Abstract
Optimum concentrations of heavy metals like copper, cadmium, lead, chromium, and zinc in soil are essential in carrying out various cellular activities in minimum concentrations and hence help in sustaining all life forms, although higher concentration of these metals is lethal to most of the life forms. Galerina vittiformis, a macrofungus, was found to accumulate these heavy metals into its fleshy fruiting body in the order Pb(II) > Cd(II) > Cu(II) > Zn(II) > Cr(VI) from 50 mg/kg soil. It possesses various ranges of potential cellular mechanisms that may be involved in detoxification of heavy metals and thus increases its tolerance to heavy metal stress, mainly by producing organic acids and phytochelatins (PCs). These components help in repairing stress damaged proteins and compartmentalisation of metals to vacuoles. The stress tolerance mechanism can be deduced by various analytical tools like SEM-EDX, FTIR, and LC-MS. Production of two kinds of phytochelatins was observed in the organism in response to metal stress.
1. Introduction
Metal pollutants are released into the environment in many ways at potentially harmful levels [1]. In some areas, origin of heavy metals and metalloids in food chain is geological rather than anthropogenic; hence microorganisms in such areas develop effective strategy to cope with the harmful consequence of metal and metalloid exposures. Generally the heavy metal ions like Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) are very reactive. Many of them possess high affinity especially for sulfhydryl groups in proteins and small biological molecules. As a consequence, the metal can critically affect function in many biological systems, as enzyme inhibitors or in other ways disturbing the pathways causing various lethal disorders.
Over the last decades, biosorption has emerged as a promising low cost methodology for the removal of metals from the environment, where biological components are employed to remove and recover heavy metals from aqueous solutions [2–4]. The metal removal mechanism is a complex process that depends on the chemistry of metal ions, cell wall compositions of microorganisms, physiology of the organism, and physicochemical factors like pH, temperature, time, ionic strength, and metal concentration [5]. The biosorption of heavy metals from soil process through phytoremediation is widely discussed. Researchers have studied phytoremediation thoroughly within last few decades and have understood that phytoremediation solely cannot solve all issues regarding heavy metal pollution because of its limitations like selectivity of plant, climatic inhibitions, tolerance to heavy metals, and back contamination by depuration or from ashes of fire woods; hence there is a need of a robust methodology which can go hand in hand with other techniques to remediate heavy metal contaminated areas more quickly, effectively, and economically.
Mushrooms or macrofungi can act as effective biosorbent alternative to plants in removing toxic metals from soil and the process is referred to as mycoremediation. Mushroom mycelia can serve as biological filters since their aerial structures consist of large biomass and have a tough texture which makes them potential sorbents [6]. Mushrooms are known to have high metal/metalloid tolerance which helps them to thrive and accumulate metals from the contaminated environment. They also have shorter life cycle (30 days) and better adaptability compared to plants; hence mycoremediation can be regarded as an evolved remediation technique. To understand and to engineer the bioaccumulation efficiency a thorough study on the mechanism of metal removal is essential.
Mushrooms response to metal stress in the environment by producing stress compounds of proteinous and nonproteinous origin. The cap of the mushrooms has been found to produce stress related factors which govern in metal ion uptake, that is, metallothionein glutothionine, and plastocyanin. Fungi have evolved metal tolerance and accumulation mechanism. Compared to microfungi, the mushrooms play a major role in accumulating heavy metals. Fruiting bodies of the mushrooms are considered to be advantageous to plants as they have shorter life cycle (30 days) and better adaptability compared to plants; hence mycoremediation can be regarded as an evolved remediation technique. The major factors governing the metal uptake are bioavailability and nature of soil. The bioavailability of metals is affected by numerous soil factors, such as cation exchange capacity [7], pH [8–10], and organic matter content [11, 12]. Understanding the mechanism of metal uptake from the soil to the fruiting bodies helps us to improvise the process by biotechnological tools; hence the study on the uptake mechanism plays a significant role in developing better remediation technique.
2. Materials and Methods
2.1. Bioaccumulation Studies
Galerina vittiformis spawns were used as the inoculums for in vitro fruiting body production. The spawns were mixed with soil mixture and were incubated at 23 ± 3°C in dark conditions. Casing of spawns was carried out in trays of 25 × 20 × 5 cm dimensions, sterilized with 70% alcohol. Soils used for the study were mixed with heavy metal (as PbNO3, CdSO4, CuSO4, K2Cr2O7, and ZnNO3) solutions to attain 50 mg kg−1 concentration and were dried in an oven. The dried soil was mixed with sawdust in the ratio of 3 : 1 (w/w). Sawdust increases the porosity and helps in better mushroom production. These soil mixtures were used for all bioaccumulation studies. Spawns were cased with the soil mixture and soil layer of about 3 to 4 cm thick was prepared. Cased trays were incubated in the dark conditions at 22 ± 2°C and 85 ± 5% relative humidity for a period of 25 days with periodical monitoring. At the end of the 25th day, the fruiting bodies formed were harvested using sterile forceps and allowed to dry at room temperature. 1 g of the dried biomass samples was mixed with 2 mL of 65% HNO3 and 6 mL of HCl and then digested in a microwave digester (CEM-MARS, USA) at 600 W for 20 min. The digested mixtures were cooled and were made up to 50 mL using deionized water. The cooled mixture is then filtered using Whatman No. 1 filter paper. These samples were analyzed for metal contents using atomic absorbtion spectrometer (AAS) [13, 14].
2.2. Mechanism of Bioaccumulation
To determine the tolerance and accumulation mechanism employed by mushrooms the fruiting bodies were analysed for primary and secondary stress components produced by them. The dried fruiting bodies and their extracts were analysed by various modern techniques, namely, scanning electron microscopy with energy dispersive X-ray analysis, fourier transforms infrared spectroscopy analysis, and liquid chromatography coupled with mass spectrometry to understand their metal uptake mechanisms.
2.2.1. Scanning Electron Microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDX)
In order to understand the role of surface activity on metal accumulation the toughs of fungal mycelia were subjected to SEM and EDX. The fungal mat obtained was harvested and dried in oven at 60°C. Theses dried biomasses were treated with 10% glutaraldehyde and then incubated for about 10–12 hours at 4°C. Further the biomass was treated with alcohol gradations (10%, 30%, 50%, 80%, and 100%) for 2 min to remove the water content [15, 28–30]. The pretreated specimens were then sputtered with gold particles using a sputter coater under vacuum and then observed under a scanning electron microscope (JSM-6380; JEOL, Tokyo) at an accelerating voltage of 12 or 15 kV to capture the images. EDX of these images was performed at 20 kV.
