=117) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| <5% (n=94) | 5%-10% (n=30) | >10% (n=30) | <5% (n=72) | 5%-10% (n=19) | >10% (n=26) | |||||||||||||||||||
|
| ||||||||||||||||||||||||
| No. | % | Median ΔSS (Range) |
No. | % | Median ΔSS (Range) |
No. | % | Median ΔSS (Range) |
N | o. | % | Median ΔSS (Range) |
No. | % | Median ASS | No. | % | Median ΔSS (Range) |
||||||
| Upper extremity | ||||||||||||||||||||||||
| SLN biopsy | 28 | 67.5 | 0.5 | (−1-6) | 7 | 16.7 | 1.5 | (0-3) | 7 | 16.7 | 2 | (0-7) | 26 | 72.2 | 0 | (−1-5) | 4 | 11.1 | 1.5 | (−2-4) | 6 | 16.7 | 0 | (0-4) |
| TLND | 27 | 67.5 | 3 | (−2-10) | 8 | 20.0 | 4.5 | (0-8) | 5 | 12.5 | 7 | (0-11) | 21 | 63.6 | 1 | (−2-7) | 5 | 15.2 | 0 | (0-5) | 7 | 21.2 | 7 | (1-11) |
|
| ||||||||||||||||||||||||
| Lower extremity | ||||||||||||||||||||||||
| SLN biopsy | 22 | 81.5 | 1 | (−2-7) | 4 | 14.8 | 0.5 | (−2-4) | 1 | 3.7 | 3 | (3-3) | 16 | 80 | 0 | (−2-5) | 4 | 20 | 0.5 | (0-9) | 0 | 0.0 | - | - |
| TLND | 17 | 37.8 | 5 | (−3-11) | 11 | 24.4 | 4.5 | (−1-11) | 17 | 37.8 | 6 | (1-13) | 9 | 32.1 | 7 | (−3-11) | 6 | 21.4 | 6.5 | (2-11) | 13 | 46.4 | 6 | (−1-12) |
|
| ||||||||||||||||||||||||
| Primary site on extremity |
||||||||||||||||||||||||
| Yes | 60 | 61.9 | 1 | (−2-11) | 17 | 17.5 | 2 | (−2-11) | 20 | 20.6 | 5.5 | (0-13) | 46 | 60.5 | 0 | (−2-11) | 15 | 19.7 | 2 | (−2-11) | 15 | 19.7 | 4 | (−1-12) |
| No | 34 | 59.7 | 3 | (−3-10) | 13 | 22.8 | 2 | (0-9) | 10 | 17.5 | 3 | (0-11) | 26 | 23.4 | 0 | (−3-9) | 4 | 9.8 | 1 | (0-4) | 11 | 26.8 | 3 | (0-9) |
|
| ||||||||||||||||||||||||
| SLN positive | ||||||||||||||||||||||||
| Yes | 29 | 54.7 | 3.5 | (−3-11) | 13 | 24.5 | 4 | (0-10) | 11 | 20.8 | 6 | (1-10) | 21 | 55.3 | 1 | (−3-9) | 6 | 17.8 | 2 | (0-9) | 11 | 29.0 | 6 | (−1-9) |
| No | 65 | 64.4 | 1 | (−2-9) | 17 | 16.8 | 2 | (−2-11) | 19 | 18.8 | 3 | (0-13) | 51 | 64.6 | 0 | (−2-11) | 13 | 16.5 | 2.5 | (−2-11) | 15 | 19.0 | 3 | (0-12) |
|
| ||||||||||||||||||||||||
| LN disease | ||||||||||||||||||||||||
| Microscopic | 77 | 64.2 | 1 | (−3-11) | 24 | 20 | 2 | (−2-10) | 19 | 15.8 | 4 | (0-10) | 62 | 66.7 | 0 | (−3-9) | 14 | 15.1 | 1.5 | (−2-9) | 17 | 18.3 | 3 | (−1-9) |
| Clinical | 17 | 50 | 4 | (0-9) | 6 | 17.7 | 5 | (−1-11) | 11 | 32.4 | 5 | (0-13) | 10 | 41.7 | 5 | (0-11) | 5 | 20.8 | 5 | (0-11) | 9 | 37.5 | 6 | (1-12) |
ΔSS, change in symptom score; LN, lymph lode; NA, not available; SLN, sentinel lymph node; TLND, therapeutic lymph node dissection.
Patients with a primary melanoma on the extremity and patients with a primary melanoma on the head or neck or trunk had similar changes in symptom score at both 6 and 12 months (Table 2). Clinically evident nodal disease was associated with higher median change in symptom score than microscopic nodal disease at 12 months (Table 2).
