Abstract
Bacteriovorax were quantified in US Atlantic, Gulf, and Pacific seawater to determine baseline levels of these predatory bacteria and possible seasonal fluctuations in levels. Surface seawater was analyzed monthly for 1 year from Kailua-Kona, Hawaii; the Gulf Coast of Alabama; and four sites along the Delaware Bay. Screening for Bacteriovorax was performed on lawns of V. parahaemolyticus host cells. Direct testing of 7.5 mL portions of seawater from the Atlantic, Pacific, and Gulf coasts gave mean annual counts ≤12.2 PFU. Spikes in counts were observed at 3 out of 4 sites along the Delaware Bay 1 week after Hurricane Sandy. A comparison of summer versus winter counts showed significantly more Bacteriovorax (P ≤ 0.0001) in the Delaware Bay during the summer and significantly more (P ≤ 0.0001) in the Gulf during the winter, but no significant seasonal differences (P > 0.05) for Hawaiian seawater. Bacteriovorax counts only correlated with seawater salinity and temperature at one Delaware site (r = 0.79 and r = 0.65, resp.). There was a relatively strong negative correlation between temperature and Bacteriovorax levels (r = −0.585) for Gulf seawater. Selected isolates were sequenced and identified by phylogenetic analysis as Bacteriovorax clusters IX, X, XI, and XII.
1. Introduction
Vibrio parahaemolyticus and Vibrio vulnificus are important foodborne pathogens associated with the consumption of fish and shellfish, especially oysters, which have long been known to bioconcentrate vibrios within their edible tissues [1, 2]. Vibrio vulnificus also causes life-threatening illness from wound infections acquired in the marine environment [3]. Pathogenic vibrios show seasonal predilection in seawater and shellfish, with high counts during warmer months and low to negligible counts during the colder months [2, 4, 5]. Recently, we showed that naturally occurring Bdellovibrio and like organisms (BALOs) from coastal seawater significantly reduced the levels of V. parahaemolyticus and V. vulnificus in seawater and V. parahaemolyticus in seawater and oysters [6]. Among the BALOs are marine and terrestrial forms, with the marine forms associated with Bacteriovorax, which are exclusively saltwater predators [7, 8]. Bacteriovorax have shown preferential predation toward V. parahaemolyticus when compared to a broad range of potential host bacteria [9–12]. This suggests that Bacteriovorax may invade and kill V. parahaemolyticus in seawater more efficiently than other bacterial pathogens.
The life cycle of Bacteriovorax and other BALOs usually involve intracellular invasion of and replication within a host cell, although some are known to grow host-independently [13–16]. During the attack phase, BALOs propel themselves with a single polar flagellum to find a susceptible Gram-negative bacterium to serve as its host. The BALO enzymatically digests a hole in the host membrane, enters the periplasmic space, and, utilizing nutrients from the host, grows in a worm-like fashion in a structure known as a bdelloplast. When mature, the bdelloplast septates into multiple immature cells and are subsequently released from the host as it lyses. The immature cells develop into mature, attack phase cells to repeat the cycle all over again. Attack phase BALOs are small, with a diameter of only 0.2−0.4 μm [16], making them filterable through 0.45 μm pore size membranes, which facilitates their separation from host bacteria. Immature BALOs are about the same length but appear narrower and smoother than those in the attack phase [6], making them easily filterable as well. In natural unfiltered seawater spiked with >1 × 103 CFU/mL V. parahaemolyticus, V. parahaemolyticus counts decreased by 3-logs to nearly nondetectable levels over 72 h, while naturally occurring BALOs (Bacteriovorax) increased by 3-logs from nearly nondetectable initial levels over this same period [6].
Some studies have evaluated the seasonality of Bacteriovorax in marine systems including estuarine sediment [17] and seawater [18, 19]. The present study further evaluates the seasonality of Bacteriovorax among estuarine species. In this study, we evaluated natural seawater monthly for 1 year for total culturable Bacteriovorax that were capable of infecting Vibrio parahaemolyticus O3:K6 host cells (so called Vibrio predatory bacteria [6]) from four sites along the Delaware Bay (Figure 1(a)), one site from the Gulf Coast of Alabama (Figure 1(b)) and one site in Kailua-Kona, Hawaii (Figure 1(c)). Three of the collection sites along the Delaware Bay and the Gulf site were estuarine. We also identify seasonal patterns for Bacteriovorax abundance, evaluate the effects of temperature and salinity on Bacteriovorax levels, identify some of the Bacteriovorax phylotypes, and suggest environmental factors that may influence Bacteriovorax levels.
Figure 1.