2.2.2. Fourier Transforms Infrared Spectroscopy Analysis (FTIR)
The fruiting bodies and mycelia of G. vittiformis after the bioaccumulation studies were isolated and washed with distilled water and oven-dried at 60°C (Rotek, India). The dried biomass was then powdered and analyzed by Thermo Nicolet 6700, FTIR spectrometer to identify the functional groups and bonds present in them in response to heavy metal uptake which were responsible for the metal accumulation in cytosol.
To characterise the stress components produced in these biomass, FTIR was performed on fruiting body extracts. The stress components were extracted using Tris buffer system; 3 g of dried fruiting body was grounded using liquid nitrogen in a mortor and pestle; the homogenised extract was mixed with 3X Tris buffer (30 mM Tris, 250 mM NaCl, pH 7.6) in ice bath; centrifuged at 12,000 g for 15 min at 4°C; the supernatant was collected and stored at −20°C. The extract was then subjected to both FTIR and liquid chromatography coupled with mass spectra (LC-MS).
2.2.3. Analysis of Stress Factors Using LC-MS
Fruiting body extracts were characterised using a liquid chromatographic column equipped with Accela pump and an Accela autosampler (Thermo Fisher Scientific, San Jose, CA, USA). Separation of analytes was conducted on a Luna PFP (2) analytical column (100 mm × 2.0 mm, 3 μm). The LC mobile phases were (a) ammonium formate 0.75 mM adjusted to pH 3.5 with formic acid and (b) methanol. Separation was performed under isocratic conditions with 99% mobile phase A at flow rate of 200 μL/min and a column temperature of 35°C. Total run time per sample was 10 min and all injection volumes were 10 μL. Mass spectrometric analysis was performed using a TSQ Quantum Access (Thermo Fisher Scientific, San Jose, CA, USA) triple quadrupole mass spectrometer coupled with electrospray ionization (ESI) operated in multiple reactions monitoring (MRM) in positive mode. The MRM for GSH (m/z 308.1 → m/z 76.2 + 84.2 + 161.9) and GSSG (m/z 613.2 → m/z 230.5 + 234.6 + 354.8) were performed with collision energy optimized for each transition. The operating conditions for MS analysis were as follows: spray voltage, 2500 V; capillary temperature and voltage, 280°C and 35 V, respectively; Sheath gas and auxiliary gas flow, 30 and 5 arbitrary units, respectively; tube lens offset, 84 V for GSH and 115 V for GSSG. The mass spectrometer was employed in MS/MS mode using argon as collision gas. Data acquisition and analysis were performed with Xcalibur software, version 2.0 (Thermo Fisher Scientific, San Jose, CA, USA).
3. Result and Discussions
3.1. Bioaccumulatation in Fruiting Bodies of Mushrooms
Fruiting bodies of Galerina vittiformis produced after 25 days of incubation are shown in Figure 1. The results of bioaccumulation study are presented in Figure 2. The fruiting bodies act as the site of heavy metal accumulation and they can be easily separated from soil. This is considered to be advantageous over phytoremediation. In phytoremediation heavy metals are accumulated in plant parts like roots, twigs, and leaves and improper disposal methods increase the risk of back contamination. The levels of heavy metals accumulated in the fruiting bodies are shown in Figure 2 and are as follows: Cu(II) (800 mg kg−1), Cd(II) (852 mg kg−1), Cr(VI) (30 mg kg−1), Pb(II) (900 mg kg−1), and Zn(II) (700 mg kg−1). Thus the bioaccumulation potential of fruiting bodies of G. vittiformis was found to be in the following order: Pb(II) > Cd(II) > Cu(II) > Zn(II) > Cr(VI). Various researchers have found that organism's ability to accumulate heavy metals varies from species to species, at different stages of life cycle, amount of metal ion concentrations in the soil, nature of soil, and so forth [16, 21, 31–34].
Figure 1.

Fruiting body initials of organism Galerina vittiformis after 25 days of incubation in tray systems.
Figure 2.
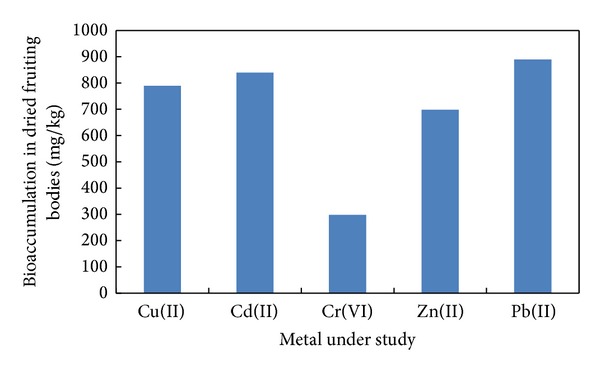
Bioaccumulation of metals by fruiting bodies of Galerina vittiformis.
The bioaccumulation potential of G. vittiformis was found to be higher than that of those mushroom species reported in the literature (summarized in Table 1). From Table 1 it is observed that nonedible mushroom species accumulate higher amounts of metal ions than the edible species. However, the bioaccumulation profile indicates that metal accumulation capability is species specific and mainly depends on its accumulation mechanism [20, 35–39].
Table 1.
Heavy metal content in fruiting body (sporocarp) of various mushrooms.
