Abstract
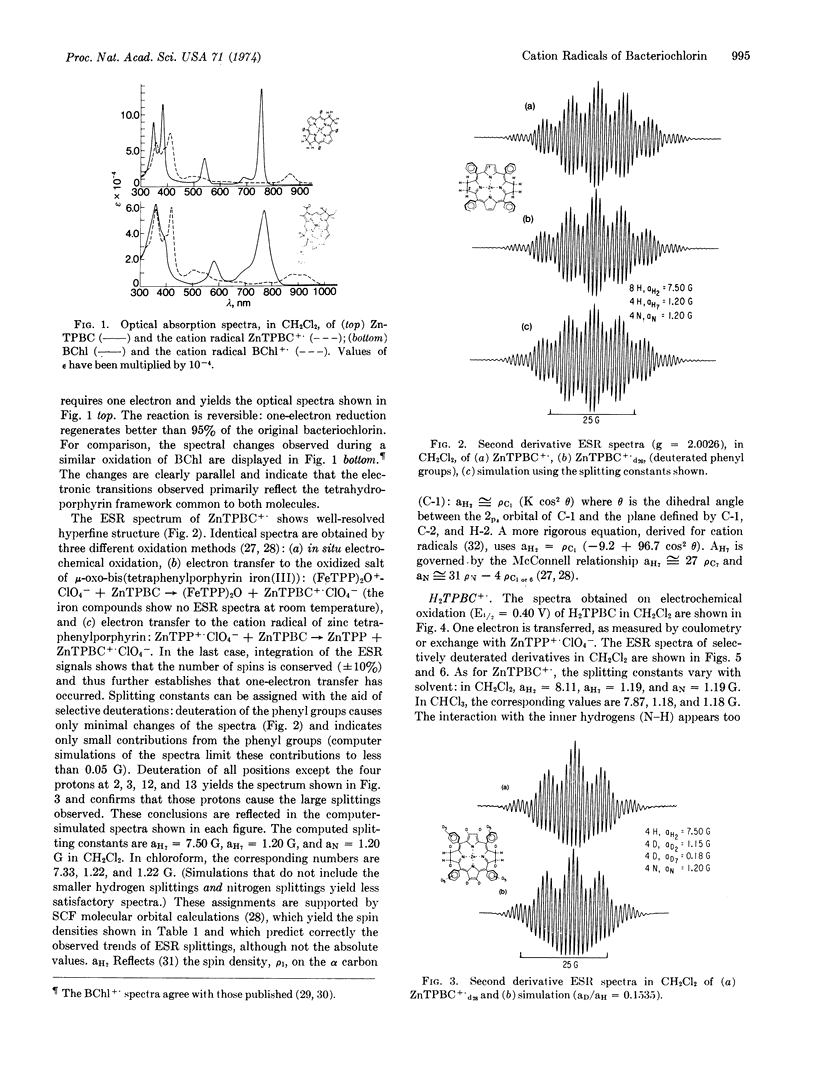
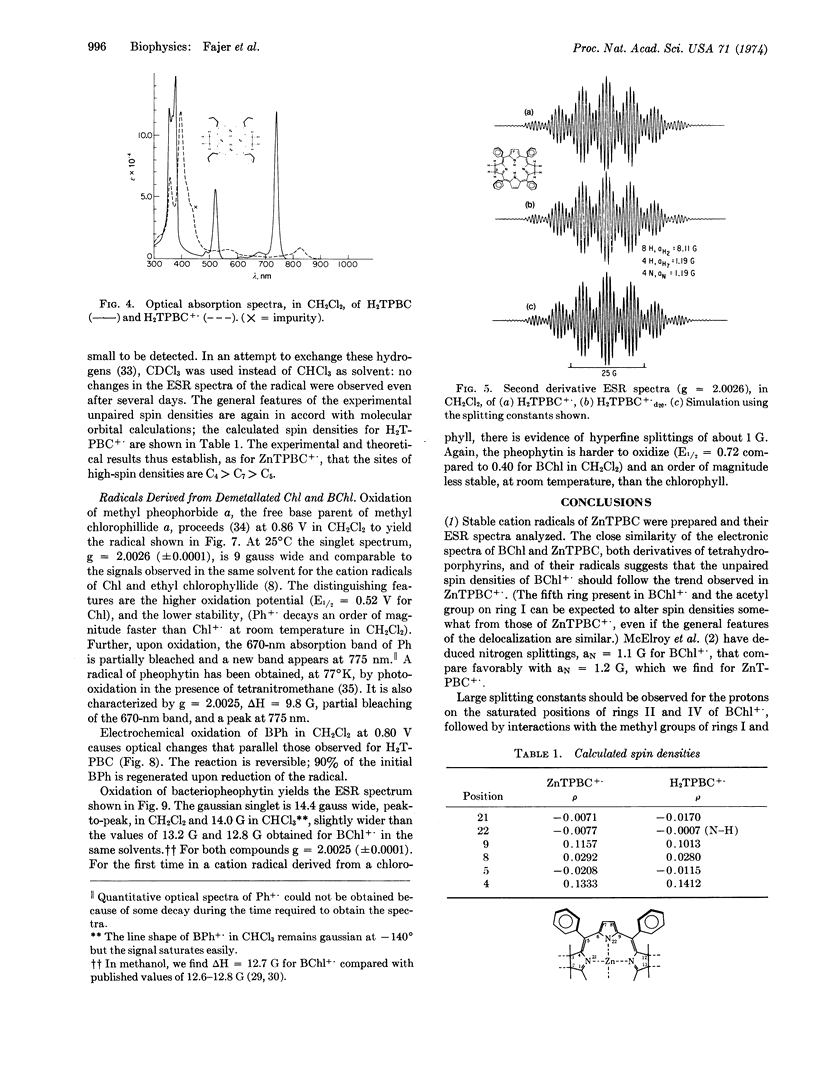
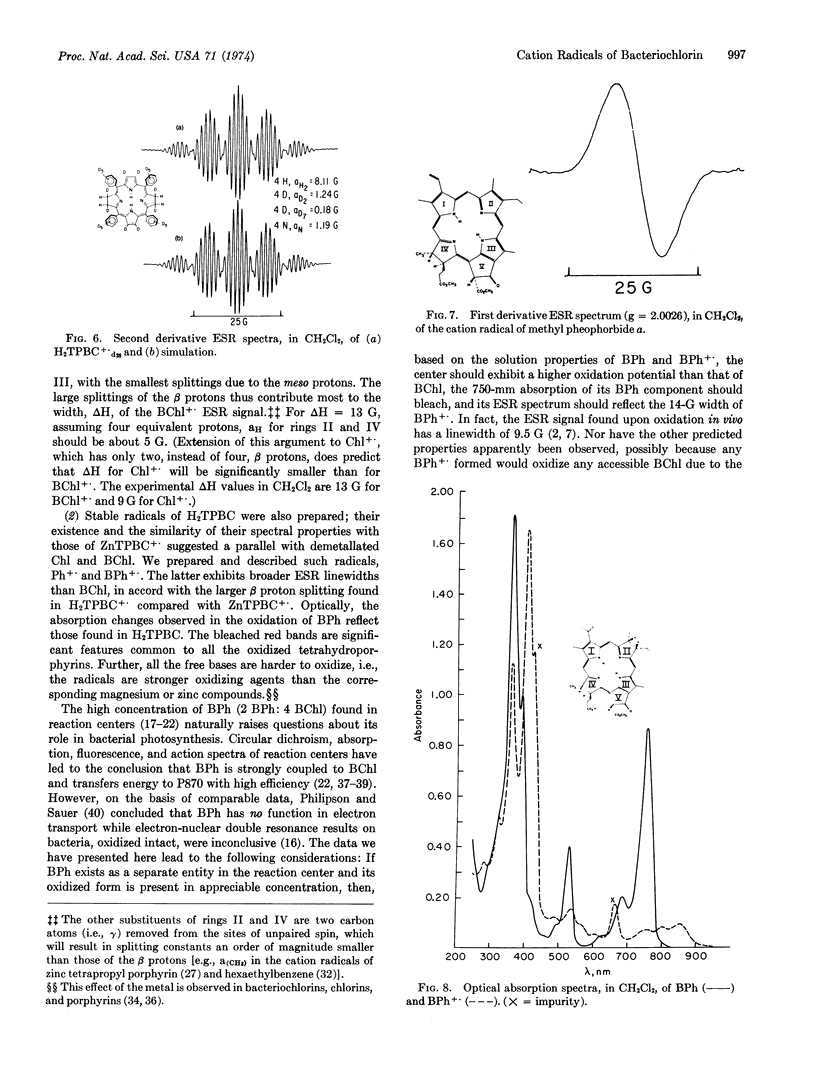
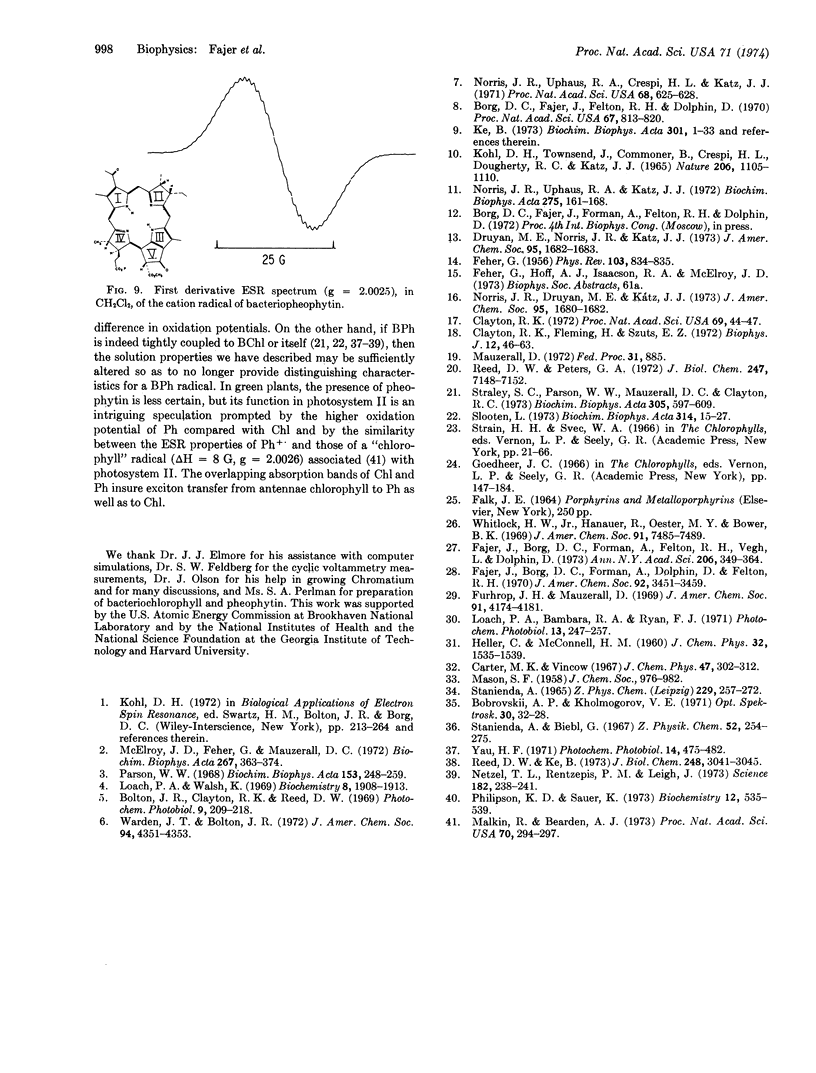
One-electron oxidation of zinc tetraphenylbacteriochlorin and its metal-free base yielded stable cation radicals. Electron spin resonance hyperfine splittings were assigned by selective deuterations. These results indicate that the protons of the saturated rings of the bacteriochlorins carry large spin densities, in accord with molecular orbital calculations. Comparison in vitro of the optical spectra of bacteriochlorins and their cation radicals with those of bacteriochlorophyll show close correspondence and suggest that the electron spin resonance data from the former may also prove a guide to the biological molecule. The surprising similarity in properties between the radicals of free base and zinc bacteriochlorins is maintained in the chlorophylls: cation radicals of bacteriopheophytin and methyl pheophorbide (the free bases of bacteriochlorophyll and methyl chlorophyllide, respectively) exhibit electron spin resonance properties similar to those of their magnesium-containing derivatives. The possibility that metal-free chlorophylls participate in photosynthesis is discussed.
