Abstract
Purpose: To determine the inclusion of women and the sex-stratification of results in moxifloxacin Clinical Trials (CTs), and to establish whether these CTs considered issues that specifically affect women, such as pregnancy and use of hormonal therapies. Previous publications about women's inclusion in CTs have not specifically studied therapeutic drugs. Although this type of drug is taken by men and women at a similar rate, adverse effects occur more frequently in the latter.
Methods: We reviewed 158 published moxifloxacin trials on humans, retrieved from MedLine and the Cochrane Library (1998–2010), to determine whether they complied with the gender recommendations published by U.S. Food and Drug Administration Guideline.
Results: Of a total of 80,417 subjects included in the moxifloxacin CTs, only 33.7% were women in phase I, in contrast to phase II, where women accounted for 45%, phase III, where they represented 38.3% and phase IV, where 51.3% were women. About 40.9% (n=52) of trials were stratified by sex and 15.3% (n=13) and 9% (n=7) provided data by sex on efficacy and adverse effects, respectively. We found little information about the influence of issues that specifically affect women. Only 3 of the 59 journals that published the moxifloxacin CTs stated that authors should stratify their results by sex.
Conclusions: Women are under-represented in the published moxifloxacin trials, and this trend is more marked in phase I, as they comprise a higher proportion in the other phases. Data by sex on efficacy and adverse effects are scarce in moxifloxacin trials. These facts, together with the lack of data on women-specific issues, suggest that the therapeutic drug moxifloxacin is only a partially evidence-based medicine.
Introduction
Fluoroquinolone antibiotics are surrounded by controversy due to their adverse effects, some of which occur more frequently in women, such as QT-interval prolongation, which can lead to torsades de pointes1 or cutaneous photosensitization.2,3 In addition, moxifloxacin has been the center of extensive debate since its authorization, mainly due to the alleged advantages it has over other drugs in its class and also due to the increased risk of cardiac disorders.4 Interestingly, the U.S. Food and Drug Administration (FDA) approved moxifloxacin despite the objections raised by some members of the FDA advisory committee and the medical review officer.5 Lastly, the FDA and European Medicines Agency (EMA) have approved its use in uncomplicated gynecological infections; a decision that has been criticized since authorization was based on a single randomized, double-blind controlled trial with 741 patients over 6 weeks. Thus, the potential for long-term complications such as infertility or extra-uterine pregnancy are unknown.4
Some fluoroquinolones have been withdrawn from the market in certain countries due to serious adverse events and safety concerns. These include temafloxacin (in 1992), which has been shown to cause hemolytic anemia, often accompanied by renal or hepatic dysfunction and/or coagulopathy6; trovafloxacin and alatrofloxacin (2001), which cause fatal liver damage7; grepafloxacin (2003), which produces adverse effects related to prolongation of the QT interval on the electrocardiogram, leading to cardiac events and sudden death—more frequently in women than men8; and gatifloxacin (2006), since it increases risk of diabetes, hallucinations, liver damage, and purpura.9 Other quinolones, including moxifloxacin, have had their licensed indications restricted due to toxicity issues.10 In 2008, alerted by the serious risks involved in the use of oral moxifloxacin, the EMA and FDA analyzed pharmacovigilance data.7,11 The EMA decided that moxifloxacin should only be prescribed in the treatment of acute bacterial sinusitis, acute exacerbation of chronic bronchitis, and community-acquired pneumonia when other antibiotics cannot be used or have failed. Furthermore, the FDA has stated that moxifloxacin shows no advantages over other antibiotics and moreover has been observed to entail a higher cardiovascular risk in women than in men.
Despite the problems associated with taking fluoroquinolones, they are now the most commonly prescribed class of antibiotics in adults.12 Nearly half of these prescriptions are to treat non-approved conditions.12 Despite the restrictions on its use, moxifloxacin has become a bestseller for Bayer—accounting for 497 million Euros ($697.3 million) worldwide in 2010.13
In 1993, the FDA published its Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs, aimed at promoting not only the inclusion of women in clinical trials (CTs) but also the analysis of gender differences.14 There has been an increase in the number of studies which have since examined the inclusion of women in CTs and conducted analyses by sex. These studies found that women only represent around 20% of subjects included in CTs of drugs for specific diseases,15 published in high impact factor journals,16–18 or funded by public institutions.19 Lastly it has been demonstrated that CTs of some drugs, such as antiretrovirals20 and aripiprazol21 have included fewer women than men. In contrast, CTs of other drugs have included more women than men, particularly in the case of anti-inflammatory drugs,22,23 which were withdrawn from the market following fatalities. However, women were significantly under-represented in the crucial first phase,22,23 where the objective is to evaluate the safety of the drug. It has been explained on the basis of the potential risk of fetal harm should women become pregnant during the CT.24 Other explanations for the exclusion of women in the CTs reported in the literature include the confounding effects related to the hormonal cycle, the higher withdrawal rate of women and interactions with other hormonal treatments.24
Due to pressure from the U.S. FDA and National Institutes of Health (NIH), feminist movements, and other lobby groups, women are now better represented in sample sizes. Nevertheless, one form of measurement bias persists as 75% of CTs that receive federal funding from NIH,25 and up to 95% of CT reports to the Spanish Medical Agency26do not include sex stratification. If analyses by sex is not considered in the design phase, it is possible that, in the subsequent analysis phase, the overall sample sizes are too small to produce valid results by sex.
The aims of this study were to determine compliance of moxifloxacin CTs with published good practice guidelines for sex and clinical trials14 and to explore editorial policies on sex differences and women-specific issues in the journal of publication. The rationale for choosing moxifloxacin was that previous publications have not specifically studied therapeutic drugs, such as antibiotics, where the benefit–risk profile differs from that of symptomatic medications. Furthermore, this drug belongs to a group of antibiotics that is consumed by both sexes to the same degree although the adverse effects occur more frequently in women.27,28
Methods
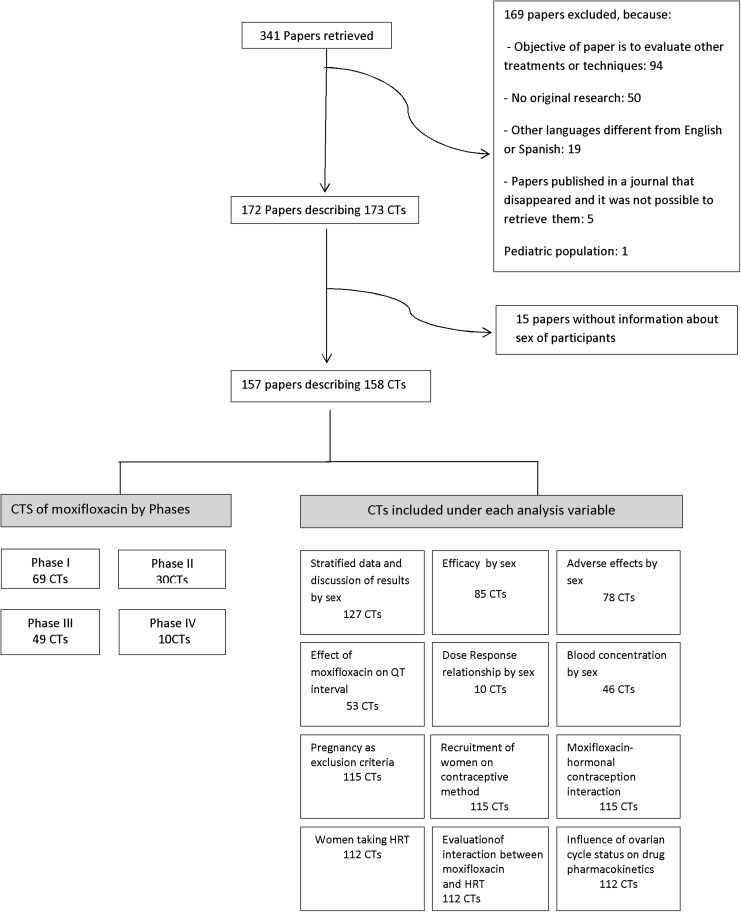
We conducted a review of moxifloxacin clinical trials, using as keywords “moxifloxacin” and “avelox,” and as limits “humans,” in Medline and the Cochrane Library. We identified a total of 173 trials published between January 1998 and December 2010 described in 172 papers of moxifloxacin on adults, published in English (171) and Spanish (1) and excluded those that gave no information about the number or frequency of women and men in the sample (15). Consequently, we retrieved and analyzed a total of 158 CTs (Fig. 1).
FIG. 1.
Diagram illustrating the review process. CTs, clinical trials; HRT, hormone replacement therapy.
In order to conduct a sex analysis of the CTs reviewed, we designed a protocol in accordance with the recommendations of the Food and Drug Administration (FDA) Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs,14 which included the following variables:
1. Demographic characteristics: inclusion frequencies for women and men, age (range, or failing that, mean), type of patient (healthy or type of infection).
-
2. CT phase
• Phase I: to test an experimental drug or treatment in a small group of people for the first time, to gather preliminary data on the agent's pharmacodynamics and pharmacokinetics, and to evaluate its safety, determine a safe dosage range, and identify side effects;
• Phase II: the experimental treatment is given to a larger group of people to see if it is effective and to further evaluate its safety;
• Phase III: treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow it to be used safely; and
• Phase IV: post-marketing studies delineate additional information, including the treatment's risks, benefits, and optimal use.
3. Objective/s of the clinical trials.
4. Limiting omissions (from the gender perspective interest).
5. Sex differences: discussion of the results by sex. Data stratified in order to enable gender analysis of the results. Analysis by sex of efficacy, adverse effects, dose–response, blood concentration–response.
6. Women-specific issues: pregnancy as an exclusion criterion, use of contraceptive methods, use of hormonal contraceptives, and use of hormone replacement therapy (HRT).
7. Excluded from the analysis: editorial policies on sex differences and women-specific issues in the journal of publication.
We analyzed the frequencies and percentages of the variables described above. The main criterion for considering whether a clinical trial fulfilled a sex-related recommendation was any mention of the sex variable in the text. The level of inter-observer agreement (authors E.C. and M.T.R.) was calculated by means of the Kappa index, and a high level of agreement was obtained (Kappa index: 95%). Any lack of agreement (5%) was resolved by a third researcher (A.P.).
The criteria used for the inclusion of clinical trials under each analysis variables were not mutually exclusive (Fig. 1):
➣ Sex stratified data and discussion of results by sex: CTs that include both sexes (127 CTs), excluding those that only included one sex or the other.
➣ Efficacy by sex: CTs that included both sexes and gave efficacy as an objective (85 CTs).
➣ Adverse effects by sex: CTs that included both sexes and gave information about adverse effects (78 CTs).
➣ Effect of moxifloxacin on QT interval: CTs that included both sexes and studied the QT interval after moxifloxacin administration (53 CTs).
➣ Dose response relationship: CTs that included both sexes and analyzed the dose-response relationship (10 CTs).
➣ Blood concentration by sex: CTs that included both sexes and gave an analysis of the dose-response relationship among their objectives (46 CTs).
For the following variables, only CTs that included women of childbearing age were considered in the analysis: Pregnancy as an exclusion criterion, recruitment of women using a contraceptive method or hormonal contraception, interaction between moxifloxacin and hormonal contraception.
• CTs that included women at menopausal age were included in the analysis of recruitment of women taking HRT, evaluation of interaction of the drug and HRT.
• CTs including women were considered in the analysis of the influence of ovarian cycle status on drug pharmacokinetics.
To determine whether a CT included women of childbearing age or women of menopausal age, we used the study population age data provided by the CTs.
Results
Tables 1 to 4 show the number of subjects (% women) and characteristics of study subjects for 69 phase I CTs29–95 (Table 1), 30 phase II CTs96–125 (Table 2), 49 phase III CTs126–174 (Table 3), and 10 phase IVCTs175–184 (Table 4). The titles of the journals that published the 158 CTs reviewed also appear. A total of 80,417 individuals participated in the CTs studied, 46.6% (37,474) of whom were women. The frequency of healthy subjects was 1,021 (31% women), while 68,289 had some kind of respiratory infection (46.8% women), 2,082 had eye infections (51.2% women), 1,964 had gastrointestinal infections (47.9% women) and 6,803 suffered other diseases (13.8% women).
Table 1.