| Sl. number | Mushroom species | Metal content in sporocarp, mg kg−1 of dry wt. | References |
|---|---|---|---|
| 1 | Agaricus bisporus 1 | Pb (4), Cd (3.48), Cu (5.) | [15] |
| Boletus edulis 1 | Cu (66.4), Cd (6.58), Pb (3.03) | ||
| Lepiota rhacodes 2 | Pb (66), Cd (3.7) | ||
| Paxillus rubicondulus 1 | Pb (0.69), Cd (0.78), Cu (51.0) Zn (16.8) | ||
|
| |||
| 2 | Agaricus bisporus 1 | Cu (107), Pb (1), Zn (57.) | [16] |
|
| |||
| 3 | Helvella leucomelaena 2 | Pb (4.8), Cd (.0) | [17] |
| Pleurotus sp. 1 | Pb (3.4), Cd (1.18), Cu (13.6), Zn (9.8) | ||
|
| |||
| 4 | Tricholoma terreum 1 | Cu (5), Zn (179), Cd (0.56), Pb (4.4) | [18] |
| Helvella leucomelaena 2 | Pb (3.1), Cd (1.1) | ||
|
| |||
| 5 | Paxillus involutus 2 | Cu (57.0), Pb (1.6.0), Fe (991), Cd (0.84), Pb (3) | [19] |
| Rhizopogonaceae luteolus 1 | Cu (13), Zn (30), Mn (13), Fe (620), Cd (0.26), Pb (2.8). | ||
| Omphalotus olearius 2 | Cu (21), Zn (27), Mn (36), Fe (95), Cd (1.3), Pb (5.2). | ||
| Hygrophorus hedyricii 2 | Cu (37), Zn (97),Mn (11), Fe (395), Cd (1.2), Pb (2.7) | ||
| Ciocybe dealbata2 | Cu (41), Zn (115), Mn (30), Fe (386), Cd (0.86), Pb (3.2) | ||
| Lepiota alba 2 | Cu (29), Zn (86), Mn (22), Fe (779), Cd (0.8), Pb (5.8) | ||
|
| |||
| 6 | Tricholoma terreum 2 | Pb (4), Cd (1.6), Cu (35.8), Zn (48.0) | [20] |
| Agaricus bisporus 1 | Pb (0.8), Cd (0.78) | ||
|
| |||
| 7 | Pseudevernia furfuracea 2 | Al (12.51), As (0.23), Cd (0.19), Cu (2.5), Cr (0.11), Pb (5.1), Zn (17.9), Mn (12.9) | [21] |
| Scorpiurus circinatum2 | Al (17.51), As (0.32), Cd (0.35), Cu (3.2), Cr (1.1), Pb (6.3), Zn (46.1), Mn (46.7) | ||
|
| |||
| 8 | Aspergillus foetidus 2 | Al (32.5), Co (5.95), Cr (6.23), Mg (44.9), Zn (2.4), Ni (189.5) | [22] |
|
| |||
| 9 | Poria sp.2 | Zn (90.3), Cu (30.8), Pb (1.0), Mn (31.3), Cd (0.1) | [23] |
| Nectria cinnabarina 1 | Zn (30.1), Cu (29.3), Pb (1.9), Cd (0.2), Mn (19.3) | ||
| Ganoderma lucidum 1 | Zn (60.1), Cu (43.8), Pb (0.7), Mn (30.4), Cd (0.31) | ||
| Paragyrodous sphaerosporous1 | Zn (115), Cu (34.4), Pb (0.4), Mn (37.3), Cd (0.2) | ||
| Polyporus frondosus 1 | Zn (120.1), Cu (34.4), Pb (0.4), Mn (37.3), Cd (0.2) | ||
|
| |||
| 10 | Phellinus badius 2 | Cd (110), Cu (60), Hg (61), Ni (56) | [3] |
| Phellinus sanguineus 2 | Cd (80), Cu (42), Hg (35), Ni (66) | ||
|
| |||
| 11 | Tricholoma terreum 2 | Pb (3.64), Cu (34.86), Cd (0.67), Zn (54.13), Cr (2.54) | [24] |
| Boletus badius 1 | Cu (44.54), Pb (4.48), Cd (0.91), Zn (34.17), Fe (264.62), Cr (2.86) | ||
| Russula delica 1 | Cu (19.55), Pb (2.02), Cd (1.22), Zn (38.5), Cr (6.95) | ||
|
| |||
| 13 | Pleurotus platypus1 | Cd (34.9), Pb (27.10) | [25] |
| Agaricus bisporus 1 | Cd (33.7), Pb (29.67) | ||
|
| |||
| 14 | Lactarius deliciosus 1 | Cd (0.26), Cr (0.12), Cu (6.15), Pb (0.73), Zn (76.7) | [26] |
| Rhizopogon roseolus 1 | Cd (0.18), Cr (0.10), Cu (21.2), Pb (2.03), Zn (36.7) | ||
| Russula delica 1 | Cd (0.42), Cr (0.27), Cu (52.2), Pb (0.77), Zn (58.2) | ||
|
| |||
| 15 | Sarcosphaera crassa 1 | Ag (0.044), As (8.03), Cd (0.016), Cr (0.98), Pb (0.02) | [27] |
| Cantharellus cibarius 1 | Ag (0.022), As (0.03), Cd (0.036), Cr (0.69), Pb (0.04) | ||
| Suillus luteus1 | Ag (0.015), As (0.15), Cd (0.034), Cr (0.15), Pb (0.06) | ||
| Morchella rigida 1 | Ag (0.087), As (0.24), Cd (0.007), Cr (0.44), Pb (0.02) | ||
| Agrocybe aegerita 1 | Ag (0.074), As (0.44), Cd (0.010), Cr (0.25), Pb (0.018) | ||
|
| |||
| 15 | Galerina sp. 2 | Cd (85), Pb (900), Cu (800), Zn (700), Cr (30) | |
1Edible, 2Nonedible.
3.2. Mechanism of Bioaccumulation
Heavy metals are known to act as a general protoplasmic poison, inducing the denaturation of proteins and nucleic acids [22, 40]. They can also break apart biological molecules into even more reactive species (such as reactive oxygen species) which will also disrupt biological processes. Hence only those species which can successfully tolerate these physiological stresses can successfully survive in heavy metal laden environment. Mushrooms respond to metal stress in the environment by producing stress compounds of proteinous and nonproteinous origin. The pileus (cap) of the mushrooms has been found to produce stress factors which help in sequestering the accumulated metal ions into their vacuoles. The most common stress components produced by plants and fungi are metallothionein (MT), glutothionine (GSH), phytochelatins (PCs), and plastocyanin [41]. From the studies of Inouhe et al. [42], Mehra et al. [43], Münger and Lerch [44], and Lerch [45], it is observed that macrofungi have evolved metal tolerance and accumulation mechanism compared to microfungi. Understanding the mechanism of the metal uptake from the soil to the fruiting bodies helps us to improvise the process by advanced molecular biology tools. Hence study on the uptake mechanism plays a significant role in developing better remediation technique.
3.2.1. Morphological Studies by Scanning Electron Microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDX)
The effect of heavy metal on the morphology of Galerina vittiformis during the bioaccumulation process was studied through SEM image analysis. Figure 3(a) revealed that the hyphae of G. vittiformis were cylindrical, septate, and branched before exposure to heavy metal. As shown in Figure 3(b) characteristic change in the morphology, curling, and formation of hyphal coils in response to Cd(II) stress is observed. Similar observations were made when exposed to other heavy metals. Cánovas et al. [46] reported that the surface of Aspergillus sp. also had rough texture due to protrusions on the hyphae on exposure to 50 mM of arsenate solution. Such modifications on the surface of fungi indicate the production of intracellular compounds due to heavy metals stress and result in increase in pressure within the mycelia leading to the outward growth of the cell wall structures [47]. Courbot et al. [48] have also observed that the impact of metal stresses had led to production of thiol compounds, especially GSH and MT due to intracellular detoxification of cadmium in the fungi, Paxillus involutus. According to them, the cell wall protrusions indicate increased formation of intracellular vacuoles that serve as storage compartments for thiol containing compounds. These compounds are responsible for the binding of metal ions into the intracellular regions and accumulate them in the vacuoles, thereby reducing their toxicity in the cytoplasm and improving tolerance levels. The energy dispersive spectroscopy (EDS) analysis was done to analyze the metal ionic concentrations in the mycelial surface indicating mycofilteration. The results of EDS analysis for the control mycelia and mycelium treated with Cd(II) are shown in Figure 4 and Table 2 and Figure 5 and Table 3, respectively. Only traces of Cd(II) were observed in EDS spectra whereas EDS of the mycelia exposed to other metals such as Cu(II), Pb(II), and Zn(II) showed no visible peaks for the metals (EDS spectra not shown), indicating the nondetectable levels of metals on the surface of the mycelia by SEM-EDS. Presence of traces or absence of metal peaks in the EDS spectra indicates that the metal removal by the mycelia of Galerina vittiformis may be attributed to vigorous intracellular bioaccumulation mechanism, rather than adsorption on the surface.