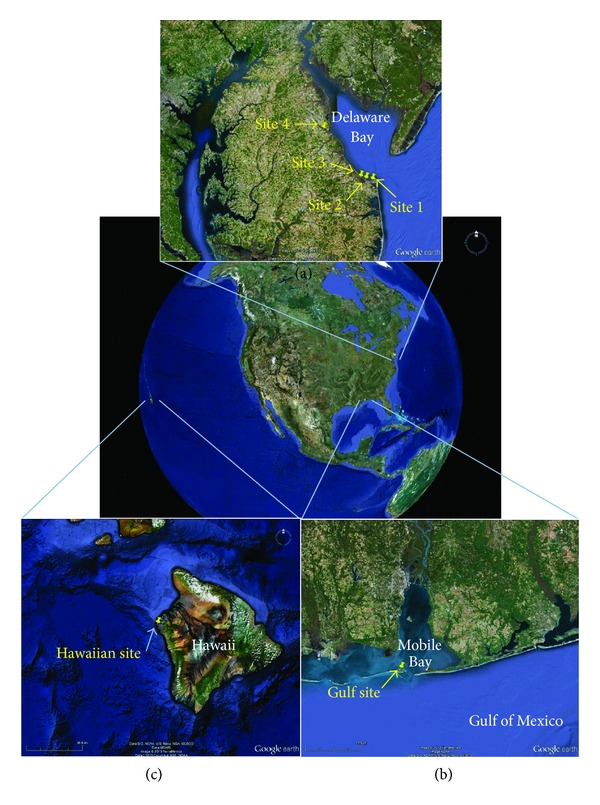
Sites of seawater collection along the (a) Delaware Bay, Delaware, (b) the Gulf Coast of Alabama, and (c) Keyhole Point near Kailua-Kona, Hawaii. Maps not to scale. Images were accessed through Google Earth.
2. Materials and Methods
2.1. Bacterial Strains
A clinical strain of V. parahaemolyticus O3:K6 known as RIMD2210633 was used as host for the assay of Bacteriovorax from seawater. This pandemic strain was originally isolated from an airport quarantine station in Japan in 1996 and caused travelers' diarrhea [20, 21]. Stock cultures of this isolate were routinely grown in Luria Bertani (LB) broth (Beckton, Dickinson and Co., Sparks, MD) supplemented with 2% NaCl (3% NaCl total) or were streaked on LB agar (Beckton, Dickinson and Co.) with 2% added NaCl (3% NaCl total).
2.2. Sampling Sites
Atlantic seawater was surface water collected along the shoreline or rivers' edges during high tide and analyzed from four Delaware Bay sites as previously described [6]. In essence, the Delaware sites were Site 1: the Cape May-Lewes Ferry Terminal in Lewes, DE (38°46′57.85′′N; 75°07′04.73′′W); Site 2: the Broadkill River, outside the University of Delaware Marine Laboratory in Lewes, DE, 0.6 km upstream from the mouth of the river (38°47′26.37′′N; 75°09′51.36′′W); Site 3: Oyster Rocks Road boat landing on the Broadkill River in Milton, DE (38°48′08.01′′N; 75°12′11.57′′W); and Site 4: Scotton Landing on the Saint Jones River in Frederica, DE (39°05′05.94′′N; 75°27′39.99′′W) (Figure 1(a)). Seawater was immediately transported to the laboratory in an insulated cooler at ambient temperature. Seawater was also collected monthly from the dock of the U.S. Food and Drug Administration Laboratory at Dauphin Island, AL (30°15′25.57′′N; 88°6′21.73′′W) (Figure 1(b)), and shipped to Delaware overnight in an insulated cooler for testing. Seawater temperature and salinity were recorded for the Delaware and Gulf sites at time of water collection. Surface seawater was also provided by Kona Coast Shellfish Co., Kailua-Kona, HI (19°43′42.9′′N; 156°3′46.2′′W) (Figure 1(c)), and was express shipped in an insulated cooler to the laboratory where it was received within 2 days of collection. Temperature of the Hawaiian water at time of collection was 24-25°C and approximately 35 ppt salinity year round. The Hawaiian and Gulf water samples were shipped with ice packs during the summer; however, the ice and seawater were physically separated in order to prevent direct contact. After delivery to the laboratory, all seawater samples were analyzed within 4 h.
2.3. Plaque Assay
Counts of Bacteriovorax in 7.5 mL portions of unfiltered or 0.45 μm filtered seawater were quantified on lawns of V. parahaemolyticus O3:K6 host cells grown in Pp20 agar (polypeptone peptone supplemented with Bacto agar), as recently described [6]. Preliminary studies showed that seawater filtration was necessary to reduce the levels of non-Bacteriovorax microbial contaminants that could otherwise overgrow the lawns of V. parahaemolyticus host cells, thus affecting the quantification of immature or attack-phase Bacteriovorax. Incubations were carried out at 22−26°C for 7 days. Potential bacteriophage plaques would be distinguished from Bacteriovorax plaques by their rapid, overnight formation; whereas, Bacteriovorax plaques require several days to form [17]. Although Bacteriovorax produce plaques on Pp20 agar, bacteriophages generally require more nutritionally complex media for plaque formation [17]. No bacteriophages were detected in this study. Since 7.5 mL of seawater was analyzed per assay, total culturable Bacteriovorax counts are listed as counts/7.5 mL seawater throughout this paper.
2.4. Scanning Electron Microscopy
Scanning electron microscopy was performed on select isolates as described previously [6] using a Quanta 200FEG microscope (FEI Co., Hillsboro, OR) after glutaraldehyde fixation, rinsing with 0.1 M imidazole buffer (pH 7.0), dehydration in graded ethanol rinses, critical point drying by vacuum (Denton, Cherry Hill, NJ), and sputter coating with gold in an argon atmosphere using an Edwards Scancoat Six sputter coater (West Sussex, United Kingdom).
2.5. Statistical and DNA Sequence Analyses
Correlation coefficients were obtained between Bacteriovorax counts and salinity or temperature, while significant differences in counts, temperatures, and salinities were determined by Student's t test. P values ≤ 0.05 were considered statistically significant while values ≤0.0001 were considered extremely statistically significant.
