Abstract
Background
Necrotizing enterocolitis (NEC) is a major cause of death and morbidity in very low birth weight infants.
Objective
To identify biomarker(s) that would predict NEC using buccal swab samples utilizing a proteomic approach.
Methods
Cumulative buccal swab samples derived from very low birthweight (VLBW) preterm infants (<32 wk gestational age and <1250g) at one, two and three weeks prior to the development of NEC and matched controls were subjected to 2D-DIGE and LC-MS/MS analysis for proteomic protein discovery. After identification of 21 altered proteins, we chose 3 candidate proteins using a broad systems biologic analysis approach that suggested several altered cellular processes that could be associated with NEC.
Results
Preliminary validation studies using Western blots on these samples and 10 additional NEC and 10 matched control buccal samples collected within 2 or 3 week before NEC diagnosis analysis showed lower Interleukin-1 receptor-antagonist (IL-1RA).
Conclusion
Our results suggest that IL-1RA is worthy of further studies to determine its utility in helping predict NEC.
Keywords: Necrotizing enterocolitis, biomarker, very low birth weight infants, proteomics, systems biology, prediction, diagnosis
INTRODUCTION
Necrotizing enterocolitis (NEC) is among the most common, devastating, and difficult to treat diseases in preterm neonates [1]. The mean prevalence of the disorder is about 7% among infants with a birth weight between 500 and 1500g in the U.S. and Canada based on multicenter neonatal databases[2,3]. The estimated rate of death associated with NEC ranges between 20 and 30%[4], with a significant risk of long-term neurodevelopmental consequences [5]. The pathophysiology of NEC is multifactorial, which renders prediction of risk a major challenge. [6–10].
There have been several studies of diagnostic biomarkers [6, 11–14] but an effective predictive approach requires the identification of highly sensitive and specific biomarkers well before the symptoms occur [6]. Very little information is available about non-invasive predictive biomarkers. An earlier attempt to develop biomarkers by our group evaluated intestinal microbiota to identify at-risk infants by using early stool samples (including meconium) of preterm infants and demonstrated that changes in the stool microbiota in these infants do occur prior to the onset of NEC [7]. However, stool samples from preterm infants are often not available for several days making microbiota based early detection difficult to implement in a clinical setting. Thus, we collected buccal swabs, which represent a minimally invasive and readily available method to obtain patient samples. Previous studies have been successful in identifying disease biomarkers in several other disease processes [15–17]. We hypothesized that predictive biomarkers of NEC could be discovered by analyzing buccal swab samples using a 2-dimensional difference gel electrophoresis (2D-DIGE) and LC-MS/MS proteomics platform. Once discovered, these could be validated using immunoblotting techniques.
METHODS
Study Design and Sample Collection
This study was approved by the Investigational Review Board of the University of Florida. IRB# is 386-2008 and the period of this study was 2010–2013. Preterm infants with birth weights ≤ 1,250 grams and gestational ages ≤ 32 weeks from three University of Florida affiliated hospitals were screened for study enrollment. Informed consent was obtained from the parents of infants that met inclusion criteria. Infants with congenital malformations, congenital conditions of the intestine or lethal conditions were excluded. Only patients with radiologic signs or direct intraoperative confirmation of intestinal pathology were considered to have met the criteria for diagnosis of NEC [1, 18]. Control infants were matched to NEC infants using gestational age, birth weight, center, and date of birth ± 2 months.
Phase 1 discovery study involved proteomic profiling, LC-MS/MS protein identification and preliminary Western blot validation of candidate biomarkers in pooled buccal swab samples from 3 time points, collected 3 weeks, 2 weeks and 1 week prior to the development of NEC on 3 NEC (n=3) and 3 control (n=3) infants.
In phase 2, further validation was done using Western blot on an additional 10 NEC and 10 matched controls with buccal samples collected but not pooled 3 weeks, 2 weeks and 1 week before the onset of NEC.