Multivariate Regression Analysis
On univariate analysis, risk factors associated with LVC greater than 5% included older age (>50 years), obesity (BMI >30), lower-extremity (vs. upper-extremity) procedure, and TLND (vs. SLN biopsy). On multivariate regression analysis, TLND (vs. SLN biopsy) was significantly associated with LVC greater than 5% (odds ratio [OR], 3.18; 95% CI, 1.75-5.78; P<0.01) (Table 3). Lower-extremity procedures were also associated with increased risk for LVC greater than 5% compared to upper-extremity procedures, but this difference was not statistically significant (OR, 1.72; 95% CI, 0.96-3.07; P<0.07).
Table 3.
Logistic regression model of factors associated with increased risk of limb volume change
| OR | 95% CI |
P Value |
|
|---|---|---|---|
| Age, years (>50 vs. ≤ 50) | 1.06 | 0.58-1.93 | 0.85 |
| BMI, kg/m2 (≥ 30 vs. <30) | 1.03 | 0.56-1.92 | 0.92 |
| Lower vs. upper extremity | 1.76 | 0.98-3.14 | 0.06 |
| TLND vs. SLN biopsy | 2.43 | 1.36-4.35 | <0.01 |
BMI, body mass index; OR, odds ratio; SLN, sentinel lymph node; TLND, therapeutic lymph node dissection.
DISCUSSION
In this study, we prospectively and objectively assessed lymphedema and associated symptoms at 6 and 12 months after surgery in a series of patients with stage I to III melanoma using LVC as a surrogate for lymphedema. Overall, at 12 months we found that, moderate lymphedema (LVC >10%) was common in patients who underwent SLN biopsy only (14.8%), as well as in patients who underwent TLND (30.4%). Our hypothesis that patient- and treatment-specific factors would be associated with higher incidences of LVC was confirmed; multivariate regression analysis identified TLND (vs. SLN biopsy) and lower extremity procedures (vs. upper extremity) as independent risk factors associated with increased risk of LVC >5% (Table 3). With regards to lymphedema-associated symptoms, we found that lower-extremity procedures were associated with increases in patient-reported symptoms, regardless of whether SLN biopsy only or TLND was performed and irrespective of the magnitude of LVC (Table 2, Figure 1b). Median change in symptom scores increased in all patient subgroups examined as early as 6 months, even in patients with LVC less than 5% (Figure 1a). Together, these data confirm our additional hypothesis that lymphedema occurs and its associated symptoms can be clinically significant in patients undergoing SLN biopsy alone.
Definitions of lymphedema vary within the literature, but generally, the threshold for a diagnosis of lymphedema is a greater than 10% to 20% change in limb volume for water displacement studies and a girth increase of greater than 2 cm at any location on a limb for circumference measurements. For this study, lymphedema was defined as LVC greater than 5% because this threshold corresponded to an increase in symptoms from baseline that varied for those with and without LVC. In addition, this LVC threshold has been identified as clinically relevant for breast cancer patients in contemporary studies [16].
Among patients who underwent TLND, at 12 months, the incidence of moderate lymphedema was higher among patients who underwent lower-extremity procedures than among those who underwent upper-extremity procedures (27.1% vs. 18.8%). Previous studies using objective measures have reported that the incidence of lymphedema in melanoma patients ranges between 7% and 17% when water displacement methods are used [3, 23] and 1% and 12.5% when circumference measurement is used [6-8, 24]. Our findings are consistent with the literature in that lower-extremity procedures (particularly inguino-femoral dissection) have been reported to be associated with higher incidences of lymphedema than upper-extremity procedures; reported incidences of lymphedema for lower-extremity procedures are 18% to 58% using water displacement methods [2, 10, 12] and 14% to 61% using circumference measurement [6-9, 11, 13, 17, 24].
A novel aspect of this report is the inclusion and characterization of patients treated with SLN biopsy only. We found that 14.8% of patients treated with SLN biopsy only had moderate lymphedema at 6 months and 14.8% had moderate lymphedema at 12 months (Table 1). When we stratified the patients who underwent SLN biopsy only by the anatomic location of surgery, we found that at 12 months, the percentage of patients meeting the criteria of mild and moderate lymphedema were 13.0% and 18.4%, respectively, among patients who underwent upper-extremity surgery, and 20.8% and 27.1%, respectively, among patients who underwent lower-extremity SLN surgery (Table 1). These rates appear to be higher than the only other reported rates of lymphedema following SLN biopsy, which are from retrospective studies: slight lymphedema (10%-20% water displacement) in 11.3% of patients undergoing upper-extremity surgery and 6% of patients undergoing lower-extremity surgery with unspecified follow-up [2, 23]. However, our findings regarding the incidence of lymphedema following upper-extremity SLN biopsy are consistent with the 1% to 17% reported incidence of lymphedema in patients undergoing SLN biopsy for breast cancer [25, 26].