For 16S rRNA sequencing, PCR was performed on 13 randomly drawn isolates using 0.2 μM final concentration of forward primer (Bac676F) and reverse primer (Bac1442R), as described by Davidov et al. [22], except there was no GC clamp on the 5′ end. Conditions were 94°C denaturation for 1 min followed by 45 cycles at 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. The cDNAs were electrophoretically purified on an 0.8% agarose gel and ethidium bromide-stained bands of ~700 bp were excised and purified using a QIAquick Gel Extraction kit (Qiagen #28704) following the manufacturer's instructions. Sequencing was performed by GENEWIZ, Inc. (South Plainfield, NJ) using primer Bac1442R. Data was trimmed at the terminal ends and sequences ~700 bp long were identified by GenBank Blast search and compared to phylotypes and clones described for similar sampling sites, as described by Pineiro et al. [9].
3. Results
Bacteriovorax levels were determined for four sites along the Delaware Bay. One site (Site 1) was at the Cape May-Lewes Ferry Terminal near the mouth of the Delaware Bay, while the other three sites were on tidal rivers in close proximity to the Delaware Bay (Figure 1(a)). Mean monthly counts of Bacteriovorax per 7.5 mL of 0.45 μm-filtered seawater were determined for 1 year and are shown in Figure 2(a). Water collection scheduled for late October 2012 was postponed because of Hurricane Sandy which impacted this area with strong tidal surge, hurricane-force winds, and extremely heavy rains during late October. The hurricane caused seawater collection for the October sampling to be delayed until November 5. Bacteriovorax counts were at their highest levels at three of the four collection sites one week after the hurricane (Figure 2(a), asterisk). The most inland site (Site 4) showed no increase after the hurricane but rather decreased from the previous month's mean count. Unlike the other sites, Site 4 experienced a dramatic increase in Bacteriovorax levels over the summer months, with a peak count of 33 PFU/7.5 mL seawater in early September. The Bacteriovorax also showed a seasonal predilection with significantly higher counts in the summer than in the winter (P ≤ 0.0001). Bacteriovorax were detected from at least one site in the Delaware Bay every month except in February 2013 when none (<0.33 PFU/7.5 mL seawater) were detected at any of the four collection sites. The lack of Bacteriovorax detection in February coincided with the lowest water temperatures (mean from 4 sites = 5°C) recorded during the year. Delaware Bay seawater temperatures over a 12-month period were similar among the four sites and showed normal seasonal fluctuations, ranging from lows of about 5°C in February and highs ≥25°C in late July (Figure 2(b)). Salinities were similar for Sites 1–3, but the mean salinity for Site 4 (Figure 2(c)) was significantly lower (P ≤ 0.0001) than the other sites.
Figure 2.

Monthly Bacteriovorax levels and water parameters at four Delaware sites over 1 year. (a) Bacteriovorax levels/7.5 mL seawater, (b) seawater temperature, and (c) seawater salinity. Bacteriovorax levels are the mean of three separate analyses for each site. The asterisk in (a) signifies Bacteriovorax counts from a sample collected 1 week after a major hurricane.
Bacteriovorax counts and corresponding temperatures and salinities are graphically overlaid for ease of comparison for each of the four sites in Figures 3(a)–3(d). Correlations between temperature and Bacteriovorax counts varied greatly between sites with r values of 0.102, 0.068, 0.053, and 0.651 for Sites 1−4, respectively. Site 4, the riverine site, was the most inland and showed a high correlation between temperature and Bacteriovorax counts. There was no correlation at the remaining sites. With one exception salinities remained >25 ppt throughout the year for three of the four sites (Figure 2(c)). Site 4 showed the lowest salinities throughout the study, ranging from a low of 4.8 ppt in February to a high of 23.4 ppt in late July (Figures 2(c) and 3(d)). In addition, there was little to no correlation between salinity and Bacteriovorax counts for Sites 1–3 with r values of −0.067, −0.119, and −0.183, respectively; however, there was a strong correlation between salinity and Bacteriovorax counts for Site 4 (r = 0.792). Spikes in counts were occasionally seen at each site (Figures 3(a)–3(d)) but were not consistently associated with temperature or salinity, except in the case of Sites 1–3, immediately after Hurricane Sandy where peaks in counts occurred as water temperatures dipped from >20°C the month before the storm to approximately 10°C after the storm (Figures 3(a)–3(c)).
Figure 3.

Overlay of Bacteriovorax level, temperature, and salinity for each of four sites along the Delaware Bay. ((a)–(d)) represent Sites 1–4, respectively. Bacteriovorax levels are the mean ± SD of three separate analyses for each site.
Topographies of the Delaware sites differed somewhat. Site 1 was the southernmost site directly along a rock-fortified shoreline near the mouth of the Delaware Bay (Figure 1(a)). In contrast, the other three sites were tidal rivers with marsh grasses growing along the banks of the rivers. The presence or absence of marshes in the vicinity of the collection sites could not be shown to influence total Bacteriovorax levels over the course of this study. Site 4 showed a different trend in total Bacteriovorax counts from those of Sites 1–3, which were similar (Figure 2(a)). This may be due more to the fact that Site 4 was the greatest distance inland from the Delaware Bay (and had lower salinity) rather than to the presence or absence of marshes in the area.