Buccal samples were collected at weekly intervals by placing and twisting a Cytobrush Collector (CooperSurgical Inc., Trumbull, CT) 360 degrees on both cheeks and the tongue to maximize protein collected. The brush was then washed into 1ml phosphate buffered saline and then immediately placed in −80ºC for later analysis.
PHASE 1: Discovery Phase
Buccal Swab Protein Extraction, 2-D Gel Electrophoresis and Image Data Analysis
Buccal swab protein was extracted in 10× RIPA buffer. The resulting protein pellet was dissolved in DIGE labeling buffer. Protein concentration was determined with EZQ Protein Quantification Kit (Invitrogen, Carlsbad, CA).
Protein labeling with CyDye was performed using the CyDye technology (GE Healthcare, Pittsburgh, PA). Equal amounts (100μg) of Cy3-control, Cy5-NEC, and Cy2-reference mixture were loaded per polyacrylamide gel (Jule INC. Milford, CT) in Ettan Daltsix Unit from GE.
Immediately after gel electrophoresis, gels were scanned using a Typhoon 9400 Variable Mode Imager (GE). The image acquired was analyzed with DeCyder 2D software(GE). In the Biological Variation Analysis, a master gel was created with information from all gels to provide statistical data on differential protein expression levels between control and NEC groups. There were 2000 spots detected and matched. Interested spots were selected by setting the fold difference threshold to 1.5 fold (NEC/control after normalized). A pick list was made and statistical confidence when two sided Student’s t-test p value was less than 0.05.
Protein Spot Excision and Protein Identification
The information obtained from DeCyder software was transferred to an automated ProPic spot picker (Genomic Solutions, Ann Arbor, MI) and was submitted for protein identification using LC-MS/MS. LC-MS/MS analysis was carried out on a LTQ Orbitrap XL mass spectrometer (Thermo Scientific, Bremen, Germany). All MS/MS spectra were analyzed using Mascot software (Matrix Science, London, UK; version 2.2.2). Scaffold-02-03-01 (Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications [19].
Database Mining – System Biology Approach
PANTHER bioinformatics software (Protein ANalysis THrough Evolutionary Relationships, University of Southern California, Los Angeles Ca) was used for gene ontology classification utilizing the human protein ontology database to classify proteins into distinct categories of molecular functions and biological processes [20]. Pathway Studio software version 8.0 (Ariadne Genomics, Rockville, MD) was used to construct pathways and identify altered protein interaction maps along with determining cellular localization of differential proteins.
Western Blot
To validate the findings from proteomic analysis, the same protein extracts used for 2D-DIGE were analyzed using Western blotting.
Equal amounts of protein were loaded to each well and separated on 4–20% acrylamide SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA) and transferred to PVDF membranes. After blocking, three separate blots were probed using different antibodies (all 1:1000, Abcam, Cambridge, MA) for IL-1RA (interleukin 1 receptor antagonist), peroxidorexin 1 or alpha 1 antitrypsin, respectively. Secondary antibodies, ECL Plex goat anti-rabbit Cy5 or goat anti-mouse Cy5 (1:3000, GE Healthcare, Pittsburgh, PA) were incubated with the blots. Images of blots were captured by a Typhoon Trio+ scanner and analyzed using ImageQuant software. Relative protein concentrations were quantitated and normalized by total protein loading with Sypro Ruby or Deep Purple staining [21].
PHASE 2: Validation Study
Two additional validation experiments were performed on samples collected two or three weeks and one week prior to onset of NEC from 10 NEC babies and 10 matched control infants. Western blot were used to evaluate IL-RA, perixidorexin 1 or alpha 1 antitrypsin as described above.