Characteristics of Published Phase-1 Clinical Trials of Moxifloxacina
| First author of study | Journal (year of publication) | No. subjects (% women) | Age | Research focus | Objective | Limiting omission |
|---|---|---|---|---|---|---|
| Ober et al.29 Germany |
J Antimicrob Chemother (2003) |
16 (50.0) |
24–76 |
Acute cholecystitis |
To determine the penetration of MXF into gallbladder tissue to evaluate its antibiotic potential in acute cholecystitis |
Enough women, sex was not considered in the design or data analysis |
| Czock et al.30 Germany |
Clin J Am Soc Nephrolr (2006) |
15 (13.3) |
25–76 |
Acute renal failure |
To determine the PK of two quinolone antibiotics in patients who had anuric acute renal failure and were being treated with extended daily dialysis |
Sex not considered in the design Not enough women |
| Fuhrmann et al.31 Austria |
J Antimicrob Chemother (2004) |
9 (22.2) |
56±17 |
Acute renal failure |
To investigate the PK of intravenous MXF in anuric critically ill patients undergoing continuous venovenous hemodiafiltration |
Sex not considered in the design Not enough women No sex analysis performed |
| Metadillis et al.32 Greece |
J Chemother (2007) |
14 (57.1) |
61.1±8.8 |
Arthroplasty |
To study levofloxacin and MXF into cancellous and cortical bone in patients who underwent routine total hip arthroplasty |
Enough women, sex was not considered in the design or data analysis |
| Breilh et al.33 USA |
J Chemother (2003) |
49 (10.2) |
42–75 |
Bronchial cancer |
To examine the lung tissue diffusion of MXF at a dose of 400 mg administered intravenously or orally once daily, and the results were correlated to microbiological data to estimate the clinical efficacy of MXF in lower community-acquired respiratory infections |
Sex not considered in the design Not enough women |
| Simon et al.34 France |
Clin Pharmcol Ther (2003) |
16 (18.8) |
27–68 |
Bronchopneumonia |
To construct a population pharmacokinetic model for MXF disposition in plasma and bronchial secretions in patients with severe bronchopneumonia who were ventilated |
Sex not included in the design Not enough women |
| Metadillis et al.35 USA |
Int J Antimicrob Agents (2006) |
8 (25.0) |
57.1±9.1 |
Cardiopulmonary bypass surgery |
To investigate plasma and bone concentrations of MXF following a single intravenous dose of 400 mg to consider its potential role in the treatment of osteomyelitis |
Sex not included in the design Not enough women |
| Garcia Saenz et al.36 Spain |
J Cataract Refract Surg (2001) |
42 (66.7) |
76 |
Cataract surgery |
To study the aqueous penetration of ciprofloxacin, levofloxacin, and MXF in patients having cataract surgery |
Enough women, sex not considered in the design or data analysis |
| Kampougeris et al.37 Greece |
Br J Ophthalmol (2005) |
21 (60.0) |
50–87 |
Cataract surgery |
To determine the PK of MXF in the anterior chamber of the human uninflamed eye |
Enough women, sex not considered in the design or analysis |
| Solomon et al.38 USA |
Ophthalmology (2005) |
52 (59.6) |
64 |
Cataract surgery |
To investigate the aqueous penetration of the topical application of three commercially available ophthalmic fluoroquinolones: gatifloxacin 0.3%, MXF 0.5%, and ciprofloxacin 0.3%, administered before cataract surgery |
Sex not included in the design Not enough women |
| Gehanno et al.39 France |
J Antimicrob Chemother (2002) |
48 (21.7) |
41.7±13 |
Chronic sinusitis |
To determine MXF concentrations in sinus tissue, after oral MXF 400 mg once daily for 5 days to patients with chronic sinusitis, undergoing elective sinus surgery |
Sex not included in the design Not enough women |
| Capitano et al.40 USA |
Chest (2004) |
47 (29.8) |
62±13 |
Diagnostic bronchoscopy |
To determine the steady-state, extracellular, and intracellular pulmonary disposition of MXF, levofloxacin, and azithromycin relative to that of the plasma over a 24-hour dosing interval |
Sex not included in the design Not enough women |
| Skalioti et al.41 Greece |
Perit Dial Int (2009) |
8 (0) |
67.59±15.94 |
Dialysis |
To investigate the effect of CAPD on plasma and peritoneal fluid concentration and PK of MXF after administration of one 400 mg dose orally to end-stage renal failure patients undergoing CAPD |
Did not include women |
| Wirtz et al.42 Germany |
J Antimicrob Chemother (2004) |
22 (27.3) |
53.5±15.6 |
Gastrointestinal tract surgery |
To assess the penetration of MXF into GI mucosal tissues to evaluate its potential role as an antimicrobial drug in bacterial infections of the GI tract |
Sex not included in the design Not enough women |
| Wise et al.43 United Kingdom |
Antimicrob Agents Chemother (1999) |
8 (0) |
26–41 |
Healthy |
To examine the PK and penetration of MXF into an inflammatory exudate were examined following a single 400-mg dose given by the oral or i.v. route |
Did not include women |
| Stass et al.44 Germany |
Br J Clin Pharmacol (2005) |
9 (0) |
23–45 |
Healthy |
To evaluate the extent to which enterohepatic recycling circulation contributes to MXF bioavailability in healthy males by administration of activated charcoal and to evaluate the efficacy of activated charcoal administration in decreasing systemic concentrations of MXF in the event of overdose |
Did not include women |
| Ballow et al.45 USA |
Clin Ther (1999) |
10 (0) |
19–39 |
Healthy |
To compare the pharmacokinetic characteristics of 100 mg of MXF given orally with those of 100 mg of MXF given i.v. (60-minute infusion) to determine the drug's absolute bioavailability |
Did not include women |
| Burkhardt et al.46 USA |
Scand J Infect Dis (2002) |
12 (0) |
24–40 |
Healthy |
To investigate the single- and multiple-dose PK of MXF and its penetration into ascitic fluid in patients with severe liver insufficiency |
Did not include women |
| Edlund et al.47 USA |
Scand J Infect Dis (2000) |
12 (0) |
24–40 |
Healthy |
To compare effects of MXF and clarithromycin on the normal intestinal microflora. |
Did not include women |
| Müller et al.48 Austria |
Antimicrob Agents Chemother (1999) |
12 (0) |
24–36 |
Healthy |
To assess the potential of MXF to penetrate peripheral target sites |
Did not include women |
| Stass et al.49 Germany |
Int J Clin Pharmacol Ther (2004) |
12 (0) |
18–39 |
Healthy |
To confirm preclinical data suggesting that MXF is not metabolized by cytochrome p450 isozymes |
Did not include women |
| Stass et al.50 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
24–45 |
Healthy |
To investigate the effect of concomitant calcium administration on the PK and tolerability of MXF |
Did not include women |
| Stass et al.51 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
21–41 |
Healthy |
To investigate the effect of concomitant Al3+(sucralfate) administration on the PK and tolerability of MXF |
Did not include women |
| Stass et al.52 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
33.7±5.9 |
Healthy |
To investigate the effect of probenecid administration on the PK and tolerability of MXF |
Did not include women |
| Stass et al.53 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
19–41 |
Healthy |
To investigate the effect of concomitant iron supplements administration on the PK and tolerability of MXF |
Did not include women |
| Stass et al.54 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
25–46 |
Healthy |
To investigate the effect of concomitant administration of dairy products on the PK and tolerability of MXF |
Did not include women |
| Stass et al.55 Germany |
Clin Pharmacokinetic (2001) |
12 (0) |
21–30 |
Healthy |
To investigate the plasma and urinary PK, safety and tolerability of theophylline and MXF after single and repeated doses of either compound administered alone or concomitantly with the other |
Did not include women |
| Stass et al.56 Germany |
J Antimicrob Chemother (1999) |
12 (0) |
23–41 |
Healthy |
To describe the single-dose PK following oral administration of ascending doses of 50–800 mg as part of the clinical evaluation of MXF |
Did not include women |
| Wagenlehner et al.57 USA |
Int J Antimicrob Agents (2008) |
12 (0) |
18–44 |
Healthy |
To investigate plasma concentrations and the penetration of MXF into prostatic fluid and ejaculate were investigated |
Did not include women Sex-specific |
| Stass et al.58 Germany |
Clin Pharmacokinetic (2001) |
24 (0) |
22–39 |
Healthy |
To determine the effect of concomitant administration of the antacid Maalox 70 or the histamine H receptor antagonist ranitidine on the bioavailability of MXF |
Did not include women |
| Modi et al.59 USA |
J Clin Pharmacol (2009) |
44 (0) |
18–45 |
Healthy |
To evaluate the PK and electrocardiographic pharmacodynamics |
Did not include women |
| Stass et al.60 Germany |
Antimicrob Agents Chemother (1998) |
45 (0) |
23–45 |
Healthy |
To describe the single-dose PK following oral administration of ascending doses of 50–800 mg as part of the clinical evaluation of MXF |
Did not include women |
| Extramiana et al.61 France |
Clin Pharmacol Ther (2005) |
48 (0) |
19–45 |
Healthy |
To assess drug-induced QT interval changes using Holter-monitoring method |
Did not include women |
| Morganroth et al.62 USA |
Am J Cardiol (2004) |
58 (0) |
45–60 |
Healthy |
To evaluate the effects of vardenafil and sildenafil on QT and corrected QT |
Did not include women |
| Sullivan et al.63 USA |
Antimicrob Agents Chemother (1999) |
15 (25.0) |
18–45 |
Healthy |
To investigate the PK, safety, and tolerability of 400 mg of MXF given once daily for a prolonged duration (10 days) in healthy male and female volunteers. A second goal was to predict the effectiveness of MXF with PK/pharmacodynamic ratios for potential efficacy |
With the small number of subjects, limited conclusions can be made about gender effects in this study |
| Weiner et al.64 USA |
Antimicrob Agents Chemother (2007) |
16 (25.0) |
30.5–53.0 |
Healthy |
To compare the PK of daily MXF without and with coadministration of rifampin. A secondary objective was to characterize the effects of MDR1 C3435T polymorphisms on MXF's PK |
Sex not included in the design Not enough women Sex as confounding factor |
| Sullivan et al.65 USA |
Clin Pharmacokinetic (2001) |
36 (33.3) |
Men: 18–45 Women: >65 |
Healthy |
To determine the effects of age and gender on PK, surrogate pharmacodynamics, safety, and tolerability of a single dose of MXF |
NA |
| Barriere et al.66 USA |
J Clin Pharmacol (2004) |
160 (41.3) |
18–40 |
Healthy |
To specifically assess the effect of telavancin on the QTc interval of two dose levels of telavancin compared to negative- and positive-control agents in healthy volunteers |
Enough women, sex was not considered in the design or data analysis |
| Joukhadar et al.67 Austria |
Antimicrob Agents Chemother (2003) |
12 (41.7) |
23–89 |
Healthy |
To determine the penetration characteristics of MXF in patients with soft tissue infections |
Enough women, sex not considered in the design or data analysis |
| Demolis et al.68 USA |
Clin Pharmcol Ther (2000) |
23 (47.8) |
19–32 |
Healthy |
To measure the actual effect of single oral doses of MXF on QT interval duration in healthy volunteers |
Enough women, sex not considered in the design or data analysis |
| Torkildsen et al.69 USA |
Clin Ther (2008) |
48 (48.0) |
40 |
Healthy |
To compare the pharmacokinetic parameters of zithromycin ophthalmic solution 1% and MXF ophthalmic solution 0.5%-in the conjunctiva of healthy volunteers after a single topical administration |
Enough women, sex not considered in the design or data analysis |
| Hart et al.70 USA |
Diagn Microbiol Infect Dis (2007) |
12 (50.0) |
18–40 |
Healthy |
To compare the serum bactericidal activity of MXF and levofloxacin against penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae |
Even sex analysis was performed, sex was not considered in the design |
| Lubasch et al.71 Germany |
Antimicrob Agents Chemother (2000) |
12 (50.0) |
21–35 |
Healthy |
Evaluated and compared the PK of six fluoroquinolones after a single oral dose in the same volunteers |
Even sex analysis was performed, sex was not considered in the design |
| Stass et al.72a Germany |
Clin Infect Dis (2001) |
24 (50.0) |
18–45 |
Healthy |
To address pharmacokinetic interactions with other medications that were selected on the basis of either their known interactions with fluoroquinolones or the adverse event risks associated with their narrow therapeutic range |
Enough women, sex not considered in the design or data analysis |
| Noel et al.73 USA |
Clin Pharmacol Ther (2003) |
48 (50.0) |
19–84 |
Healthy |
To assess the effect of levofloxacin, MXF, and ciprofloxacin on the QT and QTc interval |
Enough women, sex not considered in the design or data analysis |
| Donnenfeld et al.74 USA |
Curr Med ResOpin (2004) |
30 (56.7) |
34.4 |
Healthy |
To compare the ocular tolerability of the commercially available ophthalmic solutions of gatifloxacin and MXF |
Enough women, sex not considered in the design or data analysis |
| Price et al.75 USA |
J Cataract Refract Surg (2005) |
20 (70.0) |
24–59 |
Healthy |
To determine whether gatifloxacin 0.3% ophthalmic solution or MXF 0.5% ophthalmic solutions are toxic to the corneal epithelium when used with 1 of 2 dosing regimens in healthy human eyes |
Enough women, sex not considered in the design or data analysis |
| Shain et al.76 USA |
Clin Drug Invest (2002) |
30 (100) |
31.1±8.6 |
Healthy |
To characterize the potential for combination oral contraceptives to negatively impact the PK of oral MXF due to some similarities in metabolic pathways |
NA |
| Stass et al.72b Germany |
Clin Infect Dis (2001) |
29 (100) |
18–60 |
Healthy |
To address pharmacokinetic interactions with other medications that were selected on the basis of the frequency of their coadministration (e.g., oral contraceptives) |
NA |
| Stass et al.77 Germany |
Clin Pharmacokinet (2001) |
45 (0) |
23–45 |
Healthy and impaired renal function |
To evaluate the influence of impaired renal function on the plasma and urinary PK of MXF |
Did not include women |
| Barth et al.78 |
J Antimicrob Chemother (2008) |
9 (11.1) |
40–78 |
Hepatic impairment |
To investigate the single- and multiple-dose PK of MXF and its penetration into ascitic fluid in patients with severe liver insufficiency |
Sex not included in the design Not enough women |
| Stass et al.79 Germany |
Br J Clin Pharmacol (2007) |
32 (62.5) |
22–44 |
Impaired renal function |
To investigate single dose and steady-state PK of MXF in eight venovenous hemodialysis patients |
Enough women, sex not considered in the design or data analysis |
| Rink et al.80 Germany |
Clin Drug Invest (2008) |
8 (62.5) |
36–83 |
Intra-abdominal abscess |
To examine the penetration of MXF into abdominal abscess fluid in patients with an intra-abdominal abscess |
Enough women, sex not considered in the design or data analysis |
| Malincarne et al.81 Italy |
J Antimicrob Chemother (2006) |
30 (76.7) |
ND |
Osteomyelitis |
To determine plasma and bone MXF concentrations following intravenous administration of a single 400-mg dose to evaluate the potential role of MXF in the treatment of bone infections |
Enough women, sex not considered in the design or data analysis |
| Wacke et al.82 USA |
J Antimicrob Chemother (2006) |
60 (21.7) |
25–80 |
Pancreas carcinoma or chronic pancreatitis |
To study the penetration of MXF into pancreatic tissue in patients |
Sex not included in the design Not enough women |
| Stass et al.83 Germany |
Int J Gynaecol Obstet (2008) |
40 (100) |
28–60 |
Pelvic inflammatory disease |
To determine whether MXF penetrates the uterine tissue and accumulates at levels sufficient to eradicate the major pathogens causing pelvic inflammatory disease |
NA |
| Pea et al.84 USA |
Clin Pharmacokinet (2003) |
14 (57.1) |
68–86 |
Pneumonia |
PK and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia |
Enough women, sex was not considered in the design or data analysis |
| Stass et al.85 Germany |
J Antimicrob Chemother (2006) |
10 (30.0) |
20–78 |
Peritonitis |
To investigate the penetration of MXF into peritoneal exudate in patients with complicated intra-abdominal infections |
Sex variable not included in the design Not enough women |
| Leone et al.86 France |
Antimicrob Agents Chemother (2004) |
17 (17.6) |
46±10 |
Pneumonia |
To determine the MXF concentrations in bronchial secretions, compared with those in plasma, up to 24 hours after multiple 400-mg i.v. administrations of the drug to mechanically ventilated patients with pneumonia |
Sex variable not included in the design Not enough women |
| Dinis et al.87 Portugal |
Ann Otol Rhinol Laryngol (2004) |
20 (50.0) |
51.8 |
Recalcitrant chronic sinusitis |
To assess MXF distribution pattern into the paranasal sinuses |
Enough women, sex not considered in the design or data analysis |
| Esposito et al.88 Italia |
J Antimicrob Chemother (2006) |
29 (44.8) |
18–58 |
Tonsillectomy |
To determine the MXF concentrations in plasma and tonsillar tissue after the administration of three doses of MXF 400 mg to adult patients with chronic or recurrent tonsillitis undergoing tonsillectomy |
Enough women, sex not considered in the design or data analysis |
| Alffenaar et al.89 Netherlands |
Clin Infect Dis (2009) |
4 (0) |
30–65 |
Tuberculosis |
To measure plasma and cerebrospinal fluid concentrations of MXF over time to establish the optimal dose for treatment of tuberculosis |
Did not include women |
| Johnson et al.90 USA |
Int J Tuberc Lung Dis (2006) |
45 (5.0) |
18–65 |
Tuberculosis |
To evaluate the early bactericidal activity of the new fluoroquinolones levofloxacin, gatifloxacin and MXF in patients with pulmonary tuberculosis |
Sex not included in the design Not enough women |
| Nijland et al.91 Indonesia |
Clin Infect Dis (2007) |
19 (31.6) |
18–55 |
Tuberculosis |
To assess the interaction between rifampicin and MXF |
Sex not included in the design Not enough women |
| Valerio et al.92 Italy |
J Chemother (2003) |
20 (40.0) |
21–81 |
Tuberculosis |
To observe in compliant patients, the effect of 6 months of therapy with MXF, isoniazid, and rifampin |
Enough women, sex not considered in the design or data analysis |
| Fuller et al.93 USA |
Am J Ophtalmol (2007) |
24 (54.2) |
27–83 |
Vitrectomy |
To assess the vitreal penetration of topical, oral, and combined topical and oral MXF |
Enough women, sex was not considered in the design or data analysis |
| Lott et al.94 USA |
Retina (2008) |
24 (58.3) |
37–88 |
Vitrectomy |
To investigate the vitreal penetration of MXF after oral administration |
Even sex analysis was performed, sex was not considered in the design |
| Vedantham et al.95 India |
Eye (2006) | 27 (11.1) | 20–76 | Vitrectomy | To investigate intraocular penetration of MXF hydrochloride after oral administration | Even sex analysis was performed, sex was not included in the design Not enough women |
Ordered by research focus and percentage of women participating in the clinical trials.