Gulf Coast seawater ranged in salinities (4.8−28.8 ppt) and temperatures (12.2−31.1°C) (Figure 4(a)). In contrast to Delaware seawater, the Gulf Coast seawater showed low levels of Bacteriovorax (≤5 PFU/7.5 mL of seawater) throughout the summer months and significantly higher (P ≤ 0.0001) levels during the winter months (Figure 4(a)). This is opposite to the findings in Delaware, where significantly higher counts (P ≤ 0.0001) were obtained throughout the summer months. In fact, there was a relatively strong negative correlation between temperature and Bacteriovorax levels (r = −0.585) for Gulf seawater. The highest mean Bacteriovorax reading in the Gulf was obtained in December, concurrent with the highest salinity reading. As salinity levels dropped from January to February, so did the levels of total Bacteriovorax (Figure 4(a)). Nevertheless, correlation was low between salinity and Bacteriovorax counts over the course of the year (r = 0.211).
Figure 4.
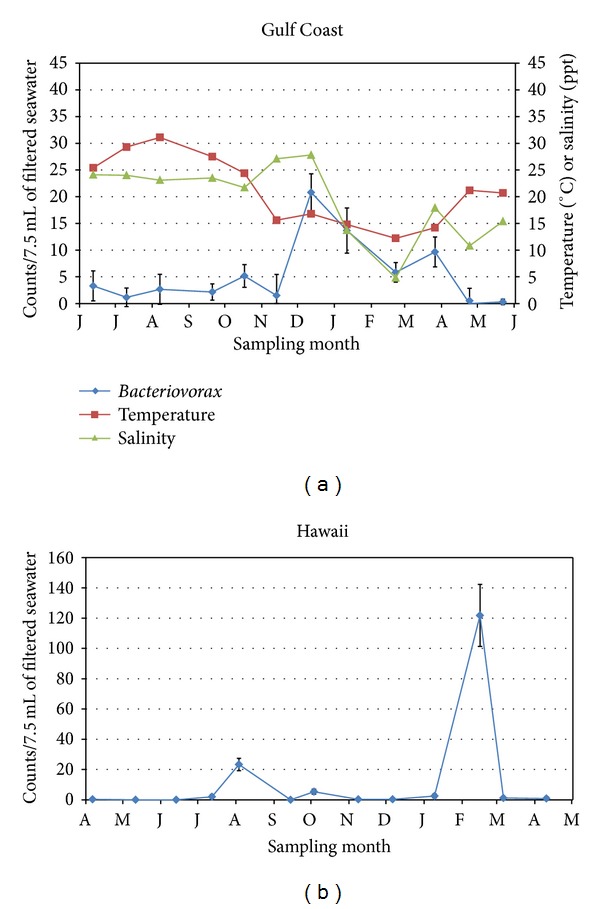
Monthly Bacteriovorax levels, seawater salinity, and temperature for (a) Gulf of Mexico site (starting in June 2012) for 1 year. (b) Bacteriovorax levels for Hawaiian site (starting in April 2012) for 13 months. Seawater temperature and salinity data are not shown for Hawaii because they remain steady between 24 and 25°C and at 35 ppt salinity. All monthly Bacteriovorax levels are the mean ± SD obtained from six samples.
Hawaiian seawater, like the Delaware Bay and Gulf Coast seawater, was surface water, but it was not subjected to seasonal temperature changes or shifts in salinities. It had nearly constant temperature and salinity (24-25°C and 35 ppt) throughout the year. Results showed two spikes in Bacteriovorax counts, one in August followed by one in February, when mean counts increased from levels generally <5 PFU/7.5 mL of seawater to 23 and 121.8 PFU/7.5 mL of seawater, respectively (Figure 4(b)). The cause for these spikes remains uncertain. Bacteriovorax counts for both Gulf Coast and Hawaiian seawater may have been affected by shipping, which took 1 and 2 days, respectively. We cannot preclude the possibility that counts may have changed during transit, especially if potential host cells were present in the seawater. Predation on natural bacteria in the seawater could account for the two spikes in monthly counts that were observed for the Hawaiian seawater (Figure 4(b)).
Sequencing of isolates showed 99.9-100% identity (maximum of 1 base difference out of 700 bp per isolate) to comparable sequence submitted to GenBank by Pineiro et al. [9]. Phylotypes and clones are shown in Table 1 and indicate that the isolates were within four different phylotype clusters (IX, X, XI, and XII). Cluster XII isolates were clones of Coco2B and Hawaii5, as described previously [9]. None of the isolates were of the lower numbered clusters, like IV and V, which are more commonly believed to be low-salt, estuarine species [9, 19, 23]. Cluster IX isolates were from two sites in the Delaware Bay near Lewes, DE, and from one site along the Gulf Coast (Table 1). Previously, Pineiro et al. [9] obtained this same isolate from the Delaware Bay in Lewes and named it as clone Lewes11. The two Delaware isolates were obtained from a medium salinity site (Site 3) and a low salinity site (Site 4).
Table 1.