Figure 3.

SEM images of organism G. vittiformis (a) untreated (b) Cd(II) treated (500–700X magnification).
Figure 4.
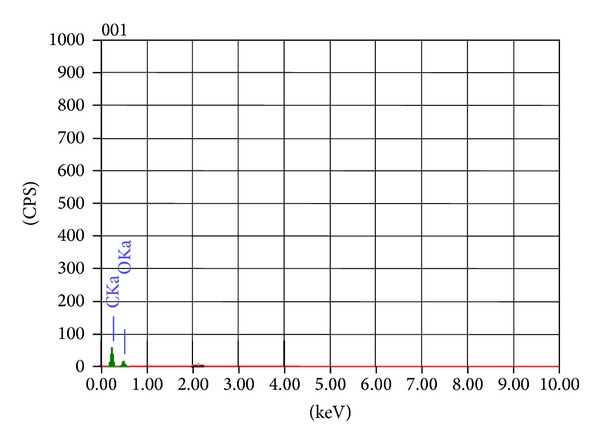
EDS analysis showing the metal content in G. vittiformis (control).
Table 2.
EDS quantitative analysis of organism G. vittiformis (control).
| Element | KeV | Mass% | Error% | At% | K |
|---|---|---|---|---|---|
| C K | 0.277 | 59.75 | 0.17 | 66.41 | 68.8722 |
| O K | 0.525 | 40.25 | 1.08 | 33.59 | 31.1278 |
|
| |||||
| Total | 100 | 100 | |||
Figure 5.
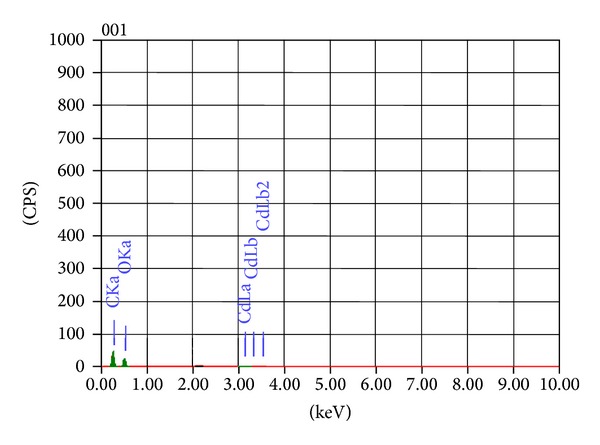
EDS analysis of organism G. vittiformis treated with Cd(II).
Table 3.
EDS quantitative analysis of organism G. vittiformis treated with Cd(II).
| Element | keV | Mass% | Error% | At% | K |
|---|---|---|---|---|---|
| C K | 0.277 | 50.25 | 0.15 | 57.62 | 53.19 |
| O K | 0.525 | 49.15 | 0.72 | 42.30 | 45.850 |
| Cd L | 3.132 | 0.60 | 0.72 | 0.07 | 0.9590 |
|
| |||||
| Total | 100 | 100 | |||
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of fruiting bodies extracts of Galerina vittiformis after bioaccumulation studies were analyzed to determine the presence and disappearance of any functional groups involved in metal accumulation mechanism. The FTIR spectrum was assessed by comparing the absorption bands of G. vittiformis grown on metal (Cu(II), Cd(II), Cr(VI), Pb(II), and Zn(II)) (50 mg/kg) contaminated soil to that of its spectrum obtained from control. Any changes in the finger print region, that is, 1000–1500 (amide-II and II regions) and 1500–1000 cm−1 (amide-III regions) which indicates the presence of higher amounts of acids, protenitous, and nonproteious compounds by G. vittiformis upon exposure to Cd(II), Cu(II), Pb(II), Cr(VI), and Zn(II). Each FTIR spectrum was studied thoroughly by comparing the peak values to their standard FTIR charts to determine the represented functional groups [32, 49–52]. The FTIR graphs obtained for all the studied metal ions are compared with control FTIR graphs to analyze the presence of stress factors produced during metal stress (Figure 6). The FTIR graphs of fruiting body obtained from Cr(VI) laden soil system showed the presence of stress related components like oxalic acid, that is, 1658 ± 5 and 1253 ± 5 and thiol group, that is, 2550 ± 5 (Figure 7) [53]. Similar peaks are also observed in the FTIR graphs of Pb(II) and Cd(II). Only thiol groups (2550 ± 5) are observed in Figure 8 for Cu(II) and Zn(II). Researchers like Qian and Krimm [54], Yang et al. [55], and Shi et al. [56] have used FTIR to detect the presence of both primary and secondary stress factors. FTIR gives only a preliminary identification of stress factors; hence the extracts are further subjected for LC-MS analysis to determine the components. Stress components present in the cellular extracts are further characterized by LC-MS analysis.
Figure 6.
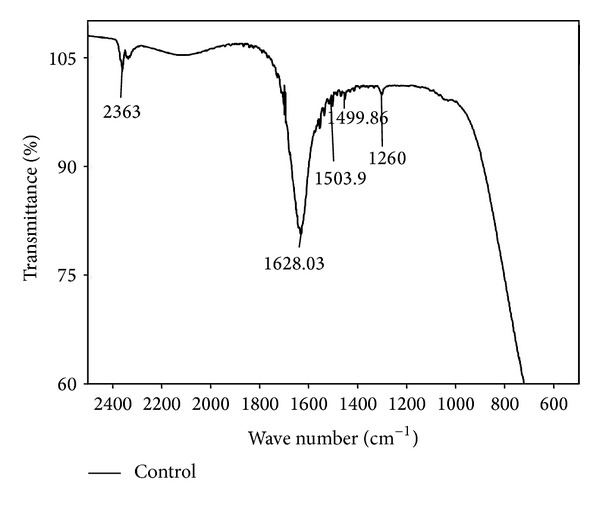
2D-FTIR results of G. vittiformis at metal free environment (control).
Figure 7.