First of two clinical trials.
Second of two clinical trials.
CAPD, continuous ambulatory peritoneal dialysis; GI, gastrointestinal; i.v., intravenous; MXF, moxifloxacin; NA, not applicable; ND, no data; PK, pharmacokinetics.
Table 2.
Characteristics of Published Phase-2 Clinical Trials of Moxifloxacina
| First author of study | Journal | No subjects (% women) | Age | Research focus | Objective | Limiting omission |
|---|---|---|---|---|---|---|
| Bozkurt et al.96 Turkey |
Allergol Immunopathol (2005) |
20 (85.0) |
38.05±14.1 |
Antibiotic hypersensitivity |
To investigate whether MXF can be among the safe alternative antibiotics determined by oral provocation tests for antibiotic intolerant patients |
Enough women, sex was not considered in the design or data analysis |
| Constantinou et al.97 Austalia |
Ophthalmology (2007) |
229 (41.5) |
12.5–98.8 |
Bacterial keratitis |
To determine the clinical efficacy and safety of MXF (1.0%) in patients with bacterial keratitis compared with patients treated with ofloxacin (0.3%) or fortified tobramycin (1.33%)/cephazolin (5%) |
Enough women, sex was not considered in the design or data analysis |
| Vasavada et al.98 India |
J Cataract Refract Surg (2008) |
145 (40.7) |
55–85 |
Cataract surgery |
To evaluate the aqueous concentration of MXF following two dosing regimens of topically administered MXF hydrochloride ophthalmic solution 0.5% |
Enough women, sex was not considered in the design or data analysis |
| Katz et al.99 USA |
Cornea (2005) |
61 (50) |
48–85 |
Cataract surgery |
To investigate the absorption of MXF into human aqueous humor after administration of MXF hydrochloride ophthalmic solution, 0.5% as base |
Enough women, sex was not considered in the design or data analysis |
| Lane et al.100 USA |
J Cataract Refract Surg |
47 (59.6) |
51–88 |
Cataract surgery |
To evaluate posterior and anterior segment safety of an intracameral injection of MXF 0.5% ophthalmic solution as prophylaxis for endophthalmitis in patients having cataract surgery |
Enough women, sex was not considered in the design or data analysis |
| Ong-Tone et al.101 Canada |
J Cataract Refract Surg (2007) |
100 (65) |
49–90 |
Cataract surgery |
To determine whether the penetration into the aqueous humor of gatifloxacin and MXF eye drops was affected by altering their concentrations in the dilating mixture in which the wick used to dilate the pupil before cataract surgery was soaked |
Enough women, sex was not considered in the design or data analysis |
| Diz Dios et al.102 Spain |
Antimicrob Agents Chemother (2006) |
221 (43) |
18–57 |
Dental extraction |
To investigate the efficacies of the prophylactic administration of amoxicillin, clindamycin, and MXF for the prevention of bacteremia following dental extractions |
Enough women, sex was not considered in the design or data analysis |
| DeRamo et al.103 USA |
Am J Ophthalmol (2006) |
42 (57) |
49–93 |
Endophthalmitis |
To study the use of gatifloxacin and MXF, and bacterial sensitivity in cases of acute postoperative endophthalmitis following cataract surgery |
Enough women, sex was not considered in the design or data analysis |
| Beyer et al.104 USA |
Eur J Clin Microbiol Infect Dis (2000) |
12 (0) |
24–40 |
Healthy |
To investigate the effect of a 7-day treatment with MXF (400 mg orally, once daily) versus clarithromycin (500 mg orally, twice daily) on the normal oropharyngeal microflora |
Did not include women |
| Man et al.105 United Kingdom |
J Antimicrob Chemother (1999) |
32 (0) |
18–45 |
Healthy |
To assess the potential of MXF to induce phototoxicity when compared with lomefloxacin and placebo |
Did not include women |
| He et al.106 USA |
J Cataract Refract Surg (2009) |
120 (50) |
24–93 |
Healthy |
To compare selection for fluoroquinolone-resistant bacteria between 1-day and 3-day application of topical MXF 0.5% |
Enough women, sex was not considered in the design or data analysis |
| Sebban et al.107 France |
Support Care Cancer (2008) |
90 (68.9) |
21–80 |
Healthy |
To evaluate MXF use in cancer patients with febrile neutropenia |
Enough women, sex was not considered in the design or data analysis |
| Cheon et al.108 Korea |
Helicobacter (2006) |
85 (44.7) |
54.3±11.7 |
H. pylori infection |
To evaluate the efficacy and tolerability of MXF-based triple therapy as an alternative second-line treatment for H. pylori infection |
Enough women, sex was not considered in the design or data analysis |
| Kilic et al.109 Turkey |
Dig Dis Sci (2008) |
120 (47.5) |
>18 |
H. pylori infection |
To evaluate the efficacy of MXF-containing regimens in the first-line treatment of H. pylori |
Enough women, sex was not considered in the design or data analysis |
| Bago et al.110 Croatia |
Wien Klin Wochenschr (2007) |
276 (48.2) |
48±13 |
H. pylori infection |
To prove the efficacy and tolerability of MXF-based treatment of H. pylori infection and to compare it with standard clarithromycin-based treatments |
Enough women, sex was not considered in the design or data analysis |
| Miehlke et al.111 Germany |
Helicobacter (2008) |
103 (65) |
21–79 |
H. pylori infection |
To investigate a 1-week once-daily triple therapy with esomeprazole, MXF, and rifabutin for rescue therapy of H. pylori infection |
Even though there are enough women, sex was not included in the design or analysis |
| Ta et al.112 USA |
J Ocul Pharmacol (2008) |
60 (31.7) |
69.3 |
Intraocular surgery |
To compare the efficacy of a 1-hour versus 1-day application of topical MXF in eliminating conjunctival bacterial flora |
Sex not included in the design Not enough women |
| Pardillo et al.113 Philippines |
Antimicrob Agents Chemother (2008) |
8 (0) |
22–49 |
Leprosy |
To evaluate bactericidal activity of MXF in human leprosy |
Did not include women |
| Schwab et al.114 Germany |
Aliment Pharmacol Ther (2005) |
20 (25.0) |
53.9±12.5 |
Obstructive cholangitis and the non-obstructed biliary tract |
To establish the secretion of MXF into obstructed and non-obstructed bile |
Sex not included in the design Not enough women |
| Hollan et al.115 USA |
Cornea (2008) |
50 (56.0) |
20–87 |
Penetrating keratoplasty |
To examine the ocular penetration of MXF |
Enough women, sex was not considered in the design or data analysis |
| Guentsch et al.116 Germany |
J Periodontol (2008) |
92 (52.2) |
49.6±9.4 |
Periodontitis |
To evaluate the impact of adjunctive systemic MXF compared to the use of adjunctive systemic doxycycline, well-established regimen, and scaling and root planning alone on the success of periodontitis treatment |
Enough women, sex was not considered in the design or data analysis |
| Burka et al.117 USA |
Am J Ophthalmol (2005) |
40 (45.0) |
21–47 |
Photorefractive keratectomy |
To compare the rate of epithelial healing following photorefractive keratectomy with gatifloxacin and MXF |
Enough women, sex was not considered in the design or data analysis |
| Moshirfar et al.118 USA |
Cornea (2005) |
46 (50.0) |
12–86 |
Photorefractive keratectomy |
To compare the effects of topical MXF and gatifloxacin on corneal reepithelialization after penetrating keratoplasty |
Enough women, sex was not considered in the design or data analysis |
| Jardim et al.119 Mexico, Chile, Brazil, Argentina, Uruguay |
Arch Bronconeumol (2003) |
84 (48.8) |
>18 |
Pneumonia |
To evaluate the efficacy and safety of treatment with either MXF or amoxicillin administered for 10 days to patients suspected of having CAP caused by a pneumococcal infection |
Even though there are enough women, sex was not included in the design or analysis |
| Ott et al.120 Germany |
Infection (2008) |
96 (27.1) |
37±14 |
Pneumonia and primary lung abscess |
To compare MXF and ampicillin/sulbactam concerning efficacy and safety in the treatment of aspiration pneumonia and primary lung abscess |
Sex variable not included in the design Not enough women |
| Campos et al.121 Brazil |
Clin Ophthalmol (2008) |
61 (72.1) |
29.1 |
Prophylaxis after LASIK |
To compare the efficacy and tolerability of a fixed-dose combination of 0.5% MXF and 0.1% dexamethasone formulation versus conventional dosing with both agents dosed separately for prophylaxis after laser-assisted in situ keratomileusis |
Women are majority, sex was not included in the design and the analysis |
| Gosling et al.122 Tanzania |
J Respir Crit Care Med (2003) |
43 (14.0) |
18–70 |
Tuberculosis |
To compare the relative activity of the drugs with control regimens examined at the same time and with historic controls. In this paper, the results of a trial comparing MXF with rifampin and isoniazid are reported |
Sex not included in the design Not enough women |
| Pletz et al.123 Germany |
Antimicrob Agents Chemother (2004) |
17 (29.4) |
50.4±8.7 |
Tuberculosis |
To assess the potency of new antituberculous drugs in clinical studies |
Sex not included in the design Not enough women |
| Burman et al.124 USA |
Am J Respir Crit Care Med (2006) |
277 (32.9) |
24–40 |
Tuberculosis |
To compare the impact of MXF versus ethambutol, both in combination with isoniazid, rifampin, and pyrazinamide, on sputum culture conversion at 2 months as a measure of the potential sterilizing activity of alternate induction regimens |
Even sex analysis was performed, sex not included in the design |
| Conde et al.125 Brazil |
Lancet (2009) | 146 (38.4) | 32.5 (11.7) | Tuberculosis | To assess the activity and safety of MXF in the initial stage of tuberculosis treatment | Even sex analysis was performed, sex not included in the design |
Ordered by research focus and percentage of women participating in the clinical trials.
Objective of phase-2 clinical trials: the experimental treatment is given to a larger group of people to see if it is effective and to further evaluate its safety.
CAP, community acquired pneumonia.
Table 3.