Phylotypes of selected Bacteriovorax isolated from seawater.
| Cluster | Strain/isolate1 | Origin2 | Isolate designation |
|---|---|---|---|
| IX | Lewes11 | Gulf Coast, Alabama | G3 |
| Delaware Site 3 | OR7 | ||
| Delaware Site 4 | S12 | ||
| X | Hawaii | Delaware Site 3 | OR3 |
| Delaware Site 4 | S13 | ||
| Delaware Site 4 | S15 | ||
| XI | Tri101 | Delaware Site 3 | OR1 |
| XII | Coco2B | Delaware Site 3 | OR2 |
| Delaware Site 2 | OS1 | ||
| Delaware Site 2 | OS2 | ||
| Delaware Site 4 | S11 | ||
| XII | Hawaii5 | Hawaii | H4 |
| Hawaii | H8 |
High levels of microbial contaminants were present in unfiltered Atlantic and Gulf Coast seawater, but Hawaiian seawater was cleaner, thus allowing a comparison of Bacteriovorax counts in unfiltered and 0.45 μm filtered samples (Table 2). In unfiltered seawater, Bacteriovorax were higher for most of the months (Table 2). Only in August were the levels in unfiltered and 0.45 μm filtered seawater essentially the same (23.5 versus 23.3 PFU/7.5 mL of seawater, resp.). In February 2013, counts in the unfiltered Hawaiian seawater were too numerous to count and far exceeded the 121.8 PFU/7.5 mL seawater that was observed for the filtered sample. These spikes in Bacteriovorax counts cannot be attributed to changes in temperature or salinity because temperature and salinity of the Hawaiian seawater remained nearly constant. Additionally, there were no unusual weather conditions to account for these high counts. Mean counts, excluding the February 2013 data, indicate the presence of 6.2 times more Bacteriovorax in unfiltered seawater than in filtered seawater.
Table 2.
Comparison of Bacteriovorax levels in unfiltered versus 0.45 μm filtered Hawaiian seawater. Each monthly count is the mean of six replicate assays.
| Date | Unfiltered seawater (PFU/7.5 mL) | Filtered seawater (PFU/7.5 mL) |
|---|---|---|
| 2012 | ||
| April 6 | 9.4 | 0.3 |
| May 11 | 6.3 | 0.0 |
| June 13 | 17.5 | 0.0 |
| July 12 | 14.2 | 2.0 |
| Aug. 3 | 23.5 | 23.3 |
| Sept. 14 | 16.5 | 0.0 |
| Oct. 3 | 17.8 | 5.3 |
| Nov. 8 | 47.8 | 0.3 |
| Dec. 6 | 23.5 | 0.3 |
| 2013 | ||
| Jan. 9 | 20.7 | 2.5 |
| Feb. 13 | TNTC (>125)1 | 121.81 |
| Mar. 6 | 19.5 | 1.2 |
| Apr. 13 | 7.5 |
0.8 |
| Mean1: | 18.7 | 3.0 |
1Abbreviation: TNTC: too numerous to count. Mean counts exclude the February 13, 2013 data.
Representative plaques on Pp20 agar plates were picked to further evaluate some of the isolates. Plaque sizes often varied from one isolate to another and plaques were generally colorless (Figure 5). Bacteriovorax were usually isolated by picking from the center of large, well-isolated plaques; enriching in host cells suspended in sterile seawater; and imaging by scanning electron microscopy, as previously described [6]. A range of morphologies were observed, as shown in Figure 6, and included small, filterable forms (immature and attack-phase stages) and larger forms within the host (bdelloplast stage). Therefore, their ability to pass through 0.45 μm filters depended on their stage in the life cycle. Areas of clearing around colonies were occasionally observed and were associated with the diffusion of inhibitory substances produced by the colonies. They were not counted as Bacteriovorax plaques. Sequencing of representative samples showed them to be Pseudoalteromonas spp. No rapidly forming plaques, representative of bacteriophages were observed during this study.
Figure 5.
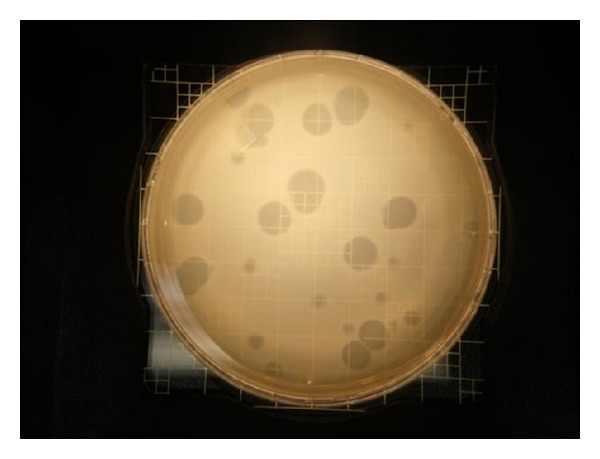
Plaques are readily visible from the assay of Bacteriovorax isolates on a lawn of V. parahaemolyticus host cells after incubation for 7 days. Larger plaques are approximately 1 cm in diameter in this image. Medium is Pp20 agar as indicated in Materials and Methods.
Figure 6.