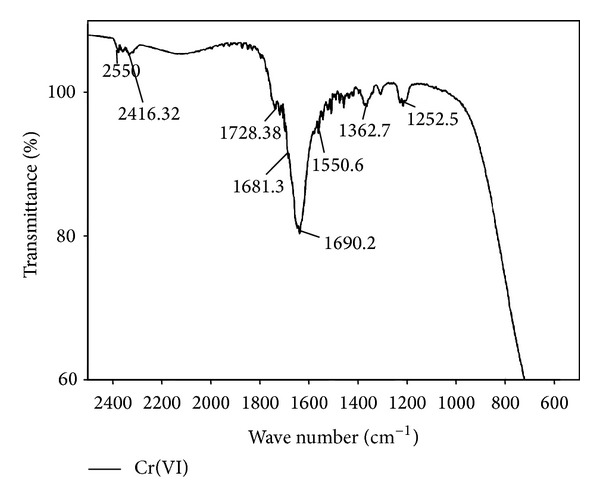
2D-FTIR results of G. vittiformis at Cr(VI) laden soil system.
Figure 8.
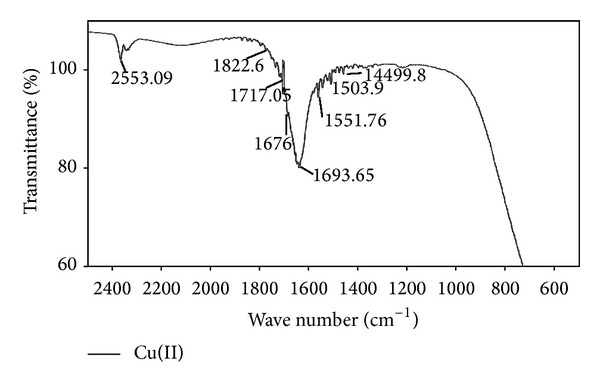
2D-FTIR results of G. vittiformis at Cu(II) laden soil system.
3.2.3. Liquid Chromatography-Mass Spectrometry Analysis (LC-MS)
Metal homeostasis requires intracellular complexation of metals when there is a cellular surplus and later release of metals to metal requiring apoproteins. The excess metal ions are stored in the storage sites within the cell, for example, vacuoles [41]. LC-MS helps to identify those proteinous and nonproteinous metal ion trafficking components of G. vittiformis cells.
The LC-MS chromatograms for Pb(II) are shown in Figures 9(a) and 9(b). The data are obtained from Luna PFP (2) analytical column using ammonium formate and methanol as eluting buffers. Figure 9(a) shows the retention time in minutes and Figure 9(b) shows the m/z ratio of each components present in fruiting body extracts. The peaks obtained in chromatograms were analyzed with the database to determine the components. On comparison with the literature it is observed that Figure 9(a) showed 2 peaks at 6–10 min retention, that is, 7.4 and 8.6 indicating the presence of cysteine (Cys) and glutamine (Glu) residues which are the subunits of phytochelatins (γ-glutamylcysteine). Figure 9(a) also indicated 2 major peaks at 14–25 min retention time, that is, 14.9 and 22.3 indicated the presence of 2 types of phytochelatins (PC2 and PC3, resp.) while Figure 9(b) showed m/z peaks of glutathione (GSH), PC2, and PC3 at 307, 538 and 679, respectively. Similar kinds of chromatograms obtained for both Cd(II) and Cr(II) indicate the presence of phytochelatins (PCs). Figures 10(a) and 10(b) show the chromatogram of Cu(II) indicating the presence of both PC2 and PC3 (539 and 679 m/z peaks) [56–59].
Figure 9.
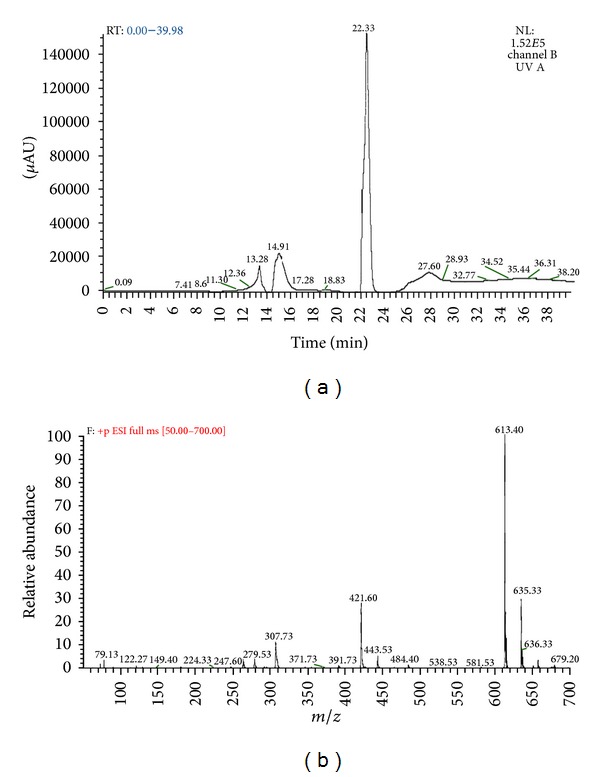
(a) Chromatogram produced by LC-MS analysis for Pb(II) at various retention times (min). (b) Chromatogram produced by LC-MS analysis for Pb(II) at various m/z ratios.
Figure 10.
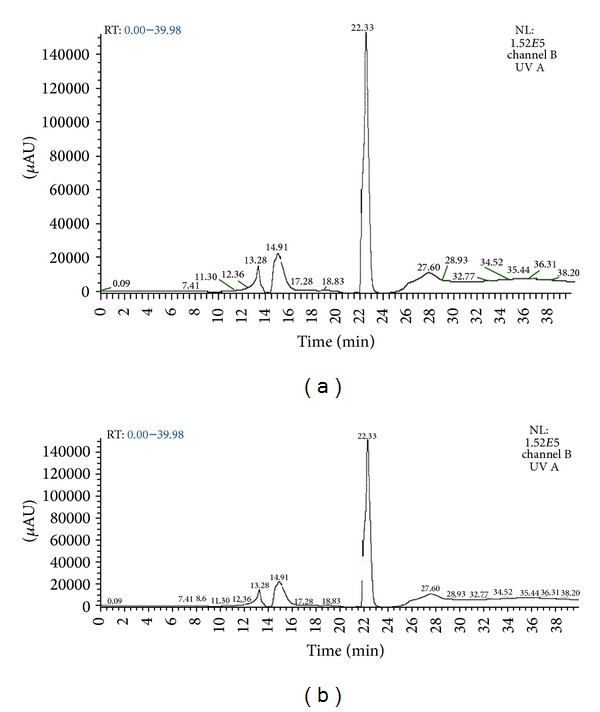
(a) Chromatogram produced by LC-MS analysis for Cu(II) at various retention times (min). (b) Chromatogram produced by LC-MS analysis for Cu(II) at various m/z ratios.