Characteristics of Published Phase-3 Clinical Trials of Moxifloxacina
| First author of study | Journal (year) | No. subjects (% women) | Age | Research focus | Objective | Limiting omissions |
|---|---|---|---|---|---|---|
| Ferguson et al.126 USA |
Otolaryngol Head Neck Surg (2004) |
322 (61.8) |
19–84 |
Acute bacterial rhinosinusitis |
To compare the clinical and bacteriologic efficacy and safety of short-duration treatment with telithromycin given for 5 days with MXF given for 10 days in adults with acute bacterial rhinosinusitis |
Enough women, sex was not considered in the design or data analysis |
| Siegert et al.127 Finland, France, Spain, Sweden Germany, Greece, Israel |
Respir Med (2000) |
493 (55.4) |
40.4±14.6 |
Acute bacterial sinusitis |
To compare the efficacy and safety of MXF with that of cefuroxime axetil for the treatment of acute bacterial sinusitis in adults |
Enough women, sex was not considered in the design or data analysis |
| Rakkar et al.128 USA |
Int J Clin Pract (2001) |
475 (48.8) |
18–67 |
Acute maxillary sinusitis |
To evaluate MXF versus amoxicillin clavulanate in the treatment of acute maxillary sinusitis |
Enough women, sex was not considered in the design or data analysis |
| Gehanno et al.129 France |
J Int Med Research (2003) |
216 (60.6) |
18–80 |
Acute maxillary sinusitis |
To evaluate the efficacy and safety of 7-day oral MXF (400 mg/day) for treatment of acute maxillary sinusitis after first-line treatment failure, and acute sinusitis with high risk of complications |
Enough women, sex was not considered in the design or data analysis |
| Burke et al.130 USA |
Clin Ther (1999) |
457 (61.1) |
18–78 |
Acute maxillary sinusitis |
To compare the efficacy and safety of MXF with those of cefuroxime axetil for the treatment of community acquired acute sinusitis |
Even sex analysis was performed, sex not included in the design |
| Klossek et al.131 France, Spain, Greece, United Kingdom Germany, Sweden, Belgium, Lithuania, |
J Laryngol Otol (2003) |
347 (66.6) |
38.8±13.9 |
Acute maxillary sinusitis |
To compare of the efficacy and safety of MXF and trovafloxacin for the treatment of acute, bacterial maxillary sinusitis in adults |
Enough women, sex was not considered in the design or data analysis |
| Miratvilles et al.132 USA |
Respir Med (2005) |
1147 (19.0) |
68.7±9.4 |
AECB |
To identify risk factors for late recovery and failure after ambulatory treatment of exacerbations of chronic bronchitis and COPD |
Sex not included in the design Not enough women |
| Chodosh et al.133 USA |
Respir Med (2000) |
936 (22.0) |
18–90 |
AECB |
to compare the safety and efficacy of moxifloxacin with clarithromycin for the treatment of patients with AECB |
Even sex analysis was performed, sex not considered in the design |
| Wilson et al.134 Austria, Spain, France, Germany, Greece, United Kingdom, Switzerland, |
Chest (2004) |
1935 (36.0) |
63.2±9.8 |
AECB |
To compare the effectiveness of oral MXF with standard antibiotic therapy in AECB |
Sex not included in the design Not enough women |
| Starakis et al.135 USA |
Int J Antimicrob Agents (2004) |
162 (36.4) |
61.3±13.5 |
AECB |
To compare the efficacy and safety of a 5 day course of MXF 400 mg orally with that of a 7-day course of clarithromycin 500 mg orally in 750 patients with AECB |
Sex not included in the design Not enough women |
| Niederman et al.136 USA |
Respir Med (2006) |
441 (37.2) |
19–90 |
AECB |
To determine the rate of bacterial eradication of Haemophilus influenzae during AECB treated with either macrolides or MXF |
Enough women, sex was not considered in the design or data analysis |
| Shaberg et al.137 USA |
J Int Med Res (2001) |
575 (39.3) |
61.3±13.5 |
AECB |
To compare the efficacy and safety of once daily dosing with MXF with that of co-amoxiclav given three times daily for the treatment of AECB |
Enough women, sex was not considered in the design or data analysis |
| Wilson et al.138 USA |
Chest (1999) |
649 (41.1) |
60±14 |
AECB |
To compare the efficacy and safety of MXF and levofloxacin for the treatment of patients with AECB |
Enough women, sex was not considered in the design or data analysis |
| DeAbate et al.139 USA |
Respir Med (2000) |
464 (45.0) |
19–88 |
AECB |
To compare the safety and efficacy of MXF to azithromycin for the treatment of patients with AECB of suspected bacterial origin |
Even sex analysis was performed, sex not considered in the design |
| Urueta-Robledo et al.140 Argentina, Peru, Brazil, Mexico, Colombia |
Respir Med (2006) |
437 (49.7) |
59±15 |
AECB |
To compare the efficacy and safety of MXF and levofloxacin for the treatment of patients with AECB |
Enough women, sex was not considered in the design or data analysis |
| Portier et al.141 France |
Eur J Clin Microbiol Infect Dis (2005) |
346 (27.5) |
59.3±17.9 |
CAP |
To assess the efficacy and safety of MXF versus amoxicillin/clavulanate plus roxithromycin in adult CAP patients with risk factors. |
Sex not include in the design Not enough women |
| Lode et al.142 Europe, Israel South Africa, USA |
Respir Med (2003) |
479 (33.8) |
18–93 |
CAP |
To evaluate IV/PO MXF in the treatment of hospitalized patients with severe CAP |
Sex not include in the design Not enough women |
| Petitprezt et al.143 Argentina, Brazil, Czech Republic, Spain, Estonia, France, Hong Kong, Hungary, Lithuania, México, Portugal, Chile, Russia, Slovenia, South Africa, Turkey, Ukrania United Kingdom, Uruguay. |
Chest (2001) |
200 (38.0) |
52.0±20.5 |
CAP |
To compare of the efficacy and safety of MXF vs amoxicillin for treatment of mild-to-moderate, suspected pneumococcal CAP in adult patients |
Enough women, sex was not considered in the design or data analysis |
| Welte et al.144 Germany |
Clin Infect Dis (2005) |
397 (37.5) |
≥18 |
CAP |
To compare the efficacy, safety, and speed and quality of defervescence of sequential intravenous or oral MXF and high-dose ceftriaxone with or without erythromycin for patients with CAP requiring parenteral therapy |
Sex analysis performed sex not considered in the design |
| Hoefken et al.145 Austria, Australia, Germany, Great Britain, Greece, Hong Kong, Israel, Indonesia, New Zealand, Norway, Philippines, Taiwan, South Africa, Sweden, Switzerland |
Respir Med (2001) |
675 (38.2) |
48.4±20.6 |
CAP |
To compare oral MXF (200 mg or 400 mg once daily for 10 days) with oral clarithromycin (500 mg, twice daily for 10 days) in the treatment of CAP |
Enough women, sex was not considered in the design or data analysis |
| Patel et al.146 USA |
Respir Med (2000) |
196 (42.3) |
18–85 |
CAP |
To evaluate the safety and efficacy of MXF in the treatment of patients with CAP |
Enough women, sex was not considered in the design or data analysis |
| Katz et al.147Germany |
J Emerg Med(2004) |
235 (47.2) |
18–89 |
CAP |
To evaluate in the treatment of hospitalized patients with severe CAP |
Enough women, sex was not considered in the design or data analysis |
| Marrie et al.148 Canada |
J Infect (2004) |
399 (48.0) |
45.7±15.8 |
CAP |
To describe the resolution of five symptoms commonly associated with CAP |
Enough women, sex was not considered in the design or data analysis |
| Morganroth et al.149 USA |
Chest (2005) |
394 (49.0) |
78 |
CAP |
To assess the cardiac rhythm safety of MXF versus levofloxacin in elderly patients hospitalized with CAP |
Sex analysis performed Sex not considered in the design |
| Anzueto et al.150 USA |
Clin Infect Dis (2006) |
281 (50.2) |
77.4±7.7 |
CAP |
To determine the efficacy and safety of MXF versus that of levofloxacin for the treatment of CAP in hospitalized elderly patients |
Sex was included in the design as confounding factor |
| Torres et al.151 Spain |
Eur Respir J (2003) |
553 (51.2) |
52.7±18.7 |
CAP |
To evaluate the effectiveness of a MXF compared with standard antimicrobial regimens, in conditions relating as closely as possible to the real world setting |
Enough women, sex was not considered in the design or data analysis |
| Marrie et al.152 Canada |
Can Respir J (2004) |
76 (60.5) |
38.1±14.4 |
CAP |
To determine the time course of the resolution of symptoms in Mycoplasma pneumoniae pneumonia |
Enough women, sex was not considered in the design or data analysis |
| McDonald et al.153 USA |
Ophthalmology (2009) |
533 (53.3) |
1–100 |
Conjunctivitis |
To compare the clinical and antimicrobial efficacy of besifloxacin ophthalmic suspension 0.6% with that of MXF ophthalmic solution 0.5% for the treatment of bacterial conjunctivitis |
Enough women, sex was not considered in the design or data analysis |
| Lipsky et al.154 USA |
J Antimicrob (2007) |
127 (28.4) |
57.0±11.8 |
Diabetic foot infections |
To assess the efficacy of MXF for treating diabetic foot infections |
Sex variable not included in the design Not enough women |
| Malangoni et al.155 USA, Canada, Israel |
Ann Surg (2006) |
379 (35.4) |
47.4±16.7 |
Intraabdominal infections |
To compare the safety and efficacy of sequential i.v. to p.o. MXF against a standard antimicrobial regimen of intravenous piperacillin– tazobactam followed by oral amoxicillin– clavulanate for the treatment of adults with complicated intra-abdominal infections |
Sex variable not include in the design Not enough women |
| Kang et al.156 Korea |
Helicobacter (2007) |
192 (45.3) |
22–80 |
H. pylori infection |
To test the efficacy of 10-day MXF-based triple therapy versus 2-week quadruple therapy for the second-line treatment of H. pylori infection |
Enough women, sex was not considered in the design or data analysis |
| Nista et al.157 Italy |
Aliment Pharmacol Ther (2005) |
320 (45.6) |
18–65 |
H. pylori infection |
To compare the efficacy of two 1-week MXF-based H. pylori eradication regimens with two standard treatments |
Enough women, sex was not considered in the design or data analysis |
| Höffken et al.158 USA |
Respir Med (2007) |
161 (46) |
18–95 |
Hospital-acquired pneumonia |
To evaluate the efficacy and safety of MXF versus ceftriaxone in patients with HAP without risk of infections with Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. |
Enough women, sex was not considered in the design or data analysis |
| Bago et al.159 Croacia |
Wien Klin Wochenschr (2009) |
160 (48.1) |
48±15 |
H. pylori infection |
To test the efficacy of 10-day MXF-based triple therapy versus 2-week quadruple therapy for the second-line treatment of H. pylori infection |
Enough women, sex was not considered in the design or data analysis |
| Yoo et al.160 Korea |
Helicobacter (2009) |
361 (49.0) |
55.3 |
H. pylori infection |
To evaluate the efficacy of a MXF-containing triple therapy as second-line treatment for H. pylori infection. We also investigated the effect of treatment duration and antibiotic resistance on the eradication rate of this therapy |
Enough women, sex was not considered in the design or data analysis |
| Di Caro et al.161 Italy |
Aliment Pharmacol Ther (2002) |
120 (58.3) |
18–65 |
H. Pylori infection |
To compare the efficacy of different 1-week MXF-based H. pylori eradication regimens |
Enough women, sex was not considered in the design or data analysis |
| Sezguin et al.162 Turkey |
Helicobacter (2007) |
71 (62.0) |
17–65 |
H. pylori infection |
To investigate the effectiveness of a new second-generation fluoroquinolone, MXF-containing triple therapy in H. pylori eradication |
Enough women, sex was not considered in the design or data analysis |
| Arrieta et al.163 Argentina, Brazil, Chile, México |
Am J Otolaryngol (2007) |
459 (62.5) |
37±14 |
H. pylori infection |
To compare the efficacy and safety of MXF with that of amoxicillin/ clavulanate for the treatment of acute bacterial sinusitis in adult |
Enough women, sex was not considered in the design or data analysis |
| Solomkin et al.164 China, Indonesia, South Korea, Malaysia, Hong Kong |
Int J Antimicrob Agents (2009) |
361 (30.7) |
40.3±16.0 |
Intraabdominal infections |
To compare the efficacy and safety of MXF monotherapy and ceftriaxone/metronidazole combination therapy in adults with confirmed or suspected complicated intra-abdominal infections. Patients received surgical intervention and either i.v. MXF 400mg once daily or i.v. ceftriaxone 2 grams once daily plus i.v. metronidazole 500 mg twice daily |
Sex not included in the design Not enough women |
| Weiss et al.165 Germany |
J Chemother (2009) |
511 (40.3) |
17–94 |
Intraabdominal infections |
To compare the efficacy and safety of sequential i.v. to p.o. MXF 400 mg once daily, with that of i.v. ceftriaxone 2 g once daily, plus metronidazole 500 mg three times daily, followed by p.o. amoxicillin/clavulanate 625 mg three times daily |
Enough women, sex was not considered in the design or data analysis |
| Halachimi-Eyal et al.166 Israel |
J Cataract Refract Surg (2009) |
464 (51.1) |
18–91 |
Intraocular surgery |
To assess the effectiveness of adding topical MXF 0.5% to topical povidone-iodine 5.0% for preoperative reduction of bacterial recovery from the conjunctiva |
Enough women, sex was not considered in the design or data analysis |
| Bradshaw et al.167 Australia |
Plos One (2008) |
313 (16.9) |
ND |
Mycoplasma genitalium |
To determine clinical outcomes and cure rates for Mycoplasma genitalium genital infection in men and women following azithromycin 1 gram |
Sex analysis was performed, sex was not considered in the design |
| Ross et al.168 Denmark, Finland, France, Sweden Rusia Germany, Greece, Hungary, Lithuania, Poland, South Africa, United Kingdom, Italy, |
Sex Transm Infect (2006) |
741 (100) |
30.1±8.4 |
Pelvic Inflammatory disease |
To compare the efficacy and safety of MXF monotherapy with ofloxacin plus metronidazole in women with uncomplicated pelvic inflammatory disease |
Sex specific |
| Heystek et al.169 USA |
Int J STD AIDS (2009) |
686 (100) |
29.0±7.3 |
Pelvis inflammatory disease |
To demonstrate non-inferiority of once-daily oral MXF compared with combination therapy in the management of acute, uncomplicated pelvic inflammatory disease |
Sex specific |
| Wenisch et al.170 USA |
Infection (2006) |
63 (69.8) |
69±15 |
Pneumonia |
To compare standard therapy with MXF regarding clinical cure/failure rates after start of intrahospital therapy and cure/failure rates of intrahospital therapy 28 days after initiation of intrahospital therapy |
Enough women, sex was not considered in the design or data analysis |
| Morovic et al.171 Croatia |
Am J Trop Med Hyg (2005) |
77 (14.3) |
11–66 |
Q fever pneumonia |
To compare efficacy of clarithromycin, MXF, and doxycycline in the treatment of Q fever pneumonia |
Sex not included in the design Not enough women |
| Giordano et al.172 USA |
Int J Antimicrob Agents (2005) |
617 (34.6) |
18–90 |
Skin infection |
To evaluate the clinical and bacteriological efficacy and tolerability of sequential i.v./p.o. MXF compared with a control regimen of i.v. piperacillin-tazobactam followed by p.o. amoxicillin/clavulanate for the treatment of hospitalized patients with complicated skin and skin structure infections |
Sex analysis performed Sex was not considered in the design |
| Vick- Fragoso et al.173 Philippines, Taiwan, Germany, Hungary, Spain, Chile, Israel, Argentina, |
Infection (2009) |
804 (39.4) |
51.8 |
Skin infection |
To compare sequential intravenous/oral (i.v./p.o.) MXF, 400 mg once daily, and i.v. amoxicillin/clavulanate, 1,000 mg/200 mg three times daily followed by p.o. amoxicillin/clavulanate, 500 mg/125 mg three times daily, for 7–21 days in hospitalized patients |
Enough women, sex was not considered in the design or data analysis |
| Parish et al.174 USA |
Int J Clin Pract (2000) | 351 (51.6) | 44 | Skin infections | To compare the efficacy and safety of oral MXF (400 mg once daily, 7 days) versus cephalexin (500 mg three times daily, 7 days) in uncomplicated skin infections | Sex analysis performed Sex not considered in the design |
Ordered by research focus and percentage of women participating in the clinical trials.
Objective of phase 3: treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it with commonly used treatments, and collect information that will allow it to be used safely.
AECB, Acute exacerbation of chronic bronchitis; COPD, chronic obstructive pulmonary disease; HAP, hospital acquired pneumonia; i.v., intravenous; p.o., oral.
Table 4.