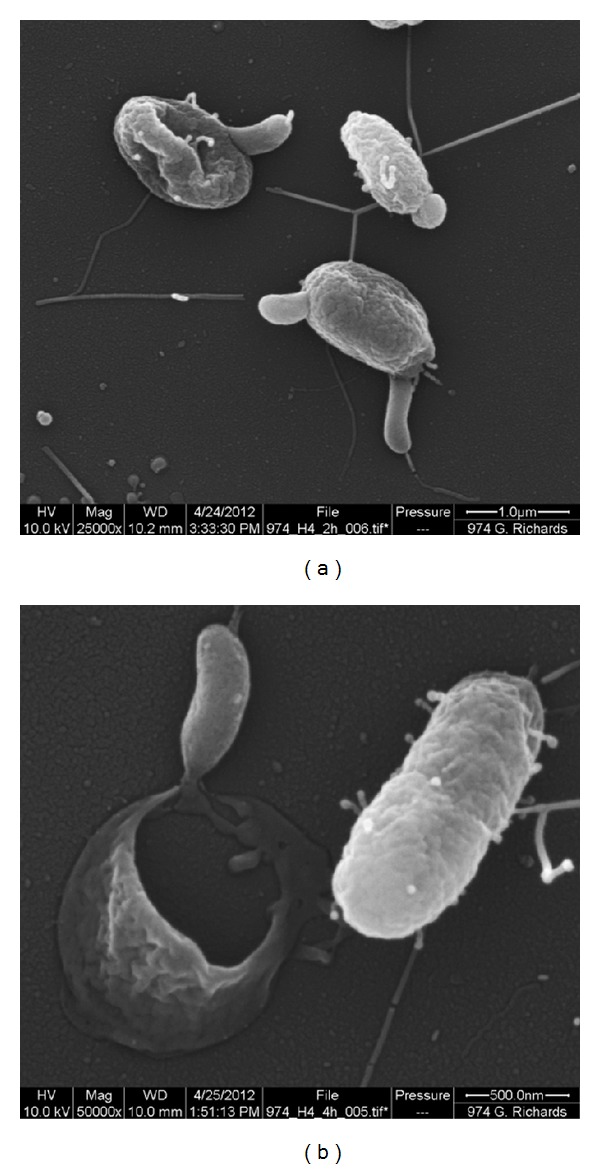
Scanning electron micrographs of Bacteriovorax infecting Vibrio parahaemolyticus. (a) Three vibrios shown with Bacteriovorax apparently entering (infecting) the Vibrio (lower right). The upper left cell shows a late-stage-infected Vibrio with the immature Bacteriovorax emerging from the cell. Note the appearance of the remaining wormlike Bacteriovorax within the partially shrunken Vibrio. (b) Immature stage Bacteriovorax (upper left) emerging from a dead Vibrio. Note the single hole in the Vibrio from which multiple immature Bacteriovorax would have emerged. An apparently uninfected Vibrio is shown on the right.
4. Discussion
Over the course of a year, Bacteriovorax were detected at the highest levels in the Delaware Bay during the summer and along the Gulf Coast during the winter. This latter finding may be surprising at first, since Vibrio levels are typically very low during the winter months. However, Bacteriovorax are known to have broad host specificities against a wide variety of Gram-negative bacteria [9–12]. Although this study concentrated on culturable Bacteriovorax that infected V. parahaemolyticus and hence may be referred to as Vibrio predatory bacteria [6], it would be expected that the Bacteriovorax likely preyed on many other Gram-negative bacteria outside the Vibrionaceae family, particularly when Vibrio levels were low. Bacteriovorax were isolated nearly year round from small volumes (7.5 mL) of seawater without the need for enrichment. It should be recognized that the above counts represent the number of culturable Bacteriovorax in 0.45 μm-filtered seawater and may underrepresent the total number of Bacteriovorax within the water. Figure 6 shows the relative size of Bacteriovorax compared to its Vibrio parahaemolyticus host. Although the immature and attack-phase Bacteriovorax are small enough to pass through a 0.45 μm filter, an appreciable number of Bacteriovorax might not; thus counts expressed throughout this paper should be considered as minimal counts derived principally from immature and attack phase Bacteriovorax. Larger Bacteriovorax, that is, those maturing as bdelloplasts within the host cell or the so-called “host independent” strains that occasionally grow as elongated chains, would readily be filtered out. By comparing levels of Bacteriovorax in filtered and unfiltered Hawaiian seawater (Table 2), we estimate that 84% of the Bacteriovorax in seawater were unable to pass through a 0.45 μm filter. This highlights a shortcoming of using filtered seawater for the quantification of Bacteriovorax in culture-dependent methods. It is uncertain if season has any influence on the prevalence of different life forms or sizes of Bacteriovorax.
The presence of a variety of Bacteriovorax phylotypes having different preferences for both salinity and temperature was reported in a study of the Cheaspeake Bay where some phylotypes (clusters IV and V) were low to medium salinity (estuarine) species, whereas others (clusters IX and XII) were recovered from medium or high salinity sites [9, 23]. Sequence analysis of 13 samples in this present study showed that all the isolates were in clusters IX, X, XI, or XII (Table 1), regardless of the isolates' origin. As mentioned previously, four of the sequenced isolates obtained from the lowest salinity site in Delaware (Site 4) were clusters IX, X, or XII—clusters more commonly associated with higher salinity seawater [23]. In the present study, salinities did not deviate greatly for three of the Delaware Bay sites or for seawater obtained from Hawaii; however, seawater from the Gulf Coast and from Delaware Bay Site 4 varied appreciably. When the salinities were the highest at these two sites, the Bacteriovorax levels peaked (Figures 3(d) and 4(a)). We showed that salinity and total Bacteriovorax counts correlated well (r = 0.792) at the lowest salinity Delaware Bay site (Site 4) but not at the higher salinity Delaware sites. Compared to the other sites, Site 4 had relatively low and widely varying salinities; therefore, the strong correlation between salinity and Bacteriovorax counts at this site must be tempered by the fact that this riverine location never reached the higher salinity levels found in the other Delaware Bay sites. Pineiro et al. detected clusters IV and V in low to moderate salinity areas of the Delaware Bay and in the nearby Chesapeake Bay [23]. We did not identify any cluster IV or V isolates in Delaware or elsewhere, even though these clusters are considered more estuarine species [9, 23]. Part of the reason may be that our sampling was very limited and was not intended to be a comprehensive analysis of phylotypes, since the dynamic mixing of various proportions of fresh or low-salinity water and seawater in these areas during high tide would be expected to continually alter the phylotype profiles. The identification of only phylotypes IX, X, XI, and XII in the Delaware Bay does not preclude the presence of other undetected phylotypes from among the many unsequenced Bacteriovorax that were enumerated over the year.