Our study has a number of strengths and makes novel contributions to the melanoma literature. By using infrared perometry, we were able to objectively quantify the change in limb volume over time in a prospective fashion. To our knowledge, this is the first published report of objective measurement of lymphedema in patients with melanoma undergoing SLN biopsy. In addition, we examined the relationships between the degree of LVC and the increase in the number of lymphedema-associated symptoms (Table 2). We found that in most of the LVC subgroups, symptom scores at both 6 and 12 months were higher than symptom scores at baseline. When we compared our findings to findings from a similar prospective analysis of lymphedema in breast cancer patients undergoing axillary procedures [16], we found similar increases in symptom scores from baseline which corresponded with increasing severity of LVC (Table 2, Figure 1), and we found that the most commonly-reported increased symptoms (numbness, firmness/tightness, swelling, and tenderness) were similar between melanoma and breast cancer patients, as well. In addition, we found significant variability in reported symptoms among patients without mild or moderate LVC changes. This is likely related to post-surgical changes such as scarring which may cause limited movement of the affected extremity as well as residual numbness, tenderness and swelling potentially associated with the surgical procedures required to treat melanoma. Within the melanoma literature, lymphedema-associated symptoms have been variably defined and inconsistently reported; however, the symptoms described in our study largely overlap with the commonly-described symptoms of dysesthesia, perception of swelling, pain, fatigue, and functional limitation [2, 3, 23, 24, 27].
Despite its strengths, our study has some limitations. The first is the relatively small number of patients enrolled to date, which limited analyses of several cohorts, especially at the 12-month follow-up for lower-extremity procedures. . As more time elapses, more patients will be accrued for all procedures, and the end point for the follow-up of LVC will be 30 months. In addition, the relatively small number of patients who underwent radiation therapy did not allow for evaluation of the effect of radiation therapy on the development of lymphedema among melanoma patients. Furthermore, the authors did not stratify patients relative to specific surgical procedure and use of flaps in closure which has been shown to influence lymphedema incidence [28]. Caution should be used in generalizing conclusions based on 12 months of follow-up, as the breast cancer literature report new diagnoses of lymphedema up to and even beyond 24 months after surgery [29, 30].
CONCLUSIONS
We believe that our methods and findings provide a blueprint for future prospective studies evaluating post-operative lymphedema in cancer patients. Early detection of lymphedema with early referral for intervention has been demonstrated to halt progression and alleviate symptoms and associated complications of lymphedema. Unfortunately, measurement tools such as the perometer remain cost prohibitive for widespread implementation and less expensive techniques report high inter-rater reliability but can be time consuming in a patient care setting [31, 32]. Early intervention with compression and physical therapy has been reported to improve the quality of life of breast cancer patients with LVC as low as 3% LVC [33]. The detection of measurable lymphedema and associated symptoms in melanoma patients who have undergone SLN biopsy and/or TLND mandates discussion of the risks of developing clinically significant lymphedema with its commonly reported symptoms such as numbness, firmness/tightness, swelling, and tenderness. These issues should be raised as an essential part of the informed consent process for patients with melanoma for whom surgery is recommended. This on-going study will continue to accrue patients to further clarify the risk of lymphedema and associated symptoms for this at-risk population.
Table 4.
Logistic regression model of factors associated with increased risk of limb volume change
| OR | 95%CI | P value |
|
|---|---|---|---|
| Age (>50 vs. ≤50) | 1.09 | 0.60-1.98 | 0.77 |
| BMI (≥30 vs. <30) | 1.06 | 0.57-1.98 | 0.84 |
| LE vs.UE | 1.72 | 0.96-3.07 | 0.07 |
| TLND vs. SLNB | 3.18 | 1.75-5.78 | <0.01 |
Acknowledgements
Source of Funding: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672. The authors would like to acknowledge Stephanie Deming for her editorial assistance.