Characteristics of Published Phase-4 Clinical Trials of Moxifloxacina
| First author | Journal | No. subjects (% women) | Age | Research focus | Objective | Limiting omission |
|---|---|---|---|---|---|---|
| Grassi et al.175 USA |
J Chemother (2002) |
423 (21.3) |
69.3±8.4 |
AECB |
To compare the efficacy and tolerability of a 5-day treatment course with oral MXF vs a 7-day course with intramuscular ceftriaxone in 476 patients with acute exacerbations of chronic bronchitis, and to conduct a cost minimization analysis of the two treatments from the perspectives of both the Italian National Health Service and society |
Sex not included in the design Not enough women |
| Miratvilles et al.176 Spain |
Int J Clin Pract (2001) |
5,736 (35.4) |
66 |
AECB |
To examine the clinical effect of oral MXF on patients' signs and symptoms of AECB |
Sex not included in the design Not enough women |
| Lorenz et al.177 USA |
J Int Med Res (2001) |
332 (46.1) |
16–93 |
AECB |
To compare with the macrolides azithromycin, clarithromycin and roxithromycin in a cohort study to assess clinical, safety, and health-related outcomes of these antimicrobials in general practice settings |
Enough women, sex was not considered in the design or data analysis |
| Koch et al.178 USA |
Clin Drug Investig (2004) |
1,146 (47.3) |
54.3±18.2 |
CAP |
To assess the efficacy, safety and tolerability of oral MXF in outpatients with respiratory tract infections treated in general practices in Germany with the focus on CAP |
Sex analysis was performed Sex not considered in the design |
| Landen et al.179 Germany |
J Int Med Res (2001) |
15,959 (48.7) |
14–60 |
CAP |
To study of the speed, efficacy and tolerability of MXF when used in clinical practice for the treatment of CAP or AECB |
Enough women, sex was not considered in the design or data analysis |
| Barth et al.180 Germany |
Clinical Drug Investigation (2005) |
1,749 (43.5) |
43.4±14.3 |
Pneumonia |
To investigate the efficacy, safety, and tolerability of sequential i.v./o.p. therapy with MXF in pneumonia under general hospital treatment conditions. |
Enough women, sex was not considered in the design or data analysis |
| Liu et al.181 China |
Int J Clin Pract (2007) |
3,184 (41.0) |
20–79 |
Respiratory tract infections |
To assess the efficacy and tolerability of MXF post marketing |
Sex analysis performed Sex not considered in the design. |
| Chen et al.182 China |
Clin Drug Investig (2006) |
855 (41.1) |
50.4±18.5 |
Respiratory tract infections |
To assess the efficacy, safety and tolerability of oral MXF in patients with respiratory tract infections treated by attending physicians in routine clinical practice in China |
Sex analysis performed Sex not considered in the design |
| Faich et al.183 USA |
Ann Pharmacother (2004) |
18,299 (61.9) |
6–97 |
Respiratory tract infections |
To further investigate MXF's general and cardiac safety and evaluate its efficacy in the community practice setting in a large surveillance study |
Enough women, sex was not considered in the design or data analysis |
| Elies et al.184 Germany |
Clin Drug Investig (2004) | 2,405 (56.8) | 43.4±14.3 | Sinusitis | To assess the efficacy, safety and tolerability of MXF in patients with respiratory tract infections treated in general practice in Germany | Enough women, sex was not considered in the design or data analysis |
Ordered by research focus and percentage of women participating in the clinical trials.
Phase 4: postmarketing studies delineate additional information, including the treatment's risks, benefits, and optimal use.
Thirty-one CTs were conducted on one sex alone: 23 phase I CTs were performed with 436 men and 3 CTs with 99 women; 3 phase II CTs were conducted with 52 men; and 2 phase III CTs with 1,427 women.
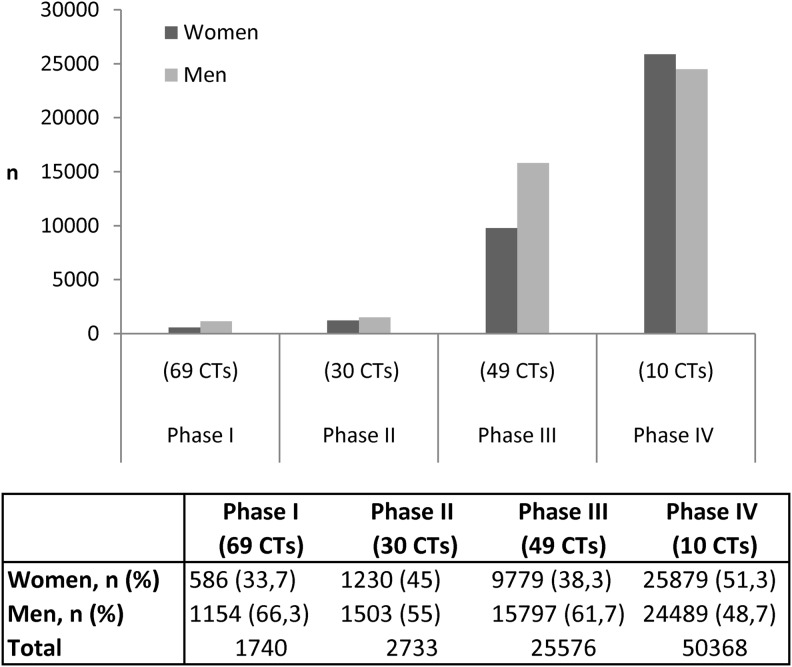
Figure 2 shows that women made up 33.7% of the enrollment in the 69 phase I CTs, 45% of the 30 phase II CTs, 38.3% of the 49 phase III CTs, and in 51.3% of the 10 phase IV CTs.
FIG. 2.
Participation of men and women in published moxifloxacin CTs.
Fifty-two (40.9%) of CTs stratified randomization by sex, accounting for 42.7% of the women (n=15,361 women) included as study subjects in the CTs (Table 5). Only 8 CTs (5.5%) discussed the results by sex (Table 5).
Table 5.
Moxifloxacin Clinical Trial Compliance with Variables Related to Sex Analysis and Women-Specific Issues
| Moxifloxacin CT | CT with sex analysis n (%) | Women/men in the CT with sex analysis n (%) | Bibliographic reference |
|---|---|---|---|
|
Stratification 127 CTs 42,471 men 35.948 women |
52 (40.9) |
15,361 (36.2)/ 18507 (43.6) |
40,66,69,73,79,87,88,93,94,97,98,100–102,108–110,120,121,124,125,132,133,135,136,142–145,150,151,153–156,159,160,164–166,168,170,172,173,177,179,182,184 |
|
Discussion 127 CTs 42,471 men 35,948 women |
8 |
356 (1.0)/ 627 (1.5) |
63,65,68,70,72,84,142,144 |
|
Analysis of efficacy 85 CTs 41,703 men 35,344 women |
13 (15.3) |
2,304 (11.0)/ 4,501 (10.8) |
11 CTs did not detect any differences70,124,125,128,130,133,139,144,147,167,172,174,182 1 CT detected sex differences at the time of peak serum bactericidal activity70 1 CT found the drug to be more effective in men than in women182 |
|
Analysis of adverse effects 78 CTs 37,785 men 33,651 women |
7 (9.0) |
14,055 (42.7)/ 10,648 (28.2) |
4 CTs found no sex differences47,65,181,183 1 CT identified being male as a risk factor142 1 CT reported better tolerance in men182 1 CT reported the possibility of paralytic ileus in 1 woman178 1 CT and 6 women and 3 men presented mild-to-moderate effects on their central nervous system in another27 |
|
Analysis of moxifloxacin effect on QT interval 53 CTs 17, 774 men 15,697 women |
3 (7.4) |
269 (1.7)/ 308 (1.7) |
No sex difference94,29,65 |
|
Analysis of dose-response 10 CTs 5,964 men 2,808 women |
0 |
0 |
NA |
|
Analysis of blood concentration response 46 CTs 4,528 men 2,762 women |
3 (8.7) |
25 (1.7)/ 77 (1.7) |
29,65,94 |
|
Pregnancy as exclusion criteriona 115 CTs 35,828 subjects |
60 (52.1) |
21,298 (59.4)/ NA |
38,40,63,64,66,67,69,70,72,76,78,82,83,86,91,94,95,97,108,110,114–116,118–121,124,125,129,130,131,134,135,138–141,144–148,151,152,155,157–159,161,164,172,173,178,180–184 |
|
Recruitment of women using a contraceptive method*,a 115 CTs 35,828 subjects |
21 (18.3) |
4,907 (13.7)/ NA |
39,63,64,66,68,71,72,76,127,128,130,131,137,143,145,169,174,175,177,182 |
|
Recruitment of women using hormonal contraceptiona 115 CTs 35,828 subjects |
3 (2.6) |
64 (0.2)/ NA |
63,72,76 |
|
Drug and hormonal contraception interactiona 115 CTs 35,828 subjects |
2 (1.7) |
59 (0.2)/ NA |
1 CT Hormonal contraceptives lowered the plasma concentration of MXF76 1 CT MXF does not interfere with hormonal contraception72 |
|
Recruitment of women taking HRTb 112 CTs 35,327 subjects |
0 |
0/ NA |
NA |
|
Interaction of the drug and HRT is evaluatedb 112 CTs 35,327 subjects |
0 |
0/ NA |
NA |
|
Influence of ovarian cycle status on pharmacokinetics of the drug†,c 132 CTs 36,651 subjects |
1 (1.5) | 30 (0.1)/ NA |
NA |
Adopted from the FDA Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs.14
Either oral or barrier contraception.
Including comparison between pre and postmenopausal patients.
CTs including women of childbearing age.
CTs including women of menopause age.
CTs including women.
CT, clinical trial; HRT, hormone replacement therapy.
Of the 85 CTs studying efficacy, 15.3% (n=13) conducted an analysis by sex (Table 5). Specifically, 11% of women and 10.8% of men were included. The results of these CTs showed that 11 CTs did not detect any differences (Table 5), 1 CT detected sex differences at the time of peak serum bactericidal activity, and 1 CT found the drug to be more effective in men than in women. Nine percent (n=7) of the 78 CTs that studied adverse effects conducted an analysis by sex (Table 5). These CTs included 42.7% of women, compared with 28.2% of men. However, only 3 CTs (7.4%) analyzed the QT interval by sex, accounting for 1.7% of study subjects of both sexes (269 women and 308 men): 1 CT did not detect significant differences between men and women, but 2 reported increased QT intervals in women (Table 5).
No sex differences were detected in the 3 CTs (8.7%) that analyzed blood concentration by sex (Table 5) and included 1.7% of study subjects of both sexes (25 women and 77 men). None of the CTs studied dose response by sex.
Table 5 shows the FDA recommendations related to women-specific issues. The most frequently observed recommendation was to consider pregnancy as an exclusion criterion in women of childbearing age, used in 52.1% of CTs (n=60). Regarding the recommendations related to contraception, 18.3% of CTs (n=21) included the recommendation to take measures to avoid pregnancy during the trial (either barrier or hormonal contraceptive methods). These CTs only included 13.5% (n=4,907) of women participating in CTs with women of childbearing age.
Only 3 CTs mentioned the inclusion of women using hormonal contraceptives (64 women) (Table 5). Furthermore, only 2 CTs conducted a comparison of women taking hormonal contraception and those who were not and analyzed the possible interactions between hormonal contraceptives and moxifloxacin (59 women) (Table 5). None of the CTs involving women of menopausal age specified whether the women were taking HRT. One CT studied the influence of menstrual status, including a comparison between pre- and postmenopausal women (Table 5).
The 158 CTs were published in 59 journals. Of these, only 466,73,104,167 CTs were published in 3 journals that recommended analyzing data by sex in their instructions for authors: Current Medical Research Review, Ophthalmology, and PLoS One. One of the 4 provided efficacy results by sex,167 while the other 3 stratified the study sample by sex.66,73,104
Discussion
The main findings of this review of published CTs on moxifloxacin were that fewer women participated in phase I trials. This is significant, as results from this initial phase are used to determine dosage in subsequent phases. The study also revealed that CTs that examined adverse effects included more women than men, and that although a considerable number of CTs stratified by sex in the design phase, only a very small proportion analyzed the results by sex. Moreover, very little attention was paid to the influence of hormones on the action of moxifloxacin. Although an increasing number of policies exist aimed at ensuring the inclusion of women in CTs and the analysis of sex differences, and organizations such as the FDA, NIH,185 or the General Accounting Office 186 have made considerable efforts in this respect, in practice implementation of such policies is still insufficient according to the information published on CTs of therapeutic drugs such as moxifloxacin.
Since the CT is the paradigm of clinical research, and the fundamental tool for evaluating drugs, the distribution of CT patients by sex should reflect the population of patients that will use the drug once it is on the market.14 The moxifloxacin CTs included equal numbers of men and women, coinciding with the number of users according to phase IV or post-marketing data.147,178–186 However, women were underrepresented in phase I, which implies the loss of information necessary for the design of subsequent CT phases.18
Few CTs conducted an analysis of efficacy by sex, and these included only a small proportion of men and women with respect to the total number of efficacy CTs. This raises the question of whether failure to take sex into account in the design of the sample size reduces a CT's power to detect differences between the two sexes in the subsequent sex-stratified analysis. The case of the adverse effects of moxifloxacin is different. Although few CTs were aimed at identifying adverse effects by sex, these did include a high proportion of both men and women. The results of these CTs were heterogeneous and thus no specific conclusions can be drawn. The variability of results for the occurrence of adverse effects is important, since women have been reported to experience adverse effects with greater frequency.187 Specifically, four CTs found no sex differences.47, 65,181,183 In contrast, one CT identified being male as a risk factor,142 whereas another reported better tolerance in men.182 One CT178 reported the possibility of paralytic ileus in one woman, and six women and three men presented mild-to-moderate effects on their central nervous system in another.27 The Summary of Product Characteristics (SPC)3 for moxifloxacin indicates that women may be more sensitive to drugs that prolong the QT interval; however, only a few trials have studied differences by sex for this effect. This may be because the effect is already known.188 The SPC for moxifloxacin indicates that plasma concentration may be higher in women,3 although the published moxifloxacin CTs did not give pharmacokinetic data by sex.
As regards women-related issues, the use of moxifloxacin is contraindicated in pregnancy, since reversible joint damage has been described in children who had absorbed quinolones.3 Furthermore, although the reproductive toxicity of moxifloxacin in humans is unknown, other fluoroquinolones have been associated with an increased frequency of miscarriage in pregnant women.189 According to this information, all CTs that include women of childbearing age should specify pregnancy as an exclusion criterion. However, nearly half did not provide information on whether pregnancy was used as an exclusion criterion, let alone whether subjects were required to take steps to avoid pregnancy during the CT.
The possible influence of hormones on the results of the published moxifloxacin CTs is barely addressed, and thus there is a clear lack of information on the concomitant use of this drug and HRT. When reporting data about interactions between hormone levels and moxifloxacin, the information was sometimes incomplete. This was the case with one CT, which indicated that the use of oral contraceptives was permitted but did not report whether any of the women subjects were taking them nor analyzed the possible effect.63 According to the study by Stass, moxifloxacin does not interfere with hormonal contraception72; however, another study found that hormonal contraceptives lowered the plasma concentration of moxifloxacin, which is an important consideration when treating for pathogens with “borderline susceptibility” to moxifloxacin.76 Interestingly, the SPC does not mention the interaction between moxifloxacin and hormones.3
The pharmaceutical industry has made valuable contributions to the effective treatment of certain diseases and should therefore be an authority on the subject. Consequently, it is difficult to understand why it would risk losing credibility by not complying with recommendations such as considering an analysis by sex in the discussion sections of related articles. This information is easy to include but was only provided in a handful of articles on moxifloxacin. Two of these articles acknowledged that although sex-related differences had been detected, the results were of limited value due to the insufficient number of women included in the trial.68 In one CT, the authors reported being unable to explain the significance of the sex difference detected in time of peak serum bactericidal activity.70 Another reported an already well-known difference, namely that being male is a risk factor for mortality in community-acquired pneumonia.142 A further three CTs mentioned in their discussion sections that no sex-related differences had been detected.72,65,144
Regulatory bodies and funding agencies should devise new programs or strategies to improve representation of both sexes in CTs and should oblige researchers to consider sex differences during data analysis. Together with public health authorities, the pharmaceutical industry is facing the new challenge of protecting health by implementing actions that comply with the codes of good scientific practice. As the Canadian Medical Research Council's Advisory Committee on Women in Clinical Trials has suggested, if information is provided by sex for each clinical trial, it would be possible to conduct meta-analyses to determine how women respond to a particular drug.190 Furthermore, Paula A. Rochon has proposed the publication of subgroup analyses of men and women to facilitate meta-analysis.15 The recently published CONSORT statement191 has missed the opportunity to include recommendations from a gender perspective, which would have been decisive in preventing methodological biases in study designs and analyses that limit the accuracy and extrapolation of the findings.192 In the case of moxifloxacin CTs, only three journals (Current Medical Research Review, Ophthalmology, PLoS One) mentioned this requirement. Although it is possible that many CTs are well designed, based on an adequate number of patients, and include an analysis by sex of the data, this information is not published, perhaps because the researchers did not consider the information to be relevant when they detected no differences. We could improve this situation if scientific journals were to recommend (or oblige) authors to present such data in their editorial instructions, or if the authors were at least to state whether they had conducted (or not) an analysis by sex, and if so, that the results would be made available on request. Knowing that CT results reveal no sex-related differences is also important. Nevertheless, it would be useful to provide incentives to researchers to publish these results separately, and to train junior researchers and students in the skills necessary to analyze CT results, identify the omissions that have occurred (or can occur) and recognize exemplary studies.