Statistical Methods
Comparisons between NEC vs. matched controls were done on three candidate biomarkers: IL1RA, antitrypsin, and peroxiredoxin via one sample two-sided t-tests. These were selected from a wider battery of markers from three other patients, as described elsewhere in this article. As this is a pilot study, we consider those with P<0.10 worthy, of future study. The planned sample size (n=10) was not based on a power analysis, but rather on what could be accomplished in the intended time frame.
RESULTS
Clinical Characteristics
Clinical characteristics were compared between control and NEC infants, including birth weight, gestational age, Apgar scores, type of milk, mode of delivery, prenatal steroids exposure, maternal antibiotic exposure, and mode of ventilator support. There were no significant differences between NEC infants and controls (p >0.05). Table 1 showed data for the second phase validation study.
Table 1.
Baseline Characteristics of the Infants.
| Characteristic | NEC (n=10) | Control (n=10) |
|---|---|---|
|
| ||
| Birth weight – g | 916±326 | 1279±420 |
|
| ||
| Gestational age at birth – wk | 26.8±2.1 | 28.2±1.99 |
|
| ||
| Male sex – no./total no. (%) | 6/10 (60) | 6/10 (60) |
|
| ||
| Type of Milk – no./total no. (%) | ||
| Breast Milk | 4/10 (40) | 6/10 (60) |
| Formula | 2/10 (20) | 0/10 |
| Both | 4/10 (40) | 4/10 (40) |
|
| ||
| Mode of delivery – no./total no. (%) | ||
| Vaginal | 3/10 (30) | 3/10 (30) |
| C-section | 7/10 (70) | 7/10 (70) |
|
| ||
| Maternal use of antenatal corticosteroids—no./total no. (%) | 8/10 (80) | 9/10 (90) |
| Any | 2 (20) | 2 (20) |
| Full course | 6 (60) | 7 (70) |
|
| ||
| Prenatal maternal antibiotic exposure | 7/10 (70) | 8/10 (80) |
|
| ||
| Apgar score at 1 min | 3.9±2.77 | 6.1±2.88 |
|
| ||
| Apgar score at 5 min | 7±2.21 | 8.2±1.48 |
|
| ||
| Day of life at the time of NEC | 24.4±13 | |
|
| ||
| Positive pressure ventilation (bag and mask) | 8/10 (80) | 5/10 (50) |
|
| ||
| Continuous positive airway pressure (CPAP) | 3/10 (30) | 2/10 (20) |
|
| ||
| Intubation and mechanical ventilation | 7/10 (70) | 5/10 (50) |
(Mean ± SD)
Predictive Biomarkers Discovery
2D gel analysis and LC-MS/MS of NEC buccal protein levels yielded a total of 21 (8 increased and 13 decreased, all P<0.05) altered differential protein spots compared to controls. We identified three biomarkers of special interest that were of high quality and had a high fold difference between NEC and controls; one downregulated protein, IL-1RA and two upregulated proteins, peroxiredoxin 1 and alpha-antitrypsin (Table 2). These three proteins were validated using Western blot for the same samples that were used for proteomics analysis. f. Only IL-1RA showed statistical difference (p<0.05, data not shown).
Table 2.
The proteomics platform identified 21 altered proteins associated with NEC.