Footnotes
CONFLICT OF INTEREST: The authors indicated no conflict of interest
REFERENCES
- 1.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 2.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32:785–789. doi: 10.1016/j.ejso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Starritt EC, Joseph D, McKinnon JG, Lo SK, de Wilt JH, Thompson JF. Lymphedema after complete axillary node dissection for melanoma: assessment using a new, objective definition. Ann Surg. 2004;240:866–874. doi: 10.1097/01.sla.0000143271.32568.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrightson WR, Wong SL, Edwards MJ, Chao C, Reintgen DS, Ross MI, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10:676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister BH, Smithers BM, Davis S, Spry N, Johnson C, Krawitz H, Baumann KC. Radiation therapy following nodal surgery for melanoma: an analysis of late toxicity. ANZ J Surg. 2002;72:344–348. doi: 10.1046/j.1445-2197.2002.02405.x. [DOI] [PubMed] [Google Scholar]
- 6.Lawton G, Rasque H, Ariyan S. Preservation of muscle fascia to decrease lymphedema after complete axillary and ilioinguinofemoral lymphadenectomy for melanoma. J Am Coll Surg. 2002;195:339–351. doi: 10.1016/s1072-7515(02)01230-9. [DOI] [PubMed] [Google Scholar]
- 7.Bowsher WG, Taylor BA, Hughes LE. Morbidity, mortality and local recurrence following regional node dissection for melanoma. The British journal of surgery. 1986;73:906–908. doi: 10.1002/bjs.1800731119. [DOI] [PubMed] [Google Scholar]
- 8.Urist MM, Maddox WA, Kennedy JE, Balch CM. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer. 1983;51:2152–2156. doi: 10.1002/1097-0142(19830601)51:11<2152::aid-cncr2820511134>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Brouns E, Donceel P, Stas M. Quality of life and disability after ilio-inguinal lymphadenectomy. Acta Chir Belg. 2008;108:685–690. doi: 10.1080/00015458.2008.11680316. [DOI] [PubMed] [Google Scholar]
- 10.Baas PC, Schraffordt Koops H, Hoekstra HJ, van Bruggen JJ, van der Weele LT, Oldhoff J. Groin dissection in the treatment of lower-extremity melanoma. Short-term and long-term morbidity. Arch Surg. 1992;127:281–286. doi: 10.1001/archsurg.1992.01420030043008. [DOI] [PubMed] [Google Scholar]
- 11.Karakousis CP, Heiser MA, Moore RH. Lymphedema after groin dissection. Am J Surg. 1983;145:205–208. doi: 10.1016/0002-9610(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 12.James JH. Lymphoedema following ilio-inguinal lymph node dissection. Scand J Plast Reconstr Surg. 1982;16:167–171. doi: 10.3109/02844318209006586. [DOI] [PubMed] [Google Scholar]
- 13.Papachristou D, Fortner JG. Comparison of lymphedema following incontinuity and discontinuity groin dissection. Ann Surg. 1977;185:13–16. doi: 10.1097/00000658-197701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campanholi LL, Duprat JP. JHTG Fregnani: Incidence of LE due to treating cutaneous melanoma. Journal of Lymphoedema. 2011;6 [Google Scholar]
- 15.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42:161–175. [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–490. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin SA, Wright MJ, Morris KT, Sampson MR, Brockway JP, Hurley KE, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26:5220–5226. doi: 10.1200/JCO.2008.16.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 20.Földi M, Földi E, Kubik S. Textbook of lymphology: For physicians and lymphedema therapists. Elsevier; Munich, Germany: 2003. [Google Scholar]
- 21.The diagnosis and treatment of peripheral lymphedema 2009 Concensus Document of the International Society of Lymphology. Lymphology. 2009;42:51–60. [PubMed] [Google Scholar]
- 22.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 23.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after axillary sentinel lymph node biopsy in patients with cutaneous melanoma. Eur J Surg Oncol. 2005;31:778–783. doi: 10.1016/j.ejso.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Faries MB, Thompson JF, Cochran A, Elashoff R, Glass EC, Mozzillo N, et al. The impact on morbidity and length of stay of early versus delayed complete lymphadenectomy in melanoma: results of the Multicenter Selective Lymphadenectomy Trial (I) Ann Surg Oncol. 2010;17:3324–3329. doi: 10.1245/s10434-010-1203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, Fehr MK, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 27.Spillane AJ, Saw RP, Tucker M, Byth K, Thompson JF. Defining lower limb lymphedema after inguinal or ilio-inguinal dissection in patients with melanoma using classification and regression tree analysis. Ann Surg. 2008;248:286–293. doi: 10.1097/SLA.0b013e31817ed7c3. [DOI] [PubMed] [Google Scholar]
- 28.Karakousis CP. Surgical procedures and lymphedema of the upper and lower extremity. J Surg Oncol. 2006;93:87–91. doi: 10.1002/jso.20349. [DOI] [PubMed] [Google Scholar]
- 29.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43:118–127. [PMC free article] [PubMed] [Google Scholar]
- 31.Foroughi N, Dylke ES, Paterson RD, Sparrow KA, Fan J, Warwick EB, Kilbreath SL. Inter-rater reliability of arm circumference measurement. Lymphat Res Biol. 2011;9:101–107. doi: 10.1089/lrb.2011.0002. [DOI] [PubMed] [Google Scholar]
- 32.Deltombe T, Jamart J, Recloux S, Legrand C, Vandenbroeck N, Theys S, Hanson P. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40:26–34. [PubMed] [Google Scholar]
- 33.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]