The study did have some limitations. As the study was based on the sex differences and gender analyses reported in published clinical trials of moxifloxacin indexed in Medline and the Cochrane Library, it has not covered other relevant characteristics that are worthy of investigation, such as age and ethnicity. In addition, we cannot be totally sure that data by sex is not held by the regulatory authorities or pharmaceutical companies concerned. However, previous studies on reports submitted by pharmaceutical companies to regulatory authorities have indicated that this information does not exist,26,193 and the main regulatory agencies have no authority (legal means) to compel disclosure of drug effects by gender.
Disclosure Statement
No financial conflicts exist.
References
- 1.Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: Differences and dilemmas. Clin Infect Dis 2001;32:S72–79 [DOI] [PubMed] [Google Scholar]
- 2.Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001;2:349–351 [DOI] [PubMed] [Google Scholar]
- 3.Agencia Española de Medicamentos y Productos Sanitarios [Summary of Product Characteristics] 2010
- 4.Moxifloxacin: New indication. Do not use in gynaecological infections or other conditions. Prescrire Int 2009;18:207. [PubMed] [Google Scholar]
- 5.Food and Drug Administration (FDA) Center for Drug Evaluation and Research Anti-infective drugs advisory committee, 67th meeting Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, 1999 [Google Scholar]
- 6.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: The importance of reporting suspected reactions. Arch Intern Med 2005;165:1363–1369 [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency European Medicines Agency recommends restricting the use of oral moxifloxacin-containing medicines. London: EMEA/CHMP/382927, 2008 [Google Scholar]
- 8.Food and Drug Administration, Otsuka Pharmaceutical Co., Ltd. Withdrawal of Approval of a New Drug Application [Docket No. 2007N–0221]. 2007. Available at: www.fda.gov/OHRMS/DOCKETS/98fr/E7-11496.pdf Accessed November28, 2012
- 9.Mehlhorn AJ, Brown DA. Safety concerns with fluoroquinolones. Ann Pharmacother 2007;41:1859–1866 [DOI] [PubMed] [Google Scholar]
- 10.Moxifloxacin: New preparation. A me-too with more cardiac risks. Prescrire Int 2002;11:168–169 [PubMed] [Google Scholar]
- 11.Food and Drug Administration FDA request boxed warnings on fluoroquinolone antimicrobial drugs 08-07-2008. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2011/021085s047,021277s041lbl.pdf Accessed November28, 2012
- 12.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med 2005;118:259–268 [DOI] [PubMed] [Google Scholar]
- 13.Bayer Bayer Annual Report 2010. Bayer AG, Leverkusen, Germany: Available at: www.annualreport2010.bayer.com/ Accessed November28, 2012 [Google Scholar]
- 14.Food and Drug Administration (FDA), Center for Drug Evaluation and research Guidance for Industry. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs. Department of Health and Human Services. FDA. Federal Register; 1993;58:39409–39411 Available at: www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126835.pdf Accessed November28, 2012 [Google Scholar]
- 15.Rochon PA, Clark JP, Binns MA, Patel V, Gurwitz JH. Reporting of gender-related information in clinical trials of drug therapy for myocardial infarction. Can Med Assoc J 1998;159:321–327 [PMC free article] [PubMed] [Google Scholar]
- 16.Ramasubbu K, Gurm H, Litaker D. Gender bias in clinical trials: do double standards still apply? J Womens Health Gend Based Med 2001;10:757–764 [DOI] [PubMed] [Google Scholar]
- 17.Vidaver RM, Lafleur B, Tong C, Bradshaw R, Marts SA. Women subjects in NIH-funded clinical research literature: lack of progress in both representation and analysis by sex. J Womens Health Gend Based Med 2000;9:495–504 [DOI] [PubMed] [Google Scholar]
- 18.Fleisch J, Fleisch MC, Thurmann PA. Women in early-phase clinical drug trials: have things changed over the past 20 years? Clin Pharmacol Ther 2005;78:445–452 [DOI] [PubMed] [Google Scholar]
- 19.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med 2000;343:475–480 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz Cantero MT, Angeles Pardo M. European Medicines Agency policies for clinical trials leave women unprotected. J Epidemiol Community Health 2006;60:911–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero I, Talavera M, Ruiz I. Clinical trials with a new atypical antipsychotic (Aripiprazole): Gender specific information analysis. Women Health 2008;47:39–51 [DOI] [PubMed] [Google Scholar]
- 22.Chilet-Rosell E, Ruiz-Cantero MT, Horga JF. Women's health and gender-based clinical trials on etoricoxib: Methodological gender bias. J Public Health 2009;31:434–445 [DOI] [PubMed] [Google Scholar]
- 23.Cascales Perez S, Ruiz Cantero MT, Pardo MA. [Clinical trials with rofecoxib: Analysis of the information from the gender perspective]. Medicina Clinica (Barc) 2003;120:207–212 [DOI] [PubMed] [Google Scholar]
- 24.Bartlett C, Doyal L, Ebrahim S, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess 2005;9:iii–iv, ix-x, 1–152 [DOI] [PubMed] [Google Scholar]
- 25.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: Have we made progress? J Womens Health 2009;20:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laguna Goya N, Andrés Rodriguez-Trelles FD. Participación de las mujeres en los ensayos clínicos segúnn los informes de la Agencia Española de Medicamentos y Productos Sanitarios: 2007. Revista Española de Salud Pública 2008;82:343–350 [DOI] [PubMed] [Google Scholar]
- 27.Pfaller MA, Jones RN. Gatifloxacin Phase IV surveillance trial (TeqCES Study) utilizing 5000 primary care physician practices: report of pathogens isolated and susceptibility patterns in community-acquired respiratory tract infections. Diagn Microbiol Infect Dis 2002;44:77–84 [DOI] [PubMed] [Google Scholar]
- 28.George AP. Multicenter, phase IV evaluation of intravenous cirpfloxacin as initial therapy in patients with lower respiratory tract, urinary tract, and skin/skin structure infections. Clin Ther 1995;17:353–365 [DOI] [PubMed] [Google Scholar]
- 29.Ober MC, Hoppe-Tichy T, Köninger J, et al. Tissue penetration of moxifloxacin into human gallbladder wall in patients with biliary tract infections. J Antimicrob Chemother 2009;64:1091–1095 [DOI] [PubMed] [Google Scholar]
- 30.Czock D, Hüsig-Linde C, Langhoff A, et al. Pharmacokinetics of moxifloxacin and levofloxacin in intensive care unit patients who have acute renal failure and undergo extended daily dialysis. Clin J Am Soc Nephrol 2006;1:1263–1268 [DOI] [PubMed] [Google Scholar]
- 31.Fuhrmann V, Schenk P, Jaeger W, Ahmed S, Thalhammer F. Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration. J Antimicrob Chemother 2004;54:780–784 [DOI] [PubMed] [Google Scholar]
- 32.Metallidis S, Topsis D, Nikolaidis J, et al. Penetration of moxifloxacin and levofloxacin into cancellous and cortical bone in patients undergoing total hip arthroplasty. J Chemother 2007;19:682–687 [DOI] [PubMed] [Google Scholar]
- 33.Breilh D, Jougon J, Djabarouti S, et al. Diffusion of oral and intravenous 400 mg once-daily moxifloxacin into lung tissue at pharmacokinetic steady-state. J Chemother 2003;15:558–562 [DOI] [PubMed] [Google Scholar]
- 34.Simon N, Sampol E, Albanese J, et al. Population pharmacokinetics of moxifloxacin in plasma and bronchial secretions in patients with severe bronchopneumonia. Clin Pharmacol Ther 2003;74:353–363 [DOI] [PubMed] [Google Scholar]
- 35.Metallidis S, Charokopos N, Nikolaidis J, et al. Penetration of moxifloxacin into sternal bone of patients undergoing routine cardiopulmonary bypass surgery. Int J Antimicrob Agents 2006;28:428–432 [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Saenz MC, Arias-Puente A, Fresnadillo-Martinez MJ, Carrasco-Font C. Human aqueous humor levels of oral ciprofloxacin, levofloxacin, and moxifloxacin. J Cataract Refract Surg 2001;27:1969–1974 [DOI] [PubMed] [Google Scholar]
- 37.Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol 2005;89:628–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon R, Donnenfeld ED, Perry HD, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology 2005;112:466–469 [DOI] [PubMed] [Google Scholar]
- 39.Gehanno P, Darantiere S, Dubreuil C, et al. A prospective, multicentre study of moxifloxacin concentrations in the sinus mucosa tissue of patients undergoing elective surgery of the sinus. J Antimicrob Chemother 2002;49:821–826 [DOI] [PubMed] [Google Scholar]
- 40.Capitano B, Mattoes HM, Shore E, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 2004;125:965–973 [DOI] [PubMed] [Google Scholar]
- 41.Skalioti C, Tsaganos T, Stamatiadis D, et al. Pharmacokinetics of moxifloxacin in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 2009;29:575–579 [PubMed] [Google Scholar]
- 42.Wirtz M, Kleeff J, Swoboda S, et al. Moxifloxacin penetration into human gastrointestinal tissues. J Antimicrob Chemother 2004;53:875–877 [DOI] [PubMed] [Google Scholar]
- 43.Wise R, Andrews JM, Marshall G, Hartman G. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob Agents Chemother 1999;43:1508–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stass H, Kubitza D, Moller JG, Delesen H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br J Clin Pharmacol 2005;59:536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballow C, Lettieri J, Agarwal V, Liu P, Stass H, Sullivan JT. Absolute bioavailability of moxifloxacin. Clin Ther 1999;21:513–522 [DOI] [PubMed] [Google Scholar]
- 46.Burkhardt O, Borner K, Stass H, et al. Single- and multiple-dose pharmacokinetics of oral moxifloxacin and clarithromycin, and concentrations in serum, saliva and faeces. Scand J Infect Dis 2002;34:898–903 [DOI] [PubMed] [Google Scholar]
- 47.Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis 2000;32:81–85 [DOI] [PubMed] [Google Scholar]
- 48.Müller M, Stass H, Brunner M, Moller JG, Lackner E, Eichler HG. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother 1999;43:2345–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stass H, Nagelschmitz J, Moeller JG, Delesen H. Pharmacokinetics of moxifloxacin are not influenced by a 7-day pretreatment with 200 mg oral itraconazole given once a day in healthy subjects. Int J Clin Pharmacol Ther 2004;42:23–29 [DOI] [PubMed] [Google Scholar]
- 50.Stass H, Wandel C, Delesen H, Moller JG. Effect of calcium supplements on the oral bioavailability of moxifloxacin in healthy male volunteers. Clin Pharmacokinet 2001;1:27–32 [DOI] [PubMed] [Google Scholar]
- 51.Stass H, Schuhly U, Moller JG, Delesen H. Effects of sucralfate on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet 2001;1:49–55 [DOI] [PubMed] [Google Scholar]
- 52.Stass H, Sachse R. Effect of probenecid on the kinetics of a single oral 400mg dose of moxifloxacin in healthy male volunteers. Clin Pharmacokinet 2001;1:71–76 [DOI] [PubMed] [Google Scholar]
- 53.Stass H, Kubitza D. Effects of iron supplements on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in humans. Clin Pharmacokinet 2001;1:57–62 [DOI] [PubMed] [Google Scholar]
- 54.Stass H, Kubitza D. Effects of dairy products on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in healthy volunteers. Clin Pharmacokinet 2001;1:33–38 [DOI] [PubMed] [Google Scholar]
- 55.Stass H, Kubitza D. Lack of pharmacokinetic interaction between moxifloxacin, a novel 8-methoxyfluoroquinolone, and theophylline. Clin Pharmacokinet 2001;1:63–70 [DOI] [PubMed] [Google Scholar]
- 56.Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother 1999;43:83–90 [DOI] [PubMed] [Google Scholar]
- 57.Wagenlehner FM, Kees F, Weidner W, Wagenlehner C, Naber KG, Wagenlehner FME. Concentrations of moxifloxacin in plasma and urine, and penetration into prostatic fluid and ejaculate, following single oral administration of 400 mg to healthy volunteers. Int J Antimicrob Agents. 2008;31:21–26 [DOI] [PubMed] [Google Scholar]
- 58.Stass H, Bottcher MF, Ochmann K. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400mg dose to healthy volunteers. Clin Pharmacokinet 2001;1:39–48 [DOI] [PubMed] [Google Scholar]
- 59.Modi NB, Nath R, Staehr P, et al. Pharmacokinetic, pharmacodynamic, and electrocardiographic effects of dapoxetine and moxifloxacin compared with placebo in healthy adult male subjects. J Clin Pharmacol 2009;49:634–642 [DOI] [PubMed] [Google Scholar]
- 60.Stass H, Dalhoff A, Kubitza D, Schuhly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 1998;42:2060–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Extramiana F, Maison-Blanche P, Cabanis M-J, Ortemann-Renon C, Beaufils P, Leenhardt A. Clinical assessment of drug-induced QT prolongation in association with heart rate changes. Clin Pharmacol Ther 2005;77:247–258 [DOI] [PubMed] [Google Scholar]
- 62.Morganroth J, Ilson BE, Shaddinger BC, et al. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol 2004;93:1378–1383 [DOI] [PubMed] [Google Scholar]
- 63.Sullivan JT, Woodruff M, Lettieri J, et al. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob Agents Chemother 1999;43:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiner M, Burman W, Luo C-C, et al. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother 2007;51:2861–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan JT, Lettieri JT, Liu P, Heller AH. The influence of age and gender on the pharmacokinetics of moxifloxacin. Clin Pharmacokinet 2001;1:11–18 [DOI] [PubMed] [Google Scholar]
- 66.Barriere S, Genter F, Spencer E, et al. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J Clin Pharmacol 2004;44:689–695 [DOI] [PubMed] [Google Scholar]