| spot # | Protein Name (Symbol) | Fold case/normal | p-value | Gel mass kDa/pI | Theoretical mass kDa/pI | Mascot score | % coverage | Number of unique peptides |
|---|---|---|---|---|---|---|---|---|
| 3013 | Interleukin-1 receptor antagonist (IL-1RA) | −3.15 | 0.029 | 16.23/5.01 | 20.04/5.82 | 256 | 28 | 5 |
| 2971 | −2.19 | 0.01 | 18.09/5.05 | 525 | 24 | 4 | ||
| 2892 | Peroxiredoxin-1 (Prdx1) | 4.18 | 0.004 | 23.53/8.59 | 22.09/8.27 | 274 | 39 | 8 |
| 998 | Isoform 1 of Alpha-1-antitrypsin (A1AT) | 3.18 | 0.045 | 72.77/5.53 | 46.72/5.37 | 117 | 6 | 3 |
| 1005 | 3.48 | 0.021 | 72.49/5.59 | 127 | 6 | 4 | ||
| 1203 | Clusterin isoform (apolipoprotein J) (APOJ) | −2.16 | 0.0087 | 65.50/7.62 | 52.48/5.88 | 140 | 8.91 | 3 |
| 1146 | −1.95 | 0.019 | 67.23/7.24 | 159 | 8.9 | 3 | ||
| 2777 | Proteosome subunit alpha type 2 (PSMA2) | 1.18 | 0.047 | 26.04/5.05 | 25.90/6.91 | 85 | 13.2 | 2 |
| 854 | Gelsolin (isoform 2-cytoplasmic) (GSN2) | 1.78 | 0.019 | 81.26/5.61 | 80.62/5.58 | 698 | 22 | 11 |
| 855 | 1.76 | 0.037 | 80.66/5.68 | 810 | 23 | 16 | ||
| 1897 | Cleaved Peroxisomal multifunctional enzyme type 2 (3R)-hydroxyacyl-CoA dehydrogenase) (HSD17B4) | −3.61 | 0.0016 | 38.60/8.78 | 33.59/8.6 | 298 | 9.92 | 5 |
| 2892 | Phosphatidylethanolamine-binding protein 1 (PEBP1) | 4.18 | 0.004 | 23.53/8.59 | 21.05/7.01 | 243 | 26 | 4 |
| 1975 | Alpha-2-glycoprotein 1, zinc precursor (AZGP1) | −2.24 | 0.0062 | 38.70/5.41 | 34.26/5.71 | 112 | 26 | 9 |
| 805 | Polymeric immunoglobulin receptor (PIGR) | 2.08 | 0.0026 | 86.63/5.24 | 83.260/5.59 | 238 | 7.3 | 6 |