Studies have demonstrated that relaying of oysters to higher salinity areas reduces Vibrio levels faster than when oysters are maintained in lower salinity waters [24, 25]. High salinity relay has been proposed as a postharvest processing strategy to reduce V. vulnificus in oysters and was also shown effective in eliminating V. parahaemolyticus as well [26]. The mechanism by which high salinity regimes enhance Vibrio reductions in oysters remains uncertain. We recently hypothesized that high salinity supports the proliferation of predatory bacteria and that drought conditions in North Carolina from 2007 to 2009 may have been responsible for the apparent disappearance of V. vulnificus from seawater and oysters [6], as reported by Froelich et al. [27]. Other factors beyond salinity and temperature that may well affect Bacteriovorax levels and phylotypes within rapidly changing estuarine systems include dramatic fluctuations in flow rates due to storm events, currents and winds, runoff, physical and chemical parameters of the seawater, and perhaps proximity to marshlands, beaches, farmlands, forested or residential areas, or rocky shorelines.
The amount of nutrients and suspended solids in the water varied and resuspended sediment was commonly observed. During the winter, Bacteriovorax could not be found in top or bottom water samples from the Chesapeake Bay but were isolated during this period from sediment [23]. This may indicate that greater numbers of Bacteriovorax are present in the sediment in the winter and perhaps at other times of the year; therefore, resuspension of sediment into the water column might influence the Bacteriovorax levels detected in the water. Our finding that seawater taken from the Delaware Bay one week after Hurricane Sandy contained the highest levels of Bacteriovorax detected in the Delaware waters may be due to the resuspension of large amounts of sediment into the water column by the hurricane. These are all factors that affect shellfish growing areas and the levels of Bacteriovorax within these areas. The combination of these highly variable factors will complicate full discernment of the intricacies of predatory interactions with vibrios and other bacteria in the environment.
Oysters and other molluscan bivalve shellfish are well known for filtering pathogens and other contaminants from seawater making shellfish potential sources for illness. Bacteriovorax that may be suspended in seawater or attached to suspended matter are likely sources of food for bivalve shellfish and could concentrate within the shellfish similar to that of pathogens. Tests for Bacteriovorax quantification within shellfish tissues have not been developed to date; however, we hypothesize that Bacteriovorax within shellfish would continue to parasitize vibrios and other potential pathogens much as they do in seawater, thus rendering shellfish safer to consume when they are obtained from areas where Bacteriovorax are present in higher numbers. Our previous work indicates that V. parahaemolyticus levels drop significantly in oysters and seawater as Vibrio predatory bacteria increase in the surrounding water [6]. Our objectives over the next few years are to (a) develop methods to accurately quantify Bacteriovorax levels within oyster tissues, (b) determine the relationships between Bacteriovorax and Vibrio counts in seawater and in shellfish, and (c) develop potential commercial processing strategies using Bacteriovorax to reduce vibrios and other potential pathogens in shellfish.
Acknowledgments
The authors wish to thank Corinne Audemard (Virginia Institute of Marine Science) and David Kingsley (USDA, ARS) for critical review of this paper, Guoping Bao (USDA, ARS) for assistance with electron microscopic analyses, and David Needleman (USDA, ARS) for sequence analysis. This study was funded through a USDA, Agricultural Research Service (ARS) Specific Cooperative Agreement 58-1935-0-043 with the University of Delaware, and by USDA, ARS intramural funds. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not constitute recommendation or endorsement by the U.S. Department of Agriculture or the U.S. Food and Drug Administration, who are equal opportunity providers and employers.