- 67.Joukhadar C, Stass H, Muller-Zellenberg U, et al. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob Agents Chemother 2003;47:3099–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demolis JL, Kubitza D, Tenneze L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther 2000;68:658–666 [DOI] [PubMed] [Google Scholar]
- 69.Torkildsen G, O'Brien TP, Torkildsen G, O'Brien TP. Conjunctival tissue pharmacokinetic properties of topical azithromycin 1% and moxifloxacin 0.5% ophthalmic solutions: a single-dose, randomized, open-label, active-controlled trial in healthy adult volunteers. Clin Ther 2008;30:2005–2014 [DOI] [PubMed] [Google Scholar]
- 70.Hart D, Weinstein MP, Hart D, Weinstein MP. Cross-over assessment of serum bactericidal activity of moxifloxacin and levofloxacin versus penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae in healthy volunteers. Diagn Microbiol Infect Dis 2007;58:375–378 [DOI] [PubMed] [Google Scholar]
- 71.Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother 2000;44:2600–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stass H, Kubitza D. Profile of moxifloxacin drug interactions. Clin Infect Dis 2001;32:15. [DOI] [PubMed] [Google Scholar]
- 73.Noel GJ, Natarajan J, Chien S, et al. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin Pharmacol Ther 2003;73:292–303 [DOI] [PubMed] [Google Scholar]
- 74.Donnenfeld E, Perry HD, Chruscicki DA, Bitterman A, Cohn S, Solomon R. A comparison of the fourth-generation fluoroquinolones gatifloxacin 0.3% and moxifloxacin 0.5% in terms of ocular tolerability. Curr Med Res Opin 2004;20:1753–1758 [DOI] [PubMed] [Google Scholar]
- 75.Price MO, Price FW, Jr., Maclellan D, Price MO, Price FW, Jr., Maclellan D. Effect of gatifloxacin 0.3% and moxifloxacin 0.5% ophthalmic solutions on human corneal epithelium following 2 dosing regimens. J Cataract Refract Surg 2005;31:2137–2141 [DOI] [PubMed] [Google Scholar]
- 76.Shain CS, Whitaker AM, Amsden G. Effects of oral contraceptives on the pharmacokinetics of moxifloxacin in premenopausal women. Clin Drug Investig 2002;22:429–434 [Google Scholar]
- 77.Stass H, Kubitza D, Schuhly U. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone, after repeated oral administration. Clin Pharmacokinet 2001;1:1–9 [DOI] [PubMed] [Google Scholar]
- 78.Barth J, Jäger D, Mundkowski R, Drewelow B, Welte T, Burkhardt O. Single- and multiple-dose pharmacokinetics of intravenous moxifloxacin in patients with severe hepatic impairment. J Antimicrob Chemother 2008;62:575–578 [DOI] [PubMed] [Google Scholar]
- 79.Stass H, Bührmann S, Mitchell A, et al. The influence of continuous venovenous haemodialysis on the pharmacokinetics of multiple oral moxifloxacin administration to patients with severe renal dysfunction. Br J Clin Pharmacol 2007;64:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rink AD, Stass H, Delesen H, et al. Pharmacokinetics and tissue penetration of moxifloxacin in intervention therapy for intra-abdominal abscess. Clin Drug Investig 2008;28:71–79 [DOI] [PubMed] [Google Scholar]
- 81.Malincarne L, Ghebregzabher M, Moretti MV, et al. Penetration of moxifloxacin into bone in patients undergoing total knee arthroplasty. J Antimicrob Chemother 2006;57:950–954 [DOI] [PubMed] [Google Scholar]
- 82.Wacke R, Forster S, Adam U, et al. Penetration of moxifloxacin into the human pancreas following a single intravenous or oral dose. J Antimicrob Chemother 2006;58:994–999 [DOI] [PubMed] [Google Scholar]
- 83.Stass H, Kubitza D, Aydeniz B, et al. Penetration and accumulation of moxifloxacin in uterine tissue. Int J Gynaecol Obstet 2008;102:132–136 [DOI] [PubMed] [Google Scholar]
- 84.Pea F, Qual ED, Cusenza A, Brollo L, Baldassarre M, Furlanut M. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin Pharmacokinet 2003;42:589–598 [DOI] [PubMed] [Google Scholar]
- 85.Stass H, Rink AD, Delesen H, Kubitza D, Vestweber KH. Pharmacokinetics and peritoneal penetration of moxifloxacin in peritonitis. J Antimicrob Chemother 2006;58:693–696 [DOI] [PubMed] [Google Scholar]
- 86.Leone M, Albanese J, Sampol-Manos E, et al. Moxifloxacin penetration in bronchial secretions of mechanically ventilated patients with pneumonia. Antimicrob Agents Chemother 2004;48:638–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinis PB, Monteiro MC, Martins ML, et al. Sinus tissue concentration of moxifloxacin after a single oral dose. Ann Otol Rhinol Laryngol 2004;113:142–146 [DOI] [PubMed] [Google Scholar]
- 88.Esposito S, Noviello S, D'Errico G, et al. Concentration of moxifloxacin in plasma and tonsillar tissue after multiple administration in adult patients. [Erratum appears in J Antimicrob Chemother 2006 May;57:1022]. J Antimicrob Chemother 2006;57:789–792 [DOI] [PubMed] [Google Scholar]
- 89.Alffenaar JWC, van Altena R, Bökkerink HJ, et al. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin Infect Dis 2009;49:1080–1082 [DOI] [PubMed] [Google Scholar]
- 90.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006;10:605–612 [PubMed] [Google Scholar]
- 91.Nijland HMJ, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007;45:1001–1007 [DOI] [PubMed] [Google Scholar]
- 92.Valerio G, Bracciale P, Manisco V, Quitadamo M, Legari G, Bellanova S. Long-term tolerance and effectiveness of moxifloxacin therapy for tuberculosis: preliminary results. J Chemother. 2003;15:66–70 [DOI] [PubMed] [Google Scholar]
- 93.Fuller JJ, Lott MN, Henson NM, et al. Vitreal Penetration of Oral and Topical Moxifloxacin in Humans. Am J Ophthalmol 2007;143:338–340 [DOI] [PubMed] [Google Scholar]
- 94.Lott MN, Fuller JJ, Hancock HA, et al. Vitreal penetration of oral moxifloxacin in humans. Retina 2008;28:473–476 [DOI] [PubMed] [Google Scholar]
- 95.Vedantham V, Lalitha P, Velpandian T, Ghose S, Mahalakshmi R, Ramasamy K. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Eye 2006;20:1273–1278 [DOI] [PubMed] [Google Scholar]
- 96.Bozkurt B, Karakaya G, Kalyoncu AF. Tolerability of moxifloxacin in patients with antibiotic hypersensitivity. Allergol Immunopathol (Madr) 2005;33:38–41 [DOI] [PubMed] [Google Scholar]
- 97.Constantinou M, Daniell M, Snibson GR, et al. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology 2007;114:1622–1629 [DOI] [PubMed] [Google Scholar]
- 98.Vasavada AR, Gajjar D, Raj SM, et al. Comparison of 2 moxifloxacin regimens for preoperative prophylaxis: prospective randomized triple-masked trial. Part 2: Residual conjunctival flora. J Cataract Refract Surg 2008;34:1383–1388 [DOI] [PubMed] [Google Scholar]
- 99.Katz HR, Masket S, Lane SS, et al. Absorption of topical moxifloxacin ophthalmic solution into human aqueous humor. Cornea 2005;24:955–958 [DOI] [PubMed] [Google Scholar]
- 100.Lane SS, Osher RH, Masket S, et al. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg 2008;34:1451–1459 [DOI] [PubMed] [Google Scholar]
- 101.Ong-Tone L. Aqueous humor penetration of gatifloxacin and moxifloxacin eyedrops given by different methods before cataract surgery. J Cataract Refract Surg 2007;33:59–62 [DOI] [PubMed] [Google Scholar]
- 102.Diz Dios P, Tomas Carmona I, Limeres Posse J, Medina Henriquez J, Fernandez Feijoo J, Alvarez Fernandez M. Comparative efficacies of amoxicillin, clindamycin, and moxifloxacin in prevention of bacteremia following dental extractions. Antimicrob Agents Chemother 2006;50:2996–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deramo VA, Lai JC, Fastenberg DM, Udell IJ. Acute endophthalmitis in eyes treated prophylactically with gatifloxacin and moxifloxacin. Am J Ophthalmol 2006;142:721–725.e721 [DOI] [PubMed] [Google Scholar]
- 104.Beyer G, Hiemer-Bau M, Ziege S, Edlund C, Lode H, Nord CE. Impact of moxifloxacin versus clarithromycin on normal oropharyngeal microflora. Eur J Clin Microbiol Infect Dis 2000;19:548–550 [DOI] [PubMed] [Google Scholar]
- 105.Man I, Murphy J, Ferguson J. Fluoroquinolone phototoxicity: A comparison of moxifloxacin and lomefloxacin in normal volunteers. J Antimicrob Chemother 1999;43:77–82 [DOI] [PubMed] [Google Scholar]
- 106.He L, Ta CN, Miño de Kaspar H. One-day application of topical moxifloxacin 0.5% to select for fluoroquinolone-resistant coagulase-negative Staphylococcus. J Cataract Refract Surg 2009;35:1715–1718 [DOI] [PubMed] [Google Scholar]
- 107.Sebban C, Dussart S, Fuhrmann C, et al. Oral moxifloxacin or intravenous ceftriaxone for the treatment of low-risk neutropenic fever in cancer patients suitable for early hospital discharge. Support Care Cancer 2008;16:1017–1023 [DOI] [PubMed] [Google Scholar]
- 108.Cheon JH, Kim N, Lee DH, et al. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter 2006;11:46–51 [DOI] [PubMed] [Google Scholar]
- 109.Kilic ZM, Koksal AS, Cakal B, et al. Moxifloxacine plus amoxicillin and ranitidine bismuth citrate or esomeprazole triple therapies for Helicobacter pylori infection. Dig Dis Sci 2008;53:3133–3137 [DOI] [PubMed] [Google Scholar]
- 110.Bago P, Vcev A, Tomic M, et al. High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr 2007;119:372–378 [DOI] [PubMed] [Google Scholar]
- 111.Miehlke S, Schneider-Brachert W, Kirsch C, et al. One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2008;13:69–74 [DOI] [PubMed] [Google Scholar]
- 112.Ta CN, Chan I, Dhatt HS, et al. Prospective comparison of topical moxifloxacin in eliminating conjunctival bacterial flora following a one-day or one-hour application. J Ocul Pharmacol Ther 2008;24:427–431 [DOI] [PubMed] [Google Scholar]
- 113.Pardillo FEF, Burgos J, Fajardo TT, et al. Powerful bactericidal activity of moxifloxacin in human leprosy. Antimicrob Agents Chemother 2008;52:3113–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwab D, Grauer M, Hahn EG, Muhldorfer S. Biliary secretion of moxifloxacin in obstructive cholangitis and the non-obstructed biliary tract. Aliment Pharmacol Ther 2005;22:417–422 [DOI] [PubMed] [Google Scholar]
- 115.Holland EJ, Lane SS, Kim T, et al. Ocular penetration and pharmacokinetics of topical gatifloxacin 0.3% and moxifloxacin 0.5% ophthalmic solutions after keratoplasty. Cornea 2008;27:314–319 [DOI] [PubMed] [Google Scholar]
- 116.Guentsch A, Jentsch H, Pfister W, et al. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol 2008;79:1894–1903 [DOI] [PubMed] [Google Scholar]
- 117.Burka JM, Bower KS, Vanroekel RC, et al. The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am J Ophthalmol 2005;140:83–87 [DOI] [PubMed] [Google Scholar]
- 118.Moshirfar M, Marx DP, Kumar R. The effect of the fourth-generation fluoroquinolones on corneal reepithelialization after penetrating keratoplasty. Cornea 2005;24:833–836 [DOI] [PubMed] [Google Scholar]
- 119.Jardim JR, Rico G, de la Roza C, et al. [A comparison of moxifloxacin and amoxicillin in the treatment of community-acquired pneumonia in Latin America: results of a multicenter clinical trial]. Arch Bronconeumol 2003;39:387–393 [DOI] [PubMed] [Google Scholar]
- 120.Ott S, Allewelt M, Lorenz J, Reimnitz P, Lode H; The German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008;36:23–30 [DOI] [PubMed] [Google Scholar]
- 121.Campos M, Avila M, Wallau A, Muccioli C, Hofling-Lima AL, Belfort R. Efficacy and tolerability of a fixed-dose moxifloxacin - dexamethasone formulation for topical prophylaxis in LASIK: a comparative, double-masked clinical trial. Clin Ophthalmol 2008;2:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gosling RD, Uiso LO, Sam NE, et al. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. Am J Respir Crit Care Med 2003;168:1342–1345 [DOI] [PubMed] [Google Scholar]
- 123.Pletz MWR, De Roux A, Roth A, Neumann K-H, Mauch H, Lode H. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother 2004;48:780–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006;174:331–338 [DOI] [PubMed] [Google Scholar]
- 125.Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: A double-blind, randomised, controlled phase II trial. Lancet 2009;373:1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferguson BJ, Guzzetta RV, Spector SL, et al. Efficacy and safety of oral telithromycin once daily for 5 days versus moxifloxacin once daily for 10 days in the treatment of acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004;131:207–214 [DOI] [PubMed] [Google Scholar]
- 127.Siegert R, Gehanno P, Nikolaidis P, et al.; The Sinusitis Study Group. A comparison of the safety and efficacy of moxifloxacin (BAY 12-8039) and cefuroxime axetil in the treatment of acute bacterial sinusitis in adults. Respir Med 2000;94:337–344 [DOI] [PubMed] [Google Scholar]
- 128.Rakkar S, Roberts K, Towe BF, Flores SM, Heyd A, Warner J. Moxifloxacin versus amoxicillin clavulanate in the treatment of acute maxillary sinusitis: a primary care experience. Int J Clin Pract 2001;55:309–315 [PubMed] [Google Scholar]
- 129.Gehanno P, Berche P, Perrin A. Moxifloxacin in the treatment of acute maxillary sinusitis after first-line treatment failure and acute sinusitis with high risk of complications. J Int Med Res 2003;31:434–447 [DOI] [PubMed] [Google Scholar]
- 130.Burke T, Villanueva C, Mariano H Jr., et al.; Sinusitis Infection Study Group Comparison of moxifloxacin and cefuroxime axetil in the treatment of acute maxillary sinusitis. Clin Ther 1999;21:1664–1677 [DOI] [PubMed] [Google Scholar]
- 131.Klossek JM, Siegert R, Nikolaidis P, Arvis P, Leberre MA; Sinusitis Study Group. Comparison of the efficacy and safety of moxifloxacin and trovafloxacin for the treatment of acute, bacterial maxillary sinusitis in adults. J Laryngol Otol 2003;117:43–51 [DOI] [PubMed] [Google Scholar]
- 132.Miravitlles M, Llor C, Naberan K, Cots JMA, Molina JS. Variables associated with recovery from acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Respir Med 2005;99:955–965 [DOI] [PubMed] [Google Scholar]