From the studies of Grill et al. [60], Gekeler et al. [61], Liedschulte et al. [62], and Gill and Tuteja [63], it was revealed that the Phytochelatin of the general formula (γ-Glu-Cys)n is the principal heavy metal detoxifying component in both plant and fungal kingdom. The phytochelatins can be viewed as linear polymers of the γ-glutamylcysteine (γ-Glu-Cys) portion of glutathione. These peptides could be enzymatically produced by stepwise condensation of γ-Glu-Cys moieties to growing phytochelatin chain (PC). The PC plays a key role in maintaining cell homeostasis under heavy metal stress by binding to heavy metals like Cd, Zn, Cr, and so forth and trafficking them to vacuoles or periplasmic space for storage [63]. Hence from the result of the present study, the mechanism of metal accumulation can be summarised in Figure 11. Similar heavy metal accumulation mechanism of PC was reported in various metal resistant plants and algal species [41, 57, 60, 64–70].
Figure 11.
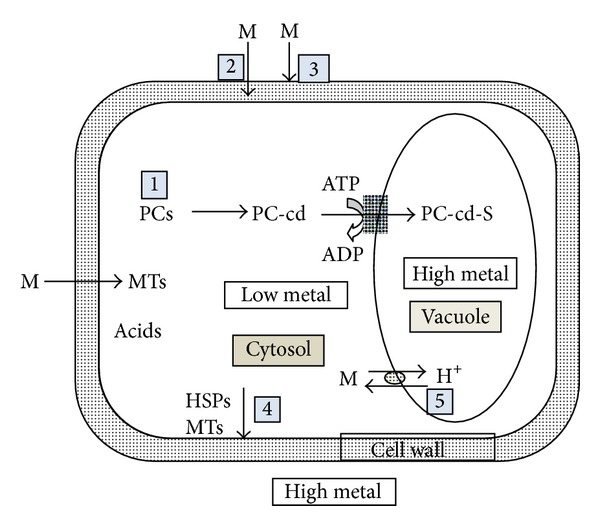
Schematic representation of the proposed mechanism of metal uptake by Galerina vittiformis. (1) Metal adsorption on fungal mycelial surface which acts as roots of fruiting bodies. (2) Uptake and storing in periplasmic space passive absorption. (3) PC and acid production in response to metal stress. (4) Acids act as HSPs (heat shock proteins) that bind to metal and store them to periplasmic space. (5) Transport and accumulation of metals in vacuole.
4. Conclusion
Removal mechanism of Cu(II), Cd(II), Cr(VI), Pb(II), and Zn(II) metals from contaminated soil using a macrofungus, Galerina vittiformis, was investigated in in vitro system.
G. vittiformis shows efficient bioaccumulation potential in removing most potent heavy metals unlike other known organisms.
Trace amounts of metal ions are observed in SEM-EDX in the surface indicating primary adsorption step in accumulation.
FTIR spectral analysis indicates the presence of secondary stress components like organic acids, phytochelatins, and so forth.
The subunits of phytochelatin chain (PC); that is, Glu-Cys moieties are observed in LC-MS analysis.
G.vittiformis produced two types of Phytochelatins, namely, PC2 and PC3 in response to Cu(II), Cd(II), Cr(VI), Pb(II), and Zn(II) metal stress.
Phytochelatins are known to transfer the excess metal ions to the vacuole of the cell, thereby reducing the cellular toxicity.
Hence from analysis reports of SEM-EDX, FTIR, and LC-MS, phytochelatin plays a major role in removing heavy metal from soil by G. vittiformis. The study of mechanism is significant as any modifications in the gene regulating phytochelatin production can be modified to increase the metal accumulation ability. Mycoremediation can be considered as an alternative to other known methods in heavy metal removal from the soil owing to its short action period and better accumulation efficiency. Integration of this technology with advanced agronomical and engineering skills can transform mycoremediation as a competitive remediation tool.
Conflict of Interests
The authors (Ms. Dilna Damodaran, Dr. Vidya Shetty k, and Dr. RajMohan) declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Avery SV. Metal toxicity in yeasts and the role of oxidative stress. Advances in Applied Microbiology. 2001;49:111–142. doi: 10.1016/s0065-2164(01)49011-3. [DOI] [PubMed] [Google Scholar]
- 2.Schnoor JL. Analysis Centre Technology Evaluation Report TE. 98-1. Pittsburgh, PA, USA: William Pitt Way; 1997. Phytoremediation: ground-water remediation technologies. [Google Scholar]
- 3.Bardan JS, Radziah O, Wahid SA, Husin A, Zadeh FS. Column bioleaching of arsenic and heavy metals from gold mine tailings by Aspergillus fumigates . Clean—Soil, Air, Water. 2012;40(6):607–614. [Google Scholar]
- 4.Morsy FM, Hassan SHA, Koutb M. Biosorption of Cd(II) and Zn(II) by Nostoc commune isotherm and kinetics studies. Clean—Soil, Air, Water. 2011;39(7):680–687. [Google Scholar]
- 5.Mishra V, Balomajumder C, Agarwal VK. Zn(II) ion biosorption onto surface of eucalyptus leaf biomass: isotherm, kinetic, and mechanistic modeling. Clean—Soil, Air, Water. 2010;38(11):1062–1073. [Google Scholar]
- 6.Volesky Z, Holan S. Biosorption of heavy metals. Biotechnology Progress. 1995;11:235–250. doi: 10.1021/bp00033a001. [DOI] [PubMed] [Google Scholar]
- 7.Moore R, Clark WD, Vodopich D. Botany. 3rd edition. Dubuque, Iowa, USA: WCB/McGraw-Hill; 1998. [Google Scholar]
- 8.Hornburg V, Welp G, Brummer G. Extraktion Mobiler Schwermetalle Mittels CaCl2 und NH4NO3. Z. Pflanzenernaehr. 2nd edition. Vol. 158. Berlin,Germany: Bodenk, Springer; 1995. Verhalten von schwermetallen in boden; pp. 137–145. [Google Scholar]
- 9.Reddy KJ, Wang L, Gloss SP. Solubility and mobility of copper, zinc and lead in acidic environments. Plant and Soil. 1995;171(1):53–58. [Google Scholar]
- 10.Schmidt U. Enhancing phytoextraction: the effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. Journal of Environmental Quality. 2003;32(6):1939–1954. doi: 10.2134/jeq2003.1939. [DOI] [PubMed] [Google Scholar]
- 11.Bliefert C. Umweltchemie. Weinheim, Germany: Wiley-VCH; 1994. [Google Scholar]
- 12.Li Z, Shuman LM. Heavy metal movement in metal-contaminated soil profiles. Journal of Soil Science. 1996;161(10):656–666. [Google Scholar]
- 13.Pakshirajan K, Worku AN, Acheampong MA, Lubberding HJ, Lens PN. Cr(III) and Cr(VI) removal from aqueous solutions by cheaply available fruit waste and algal biomass. Applied Biochemistry and Biotechnology. 2013;170(3):498–513. doi: 10.1007/s12010-013-0202-6. [DOI] [PubMed] [Google Scholar]
- 14.Oei P. Mushroom Cultivation with Special Emphasis on Appropriate Techniques for Developing Countries. 1st edition. Amsterdam, The Netherlands: Tool; 1996. [Google Scholar]
- 15.Srivastava S, Thakur IS. Biosorption potency of Aspergillus niger for removal of chromium (VI) Journal of Current Microbiology. 2006;53(3):232–237. doi: 10.1007/s00284-006-0103-9. [DOI] [PubMed] [Google Scholar]