Keywords: electron spin resonance, optical spectra, oxidized pheophytins, free radicals, photosynthesis
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- Borg D. C., Fajer J., Felton R. H., Dolphin D. The pi-Cation Radical of Chlorophyll a. Proc Natl Acad Sci U S A. 1970 Oct;67(2):813–820. doi: 10.1073/pnas.67.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Fleming H., Szuts E. Z. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. II. Interaction with external electron donors and acceptors and a reevaluation of some spectroscopic data. Biophys J. 1972 Jan;12(1):46–63. doi: 10.1016/S0006-3495(72)86070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. Physical mechanisms in photosynthesis: past elucidations and current problems. Proc Natl Acad Sci U S A. 1972 Jan;69(1):44–49. doi: 10.1073/pnas.69.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druyan M. E., Norris J. R., Katz J. J. Electron spin resonance of (25 Mg)chlorophyll a. J Am Chem Soc. 1973 Mar 7;95(5):1682–1683. doi: 10.1021/ja00786a067. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Dolphin D., Felton R. H. pi-Cation radicals and dications of metalloporphyrins. J Am Chem Soc. 1970 Jun 3;92(11):3451–3459. doi: 10.1021/ja00714a038. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Felton R. H., Vegh L., Dolphin D. ESR studies of porphyrin pi-cations: the 2A1u and 2A2u states. Ann N Y Acad Sci. 1973;206:349–364. doi: 10.1111/j.1749-6632.1973.tb43221.x. [DOI] [PubMed] [Google Scholar]
- Fuhrhop J. H., Mauzerall D. The one-electron oxidation of metalloporphyrins. J Am Chem Soc. 1969 Jul 16;91(15):4174–4181. doi: 10.1021/ja01043a027. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Townsend J., Commoner B., Crespi H. L., Dougherty R. C., Katz J. J. Effects of isotopic substitution on electron spin resonance signals in photosynthetic organisms. Nature. 1965 Jun 12;206(989):1105–1110. doi: 10.1038/2061105a0. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Walsh K. Quantum yield for the photoproduced electron paramagnetic resonance signal in chromatophores from Rhodospirillum rubrum. Biochemistry. 1969 May;8(5):1908–1913. doi: 10.1021/bi00833a021. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Detection of a Free Radical in the Primary Reaction of Chloroplast Photosystem II. Proc Natl Acad Sci U S A. 1973 Feb;70(2):294–297. doi: 10.1073/pnas.70.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. Characterization of primary reactants in bacterial photosynthesis. I. Comparison of the light-induced EPR signal (g=2.0026) with that of a bacteriochlorophyll radical. Biochim Biophys Acta. 1972 May 25;267(2):363–374. doi: 10.1016/0005-2728(72)90123-5. [DOI] [PubMed] [Google Scholar]
- Netzel T. L., Rentzepis P. M., Leigh J. Picosecond kinetics of reaction centers containing bacteriochlorophyll. Science. 1973 Oct 19;182(4109):238–241. doi: 10.1126/science.182.4109.238. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Druyan M. E., Katz J. J. Electron nuclear double resonance of bacteriochlorophyll free radical in vitro and in vivo. J Am Chem Soc. 1973 Mar 7;95(5):1680–1682. doi: 10.1021/ja00786a066. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Katz J. J. Electron spin resonance in 13 C-labelled chlorophyll and 13 C-labelled algae. Biochim Biophys Acta. 1972 Aug 17;275(2):161–168. doi: 10.1016/0005-2728(72)90036-9. [DOI] [PubMed] [Google Scholar]
- Parson W. W. The role of P870 in bacterial photosynthesis. Biochim Biophys Acta. 1968 Jan 15;153(1):248–259. doi: 10.1016/0005-2728(68)90167-9. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Comparative study of the circular dichroism spectra of reaction centers from several photosynthetic bacteria. Biochemistry. 1973 Jan 30;12(3):535–539. doi: 10.1021/bi00727a028. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Ke B. Spectral properties of reaction center preparations from Rhodopseudomonas spheroides. J Biol Chem. 1973 May 10;248(9):3041–3045. [PubMed] [Google Scholar]
- Reed D. W., Peters G. A. Characterization of the pigments in reaction center preparations from Rhodopseudomonas spheroides. J Biol Chem. 1972 Nov 25;247(22):7148–7152. [PubMed] [Google Scholar]
- Slooten L. Fluorescence excitation spectra and the relative numbers of pigment molecules in reaction centres from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jul 26;314(1):15–27. doi: 10.1016/0005-2728(73)90060-1. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]