- 133.Chodosh S, DeAbate CA, Haverstock D, Aneiro L, Church D; Short-course moxifloxacin therapy for treatment of acute bacterial exacerbations of chronic bronchitis. Respir Med 2000;94:18–27 [DOI] [PubMed] [Google Scholar]
- 134.Wilson R, Allegra L, Huchon G, et al. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004;125:953–964 [DOI] [PubMed] [Google Scholar]
- 135.Starakis I, Gogos CA, Bassaris H. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents 2004;23:129–137 [DOI] [PubMed] [Google Scholar]
- 136.Niederman MS, Anzueto A, Sethi S, et al. Eradication of H. influenzae in AECB: A pooled analysis of moxifloxacin phase III trials compared with macrolide agents. Respir Med 2006;100:1781–1790 [DOI] [PubMed] [Google Scholar]
- 137.Schaberg T, Ballin I, Huchon G, et al. A multinational, multicentre, non-blinded, randomized study of moxifloxacin oral tablets compared with co-amoxiclav oral tablets in the treatment of acute exacerbation of chronic bronchitis. J Int Med Res 2001;29:314–328 [DOI] [PubMed] [Google Scholar]
- 138.Wilson R, Kubin R, Ballin I, et al. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1999;44:501–513 [DOI] [PubMed] [Google Scholar]
- 139.DeAbate CA, Mathew CP, Warner JH, Heyd A, Church D; The Bronchitis Study Group The safety and efficacy of short course (5-day) moxifloxacin vs. azithromycin in the treatment of patients with acute exacerbation of chronic bronchitis. Respir Med 2000;94:1029–1037 [DOI] [PubMed] [Google Scholar]
- 140.Urueta-Robledo J, Ariza H, Jardim JR, et al. Moxifloxacin versus levofloxacin against acute exacerbations of chronic bronchitis: the Latin American Cohort. Respir Med 2006;100:1504–1511 [DOI] [PubMed] [Google Scholar]
- 141.Portier H, Brambilla C, Garre M, Paganin F, Poubeau P, Zuck P. Moxifloxacin monotherapy compared to amoxicillin-clavulanate plus roxithromycin for nonsevere community-acquired pneumonia in adults with risk factors. Eur J Clin Microbiol Infect Dis 2005;24:367–376 [DOI] [PubMed] [Google Scholar]
- 142.Lode H, Grossman C, Choudhri S, et al. Sequential IV/PO moxifloxacin treatment of patients with severe community-acquired pneumonia. Respir Med 2003;97:1134–1142 [DOI] [PubMed] [Google Scholar]
- 143.Petitpretz P, Arvis P, Marel M, Moita J, Urueta J; CAP5 Moxifloxacin Study Group. Oral moxifloxacin vs high-dosage amoxicillin in the treatment of mild-to-moderate, community-acquired, suspected pneumococcal pneumonia in adults. Chest 2001;119:185–195 [DOI] [PubMed] [Google Scholar]
- 144.Welte T, Petermann W, Schurmann D, et al. Treatment with sequential intravenous or oral moxifloxacin was associated with faster clinical improvement than was standard therapy for hospitalized patients with community-acquired pneumonia who received initial parenteral therapy. Clin Infect Dis 2005;41:1697–1705 [DOI] [PubMed] [Google Scholar]
- 145.Hoeffken G, Meyer HP, Winter J, Verhoef L; CAP1 Study Group The efficacy and safety of two oral moxifloxacin regimens compared to oral clarithromycin in the treatment of community-acquired pneumonia. Respir Med 2001;95:553–564 [DOI] [PubMed] [Google Scholar]
- 146.Patel T, Pearl J, Williams J, Haverstock D, Church D; Community Acquired Pneumonia Study Group. Efficacy and safety of ten day moxifloxacin 400 mg once daily in the treatment of patients with community-acquired pneumonia. Respir Med 2000;94:97–105 [DOI] [PubMed] [Google Scholar]
- 147.Katz E, Larsen LS, Fogarty CM, Hamed K, Song J, Choudhri S. Safety and efficacy of sequential i.v. to p.o. moxifloxacin versus conventional combination therapies for the treatment of community-acquired pneumonia in patients requiring initial i.v. therapy. J Emerg Med 2004;27:395–405 [DOI] [PubMed] [Google Scholar]
- 148.Marrie TJ, Beecroft MD, Herman-Gnjidic Z. Resolution of symptoms in patients with community-acquired pneumonia treated on an ambulatory basis. J Infect 2004;49:302–309 [DOI] [PubMed] [Google Scholar]
- 149.Morganroth J, Dimarco JP, Anzueto A, et al. A randomized trial comparing the cardiac rhythm safety of moxifloxacin vs levofloxacin in elderly patients hospitalized with community-acquired pneumonia. Chest 2005;128:3398–3406 [DOI] [PubMed] [Google Scholar]
- 150.Anzueto A, Niederman MS, Pearle J, et al. Community-acquired pneumonia recovery in the elderly (CAPRIE): Efficacy and safety of moxifloxacin therapy versus that of levofloxacin therapy. [Erratum appears in Clin Infect Dis. 2006 May 1;42(9):1350]. Clin Infect Dis. 2006;42(9):73–81 [DOI] [PubMed] [Google Scholar]
- 151.Torres A, Muir JF, Corris P, et al. Effectiveness of oral moxifloxacin in standard first-line therapy in community-acquired pneumonia. Eur Respir J 2003;21:135–143 [DOI] [PubMed] [Google Scholar]
- 152.Marrie T, Beecroft M, Herman-Gnjidic Z, Poulin-Costello M. Symptom resolution in patients with Mycoplasma pneumoniae pneumonia. Can Respir J 2004;11:573–577 [DOI] [PubMed] [Google Scholar]
- 153.McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology 2009;116:1615–1623.e1611 [DOI] [PubMed] [Google Scholar]
- 154.Lipsky BA, Giordano P, Choudhri S, et al. Treating diabetic foot infections with sequential intravenous to oral moxifloxacin compared with piperacillin-tazobactam/amoxicillin-clavulanate. J Antimicrob Chemother 2007;60:370–376 [DOI] [PubMed] [Google Scholar]
- 155.Malangoni MA, Song J, Herrington J, Choudhri S, Pertel P. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann Surg 2006;244:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang JM, Kim N, Lee DH, et al. Second-line treatment for Helicobacter pylori infection: 10-day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter 2007;12:623–628 [DOI] [PubMed] [Google Scholar]
- 157.Nista EC, Candelli M, Zocco MA, et al. Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2005;21:1241–1247 [DOI] [PubMed] [Google Scholar]
- 158.Höffken G, Barth J, Rubinstein E, Beckmann H; HAP Study Group. A randomized study of sequential intravenous/oral moxifloxacin in comparison to sequential intravenous ceftriaxone/oral cefuroxime axetil in patients with hospital-acquired pneumonia. Infection 2007;35:414–420 [DOI] [PubMed] [Google Scholar]
- 159.Bago J, Pevec B, Tomic M, et al. Second-line treatment for Helicobacter pylori infection based on moxifloxacin triple therapy: A randomized controlled trial. Wien Klin Wochenschr 2009;121:47–52 [DOI] [PubMed] [Google Scholar]
- 160.Yoon H, Kim N, Lee BH, et al. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: Effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter 2009;14:429–437 [DOI] [PubMed] [Google Scholar]
- 161.Di Caro S, Ojetti V, Zocco MA, et al. Mono, dual, and triple moxifloxacin-based therapies for Helicobacter pylori eradication. Aliment Pharmacol Ther 2002;16:527–532 [DOI] [PubMed] [Google Scholar]
- 162.Sezgin O, Altintas E, Ucbilek E, Tombak A, Tellioglu B. Low efficacy rate of moxifloxacin-containing Helicobacter pylori eradication treatment: In an observational study in a Turkish population. Helicobacter 2007;12:518–522 [DOI] [PubMed] [Google Scholar]
- 163.Arrieta JR, Galgano AS, Sakano E, et al. Moxifloxacin vs amoxicillin/clavulanate in the treatment of acute sinusitis. Am J Otolaryngol 2007;28:78–82 [DOI] [PubMed] [Google Scholar]
- 164.Solomkin J, Zhao YP, Ma EL, et al. Moxifloxacin is non-inferior to combination therapy with ceftriaxone plus metronidazole in patients with community-origin complicated intra-abdominal infections. Int J Antimicrob Agents 2009;34:439–445 [DOI] [PubMed] [Google Scholar]
- 165.Weiss G, Reimnitz P, Hampel B, Muehlhofer E, Lippert H; AIDA Study Group. Moxifloxacin for the treatment of patients with complicated intra-abdominal infections (the AIDA Study). J Chemother 2009;21:170–180 [DOI] [PubMed] [Google Scholar]
- 166.Halachmi-Eyal O, Lang Y, Keness Y, et al. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. [Erratum appears in J Cataract Refract Surg 2010;36:535. Halachimi-Eyal, Orly (corrected to Halachmi-Eyal, Orly)] J Cataract Refract Surg 2009;36:2109–2114 [DOI] [PubMed] [Google Scholar]
- 167.Bradshaw CS, Chen MY, Fairley CK, Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ross JD, Cronje HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sex Transm Infect 2006;82:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heystek M, Ross JD; PID Study Group. A randomized double-blind comparison of moxifloxacin and doxycycline/metronidazole/ciprofloxacin in the treatment of acute, uncomplicated pelvic inflammatory disease. Int J STD AIDS 2009;20:690–695 [DOI] [PubMed] [Google Scholar]
- 170.Wenisch C, Krause R, Szell M, Laferl H. Moxifloxacin versus standard therapy in patients with pneumonia hospitalized after failure of preclinical anti-infective treatment. Infection 2006;34:190–195 [DOI] [PubMed] [Google Scholar]
- 171.Morovic M. Q Fever pneumonia: Are clarithromycin and moxifloxacin alternative treatments only? Am J Trop Med Hyg 2005;73:947–948 [PubMed] [Google Scholar]
- 172.Giordano P, Song J, Pertel P, et al. Sequential intravenous/oral moxifloxacin versus intravenous piperacillin-tazobactam followed by oral amoxicillin-clavulanate for the treatment of complicated skin and skin structure infection. Int J Antimicrob Agents 2005;26:357–365 [DOI] [PubMed] [Google Scholar]
- 173.Vick-Fragoso R, Hernandez-Oliva G, Cruz-Alcazar J, et al. Efficacy and safety of sequential intravenous/oral moxifloxacin vs intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection 2009;37:407–417 [DOI] [PubMed] [Google Scholar]
- 174.Parish LC, Routh HB, Miskin B, et al. Moxifloxacin versus cephalexin in the treatment of uncomplicated skin infections. Int J Clin Pract 2000;54:497–503 [PubMed] [Google Scholar]
- 175.{T4}Grassi C, Casali L, Curti E, et al. Efficacy and safety of short course (5-day) moxifloxacin vs 7-day ceftriaxone in the treatment of acute exacerbations of chronic bronchitis (AECB). J Chemother 2002;14:597–608 [DOI] [PubMed] [Google Scholar]
- 176.Miravitlles M, Ros F, Cobos A, Kubin R, Tillotson G. The efficacy of moxifloxacin in acute exacerbations of chronic bronchitis: A Spanish physician and patient experience. Int J Clin Pract 2001;55:437–441 [PubMed] [Google Scholar]
- 177.Lorenz J, Thate-Waschke IM, Mast O, et al. Treatment outcomes in acute exacerbations of chronic bronchitis: comparison of macrolides and moxifloxacin from the patient perspective. J Int Med Res 2001;29:74–86 [DOI] [PubMed] [Google Scholar]
- 178.Koch H, Landen H, Stauch K. Once-daily moxifloxacin therapy for community-acquired pneumonia in general practice: Evidence from a post-marketing surveillance study of 1467 patients. Clin Drug Investig 2004;24:441–448 [DOI] [PubMed] [Google Scholar]
- 179.Landen H M M, Tillotson G, Kubin R, Höffken G. Clinical experience in Germany of treating community-acquired respiratory infections with the new 8-methoxyfluoroquinolone, moxifloxacin. J Int Med Res 2001;29:51–60 [DOI] [PubMed] [Google Scholar]
- 180.Barth J, Stauch K, Landen H. Efficacy and tolerability of sequential intravenous/oral moxifloxacin therapy in pneumonia: Results of the first post-marketing surveillance study with intravenous moxifloxacin in hospital practice. Clin Drug Investig 2005;25:691–700 [DOI] [PubMed] [Google Scholar]
- 181.Liu LY, Landen H. Treatment of respiratory tract infections with moxifloxacin: Results of postmarketing surveillance in China. Int J Clin Pract 2007;61:1509–1515 [DOI] [PubMed] [Google Scholar]
- 182.Chen W, Wu C, Li Z, Bai C. Efficacy and tolerability of moxifloxacin in patients with respiratory tract infections treated in general practice: Results of a post-marketing surveillance study. Clin Drug Investig 2006;26:501–509 [DOI] [PubMed] [Google Scholar]
- 183.Faich GA, Morganroth J, Whitehouse AB, et al. Clinical experience with moxifloxacin in patients with respiratory tract infections. Ann Pharmacother 2004;38:749–754 [DOI] [PubMed] [Google Scholar]
- 184.Elies W, Landen H, Stauch K. Efficacy and tolerability of moxifloxacin in patients with sinusitis treated in general practice: Results of a post-marketing surveillance study. Clin Drug Investig 2004;24:431–439 [DOI] [PubMed] [Google Scholar]
- 185.National Institutes of Health (NIH) Revitalization Act, Subtitle B, Part 1, Sec 1993:131–133 [Google Scholar]
- 186.General Accounting Office Problems in implementing policy on women in study populations. Bethesda, MD: National Institutes of Health, 1990. GAO/HRD-90-38 [Google Scholar]
- 187.Rodenburg EM, Stricker B, Visser L. Sex-related differences in hospital admissions attributed to adverse drug reactions in the Netherlands. Br J Clin Pharmacol 71:95–104. 10.1111/j.1365-2125.2010.03811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cubeddu LX. Sex-related differences in hospital admissions attributed to adverse drug reactions in the Netherlands. Br J Clin Pharmacol 2003;71:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: A multicenter prospective controlled study. Antimicrob Agents Chemother 1998;42:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Medical Research Council of Canada Advisory Committee on Women in Clinical Trials final report. Ottawa: The Council, 1997.
- 191.Schulz K, Altman D, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010:726–732 [DOI] [PubMed] [Google Scholar]
- 192.Chilet Rosell E, Ruiz Cantero MT. CONSORT statement 2010: What about the gender perspective? Ann Int Med 2010. Available at: www.annals.org/content/152/11/726.full/reply#annintmed_el_124620 Accessed November 28, 2012.
- 193.Pinnow E, Sharma P, Parekh A, Gevorkian N, Uhl K. Increasing participation of women in early phase clinical trials approved by the FDA. Womens Health Issues 2009;19:89–93 [DOI] [PubMed] [Google Scholar]