- 16.Turkekul I, Elmastas M, Tuzen M. Determination of iron, copper, manganese, zinc, lead, and cadmium in mushroom samples from Tokat, Turkey. Food Chemistry. 2004;84(3):389–392. [Google Scholar]
- 17.Mitra K. Studies on the uptake of heavy metal pollutants by edible mushrooms and its effects on their growth productivity and mammalian system [Ph.D. thesis] Kolkata, India: University of Calcutta; 1994. [Google Scholar]
- 18.Demirbaş A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chemistry. 2001;75(4):453–457. [Google Scholar]
- 19.Yilmaz F, Mustafa I, Melek M. Heavy metal levels in some macro fungi. Turkish Journal of Botany. 2003;27(1):45–56. [Google Scholar]
- 20.Zhu F, Qu L, Fan W, Qiao M, Hao H, Wang X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environmental Monitoring and Assessment. 2011;179(1–4):191–199. doi: 10.1007/s10661-010-1728-5. [DOI] [PubMed] [Google Scholar]
- 21.Basile A, Sorbo S, Aprile G, Conte B, Castaldo CR. Comparison of the heavy metal bioaccumulation capacity of an epiphytic moss and an epiphytic lichen. Environmental Pollution. 2008;151(2):401–407. doi: 10.1016/j.envpol.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Ge W, Zamri D, Mineyama H, Valix M. Bioaccumulation of heavy metals on adapted Aspergillus foetidus . Adsorption. 2011;17(5):901–910. [Google Scholar]
- 23.Ita BN, Essien JP, Ebong GA. Heavy metal levels in fruiting bodies of edible and non-edible mushrooms from the Niger Delta region of Nigeria. Enzyme and Microbial Technology. 2006;32:78–89. [Google Scholar]
- 24.Isildak O, Turkekul I, Elmastas M, Aboul-Enein HY. Bioaccumulation of heavy metals in some wild-grown edible mushrooms. Analytical Letters. 2007;40(6):1099–1116. [Google Scholar]
- 25.Vimala R, Das N. Biosorption of cadmium (II) and lead (II) from aqueous solutions using mushrooms: a comparative study. Journal of Hazardous Materials. 2009;168(1):376–382. doi: 10.1016/j.jhazmat.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 26.Çayir A, Coşkun M, Coşkun M. The heavy metal content of wild edible mushroom samples collected in Canakkale Province, Turkey. Biological Trace Element Research. 2010;134(2):212–219. doi: 10.1007/s12011-009-8464-0. [DOI] [PubMed] [Google Scholar]
- 27.Konuk M, Afyon A, Yağiz D. Minor element and heavy metal contents of wild growing and edible mushrooms from Western Black Sea region of Turkey. Fresenius Environmental Bulletin. 2007;16:1359–1362. [Google Scholar]
- 28.Haswell SJ. Atomic Absorption Spectrometry: Theory, Design and Applications. Amsterdam, The Netherlands: Elsevier; 1991. [Google Scholar]
- 29.Susan GW, Kaminsky AE, Tanya S. High spatial resolution surface imaging and analysis of fungal cells using SEM and AFM. Micron. 2008;39(4):349–366. doi: 10.1016/j.micron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Pakshirajan K, Swaminathan T. Biosorption of lead, copper, and cadmium by Phanerochaete chrysosporium in ternary metal mixtures: statistical analysis of individual and interaction effects. Applied Biochemistry and Biotechnology. 2009;158(2):457–469. doi: 10.1007/s12010-008-8374-1. [DOI] [PubMed] [Google Scholar]
- 31.González MC, Gonzalez RC, Wright SF, Nichols KA. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environmental Pollution. 2004;130(3):317–323. doi: 10.1016/j.envpol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Thomet M, Vogel E, Krahenbuhl U. The uptake of cadmium and zinc by mycelia and their accumulation in mycelia and fruiting bodies of edible mushrooms. European Food Research and Technology. 1999;209(5):317–324. [Google Scholar]
- 33.Chen X-H, Zhou H-B, Qiu G-Z. Analysis of several heavy metals in wild edible mushrooms from regions of China. Bulletin of Environmental Contamination and Toxicology. 2009;83(2):280–285. doi: 10.1007/s00128-009-9767-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Luo SL, Xiao X, et al. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Applied Soil Ecology. 2010;46(3):383–389. [Google Scholar]
- 35.Huang D-L, Zeng G-M, Jiang X-Y, et al. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. Journal of Hazardous Materials. 2006;134(1–3):268–276. doi: 10.1016/j.jhazmat.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Huang D-L, Zeng G-M, Feng C-L, et al. Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environmental Science & Technology. 2008;42(13):4946–4951. doi: 10.1021/es800072c. [DOI] [PubMed] [Google Scholar]
- 37.Wuyep PA, Chuma AG, Awodi S, Nok AJ. Biosorption of Cr, Mn, Fe, Ni, Cu and Pb metals from petroleum refinery effluent by calcium alginate immobilized mycelia of Polyporus squamosus . Scientific Research and Essay. 2007;2:217–221. [Google Scholar]
- 38.Niu Z-X, Sun L-N, Sun T-H, Li Y-S, Wang H. Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. Journal of Environmental Sciences A. 2007;19(8):961–967. doi: 10.1016/s1001-0742(07)60158-2. [DOI] [PubMed] [Google Scholar]
- 39.Demirbaş A. Metal ion uptake by mushrooms from natural and artificially enriched soils. Food Chemistry. 2002;78(1):89–93. [Google Scholar]
- 40.Joho M, Inouhe M, Tohoyama H, Murayama T. Nickel resistance mechanisms in yeasts and other fungi. Journal of Industrial Microbiology. 1995;14(2):164–168. doi: 10.1007/BF01569899. [DOI] [PubMed] [Google Scholar]
- 41.Hall JL. Cellular mechanism of heavy metal detoxification and tolerance: a review article. Journal of Experimental Botany. 2002;53:1–11. [PubMed] [Google Scholar]
- 42.Inouhe M, Sumiyoshi M, Tohoyama H, Joho M. Resistance to cadmium ions and formation of a cadmium-binding complex in various wild-type yeasts. Plant and Cell Physiology. 1996;37(3):341–346. doi: 10.1093/oxfordjournals.pcp.a028951. [DOI] [PubMed] [Google Scholar]