References
- 1.Earampamoorthy S, Koff RS. Health hazards of bivalve mollusk ingestion. Annals of Internal Medicine. 1975;83(1):107–110. doi: 10.7326/0003-4819-83-1-107. [DOI] [PubMed] [Google Scholar]
- 2.DePaola A, Hopkins LH, Peeler JT, Wentz B, McPhearson RM. Incidence of Vibrio parahaemolyticus in U.S. Coastal waters and oysters. Applied and Environmental Microbiology. 1990;56(8):2299–2302. doi: 10.1128/aem.56.8.2299-2302.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver JD. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiology and Infection. 2005;133(3):383–391. doi: 10.1017/s0950268805003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly MT. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Applied and Environmental Microbiology. 1982;44(4):820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motes ML, DePaola A, Cook DW, et al. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica) Applied and Environmental Microbiology. 1998;64(4):1459–1465. doi: 10.1128/aem.64.4.1459-1465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards GP, Fay JP, Dickens KA, Parent MA, Soroka DS, Boyd EF. Predatory bacteria as natural modulators of Vibrio parahaemolyticus and Vibrio vulnificus in seawater and oysters. Applied and Environmental Microbiology. 2012;78:7455–7466. doi: 10.1128/AEM.01594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piñeiro SA, Williams HN, Stine OC. Phylogenetic relationships amongst the saltwater members of the genus Bacteriovorax using rpoB sequences and reclassification of Bacteriovorax stolpii as Bacteriolyticum stolpii gen. nov., comb. nov. International Journal of Systematic and Evolutionary Microbiology. 2008;58(5):1203–1209. doi: 10.1099/ijs.0.65710-0. [DOI] [PubMed] [Google Scholar]
- 8.Williams HN, Turng B-F, Kelley JI. Survival response of Bacteriovorax in surface biofilm versus suspension when stressed by extremes in environmental conditions. Microbial Ecology. 2009;58(3):474–484. doi: 10.1007/s00248-009-9499-7. [DOI] [PubMed] [Google Scholar]
- 9.Pineiro SA, Stine OC, Chauhan A, Steyert SR, Smith R, Williams HN. Global survey of diversity among environmental saltwater Bacteriovoracaceae . Environmental Microbiology. 2007;9(10):2441–2450. doi: 10.1111/j.1462-2920.2007.01362.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor VI, Baumann P, Reichelt JL, Allen RD. Isolation, enumeration, and host range of marine Bdellovibrios . Archives of Microbiology. 1974;98(2):101–114. doi: 10.1007/BF00425273. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Amat A, Torrella F. Formation of stable bdelloplasts as a starvation-survival strategy of marine bdellovibrios. Applied and Environmental Microbiology. 1990;56(9):2717–2725. doi: 10.1128/aem.56.9.2717-2725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TD, Williams HN, Turng BF. Susceptibility of bacteria in estuarine environments to autochthonous Bdellovibrios . Microbial Ecology. 1998;35(3):256–264. doi: 10.1007/s002489900081. [DOI] [PubMed] [Google Scholar]
- 13.Burnham JC, Hashimoto T, Conti SF. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. Journal of Bacteriology. 1968;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eksztejn M, Varon M. Elongation and cell division in Bdellovibrio bacteriovorus . Archives of Microbiology. 1977;114(2):175–181. doi: 10.1007/BF00410781. [DOI] [PubMed] [Google Scholar]
- 15.Rosche TM, Smith DJ, Parker EE, Oliver JD. RpoS involvement and requirement for exogenous nutrient for osmotically induced cross protection in Vibrio vulnificus . FEMS Microbiology Ecology. 2005;53(3):455–462. doi: 10.1016/j.femsec.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Williams HN, Baer ML, Tudor JJ. Bdellovibrio stolp and starr. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology: The Proteobacteria. Vol. 2. New York, NY, USA: Springer; 2005. pp. 1040–1053. [Google Scholar]
- 17.Williams HN, Falkler WA, Jr., Shay DE. Seasonal distribution of bdellovibrios at the mouth of the Patuxent River in the Chesapeake Bay. Canadian Journal of Microbiology. 1982;28(1):111–116. doi: 10.1139/m82-011. [DOI] [PubMed] [Google Scholar]
- 18.Williams HN. A study of the occurrence and distribution of bdellovibrios in estuarine sediment over an annual cycle. Microbial Ecology. 1988;15(1):9–20. doi: 10.1007/BF02012949. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Young S, Berhane T-K, Williams HN. Predatory bacteriovorax communities ordered by various prey species. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0034174.e34174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino K, Oshima K, Kurokawa K, et al. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. The Lancet. 2003;361(9359):743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 21.Nasu H, Iida T, Sugahara T, et al. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. Journal of Clinical Microbiology. 2000;38(6):2156–2161. doi: 10.1128/jcm.38.6.2156-2161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidov Y, Friedjung A, Jurkevitch E. Structure analysis of a soil community of predatory bacteria using culture-dependent and culture-independent methods reveals a hitherto undetected diversity of Bdellovibrio-and-like organisms. Environmental Microbiology. 2006;8(9):1667–1673. doi: 10.1111/j.1462-2920.2006.01052.x. [DOI] [PubMed] [Google Scholar]
- 23.Pineiro S, Chauhan A, Brehane T-K, Athar R, Zheng G, et al. Niche partition of Bacteriovorax operational taxonomic units along salinity and temporal gradients in the Chesapeake Bay reveals distinct estuarine strains. Microbial Ecology. 2013;65:652–660. doi: 10.1007/s00248-013-0186-3. [DOI] [PubMed] [Google Scholar]
- 24.Motes ML, DePaola A. Offshore suspension relaying to reduce levels of Vibrio vulnificus in oysters (Crassostrea virginica) Applied and Environmental Microbiology. 1996;62(10):3875–3877. doi: 10.1128/aem.62.10.3875-3877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motes ML, DePaola A, Cook DW, et al. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica) Applied and Environmental Microbiology. 1998;64(4):1459–1465. doi: 10.1128/aem.64.4.1459-1465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Audemard C, Kator HI, Rhodes M, et al. High salinity relay as a postharvest processing strategy to reduce Vibrio vulnificus levels in Chesapeake Bay oysters (Crassostrea virginica) Journal of Food Protection. 2011;74(11):1902–1907. doi: 10.4315/0362-028X.JFP-11-152. [DOI] [PubMed] [Google Scholar]
- 27.Froelich BA, Williams TC, Noble RT, Oliver JD. Apparent loss of Vibrio vulnificus in North Carolina oysters coincides with drought-induced increase in salinity. Applied and Environmental Microbiology. 2012;78:3885–3889. doi: 10.1128/AEM.07855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