- 43.Mehra RK, Tarbet EB, Gray WR, Winge DR. Metalspecic synthesis of 2 metallthioneins and g-glutamyl-transferase peptides in Candida glabrata . Proceedings of the National Academy of Sciences of the United States of America. 1988;85:8815–8819. doi: 10.1073/pnas.85.23.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Münger K, Lerch K. Copper metallothionein from the fungus Agaricus bisporus chemical and spectroscopic properties. Biochemistry. 1985;24(24):6751–6756. [Google Scholar]
- 45.Lerch K. Copper metallothionein, a copper-binding protein from Neurospora crassa . Nature. 1980;284:368–370. doi: 10.1038/284368a0. [DOI] [PubMed] [Google Scholar]
- 46.Cánovas D, Vooijs R, Schat H, de Lorenzo V. The role of thiol species in the hypertolerance of Aspergillus sp. P37 to arsenic. The Journal of Biological Chemistry. 2004;279(49):51234–51240. doi: 10.1074/jbc.M408622200. [DOI] [PubMed] [Google Scholar]
- 47.Paraszkiewicz K, Bernat P, Naliwajski M, Długoński J. Lipid peroxidation in the fungus Curvularia lunata exposed to nickel. Archives of Microbiology. 2010;192(2):135–141. doi: 10.1007/s00203-009-0542-3. [DOI] [PubMed] [Google Scholar]
- 48.Courbot M, Diez L, Ruotolo R, Chalot M, Leroy P. Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus . Applied and Environmental Microbiology. 2004;70(12):7413–7417. doi: 10.1128/AEM.70.12.7413-7417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanova J, Toncheva-Panova T, Chernev G, Samuneva B. Effect of Ag2+, Cu2+ and Zn2+ containing hybrid nano matrixes on the green algae Chlorella keissleri . Journal of Applied Plant Physiolology. 2008;34:339–348. [Google Scholar]
- 50.Paraszkiewicz K, Bernat P, Naliwajski M, Długoński J. Lipid peroxidation in the fungus Curvularia lunata exposed to nickel. Archives of Microbiology. 2010;192(2):135–141. doi: 10.1007/s00203-009-0542-3. [DOI] [PubMed] [Google Scholar]
- 51.Surewicz WK, Mantsch HH, Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy. Biochemistry. 1993;32(2):389–394. doi: 10.1021/bi00053a001. [DOI] [PubMed] [Google Scholar]
- 52.Kalač P, Svoboda L. A review of trace element concentrations in edible mushrooms. Food Chemistry. 2000;69(3):273–281. [Google Scholar]
- 53.Caliskan M. Germin, an oxalate oxidase, has a function in many aspects of plant life. Turkish Journal of Biology. 2000;24:717–724. [Google Scholar]
- 54.Qian W, Krimm S. Vibrational analysis of glutathione. Biopolymers. 1994;34(10):1377–1394. doi: 10.1002/bip.360341009. [DOI] [PubMed] [Google Scholar]
- 55.Yang T-H, Dong A, Meyer J, Johnson OL, Cleland JL, Carpenter JF. Use of infrared spectroscopy to assess secondary structure of human growth hormone within biodegrable microspheres. Journal of Pharmaceutical Sciences. 1999;88(2):161–165. doi: 10.1021/js980423n. [DOI] [PubMed] [Google Scholar]
- 56.Shi Y-B, Fang J-L, Liu X-Y, Du L, Tang W-X. Fourier transform IR and fourier transform raman spectroscopy studies of metallothionein-III: amide I band assignments and secondary structural comparison with metallothioneins-I and -II. Biopolymers. 2002;65(2):81–88. doi: 10.1002/bip.10195. [DOI] [PubMed] [Google Scholar]
- 57.Camera E, Rinaldi M, Briganti S, Picardo M, Fanali S. Simultaneous determination of reduced and oxidized glutathione in peripheral blood mononuclear cells by liquid chromatography-electrospray mass spectrometry. Journal of Chromatography B. 2001;757(1):69–78. doi: 10.1016/s0378-4347(01)00081-0. [DOI] [PubMed] [Google Scholar]
- 58.Odoemelam SA, Iroh CU, Igwe JC. Copper (II), cadmium (II) and lead (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified cocoa pod husk (Theobroma cacao) waste biomass. Research Journal of Applied Sciences. 2011;6(1):44–52. [Google Scholar]
- 59.Robina S, Levequeb N, Masuyera CC, Humbertc P. LC-MS determination of oxidized and reduced glutathione in human dermis: a micro dialysis study. Journal of Chromatography B. 2011;879(30):3599–3606. doi: 10.1016/j.jchromb.2011.09.052. [DOI] [PubMed] [Google Scholar]
- 60.Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy metal complexing peptidesof higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- 61.Gekeler W, Grill E, Winnacker E-L, Zenk MH. Algae sequester heavy metals via synthesis of phytochelatin complexes. Archives of Microbiology. 1988;150(2):197–202. [Google Scholar]
- 62.Liedschulte V, Wachter A, Zhigang A, Rausch T. Exploiting plants for glutathione (GSH) production: uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation. Plant Biotechnology Journal. 2010;8(7):807–820. doi: 10.1111/j.1467-7652.2010.00510.x. [DOI] [PubMed] [Google Scholar]
- 63.Gill SS, Tuteja N. Cadmium stress tolerance in crop plants: probing the role of sulfur. Plant Signaling and Behavior. 2011;6(2):215–222. doi: 10.4161/psb.6.2.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian J-C, Sacchi GA. Heavy metal stress and sulfate uptake in Maize roots. Plant Physiology. 2006;141(3):1138–1148. doi: 10.1104/pp.105.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ammar WB, Mediouni C, Tray B, Ghorbel MH, Jemal F. Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biologia Plantarum. 2008;52(2):314–320. [Google Scholar]
- 66.Yadav SK. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany. 2010;76(2):167–179. [Google Scholar]
- 67.Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering. 2011;2011:31 pages.939161 [Google Scholar]
- 68.England R, Wilkinson KJ. Determination of phytochelatins in algal samples using LC-MS. International Journal of Environmental Analytical Chemistry. 2011;91(2):185–196. [Google Scholar]
- 69.Scheidegger C, Suter MJF, Behra R, Sigg L. Characterization of lead-phytochelatins complexes by nano electro spray ionization mass spectrometry. Frontiers in Micorbiology. 2012;3(41):1–7. doi: 10.3389/fmicb.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volland S, Schaumloffel D, Dobritzsch D, Krauss GJ, Meind UL. Identification of phytochelatins in the cadmium-stressed conjugating green alga Micrasterias denticulate . Chemosphere. 2013;91:448–454. doi: 10.1016/j.chemosphere.2012.11.064. [DOI] [PubMed] [Google Scholar]