| 2442 | cDNA FLJ75207 | −2.22 | 0.00047 | 31.15/7.35 | 29.12/8.27 | 297 | 12 | 3 |
| 2436 | −2.45 | 0.0025 | 31.12/7.0 | 326 | 12 | 4 | ||
| 3013 | Prolactin-inducible protein (PIP) | −3.15 | 0.029 | 16.23/5.01 | 16.56/8.26 | 278 | 26 | 3 |
| 3027 | −2.76 | 0.0053 | 8.85/4.63 | 172 | 4 | 2 | ||
| 1146 | Protein-glutamine gamma-glutamyltransferase E (TGM3) | −1.95 | 0.019 | 67.23/7.24 | 76.62/5.61 | 727 | 23 | 10 |
| 2892 | Neutrophil gelatinase-associated lipocalin (NGAL) | 4.18 | 0.004 | 23.53/8.59 | 22.59/9.02 | 503 | 26 | 8 |
| 2181 | N-acetylglucosamine kinase (NAGK) | −1.47 | 0.026 | 34.70/5.70 | 37.36/5.82 | 1014 | 30 | 14 |
| 1975 | cDNA FLJ53019, highly similar to Serpin B13 | −2.24 | 0.0062 | 38.70/5.41 | 45.28/5.56 | 830 | 26 | 9 |
| 1146 | cDNA FLJ54957, highly similar to transketolase | −1.95 | 0.019 | 67.23/7.24 | 68.74/7.58 | 258 | 12 | 5 |
| 1203 | −2.16 | 0.0087 | 65.50/7.62 | 222 | 8.7 | 3 | ||
| 1694 | Placental protein 11 (PP11) | −1.49 | 0.017 | 49.35/5.23 | 42.10/5.26 | 368 | 14 | 4 |
| 1173 | Isoform 1 of heat shock cognate 71 kDa protein (HSP71) | −2.37 | 0.018 | 65.92/6.3 | 70.88/5.37 | 202 | 8.2 | 4 |
| 2425 | s-formylglutathione hydrolase (esterase) (ESD) | −2.49 | 0.00027 | 31.88/6.6 | 31.45/6.54 | 86 | 7.45 | 2 |
| 2932 | Small proline-rich protein 3 (SPRR3) | 2.37 | 0.014 | 20.47/8.34 | 18.15/8.86 | 89 | 28 | 4 |
(8 increased and 13 decreased, NEC vs controls)
Systems Biology Approach
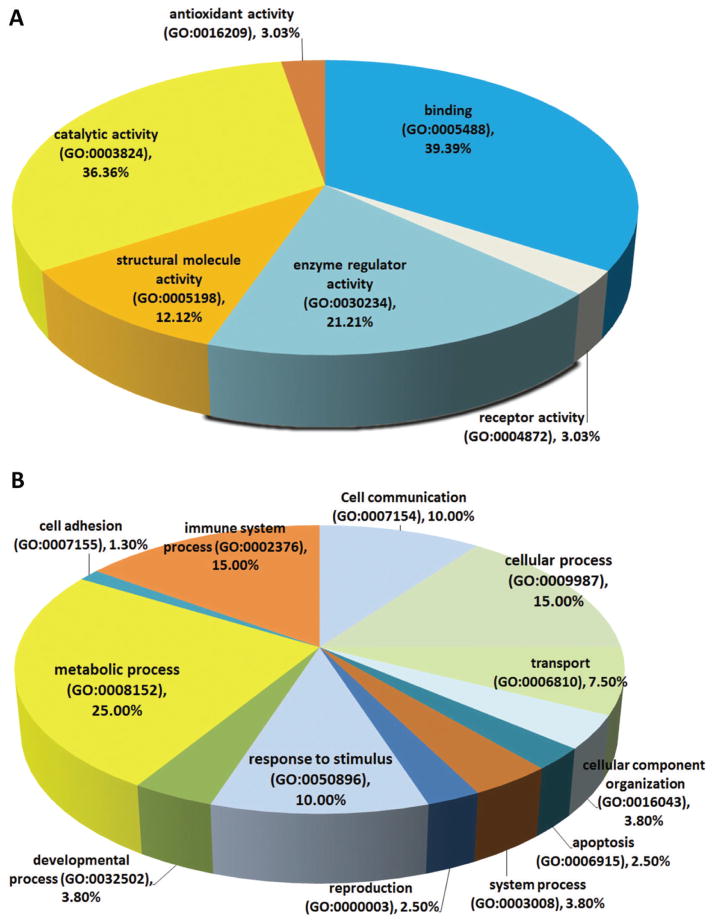
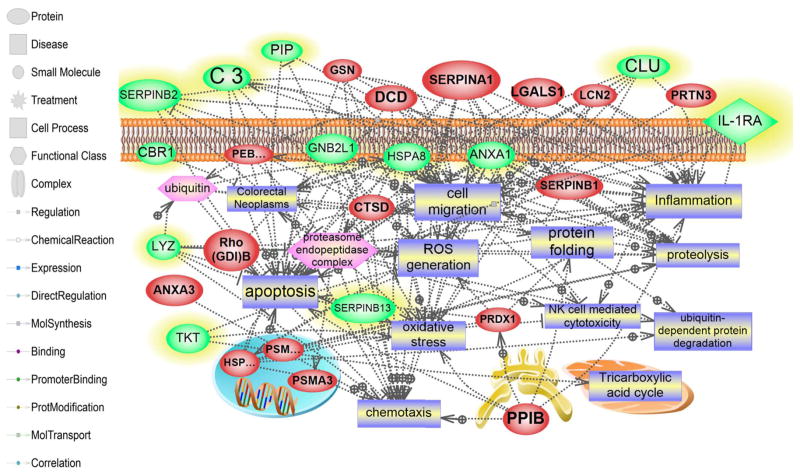
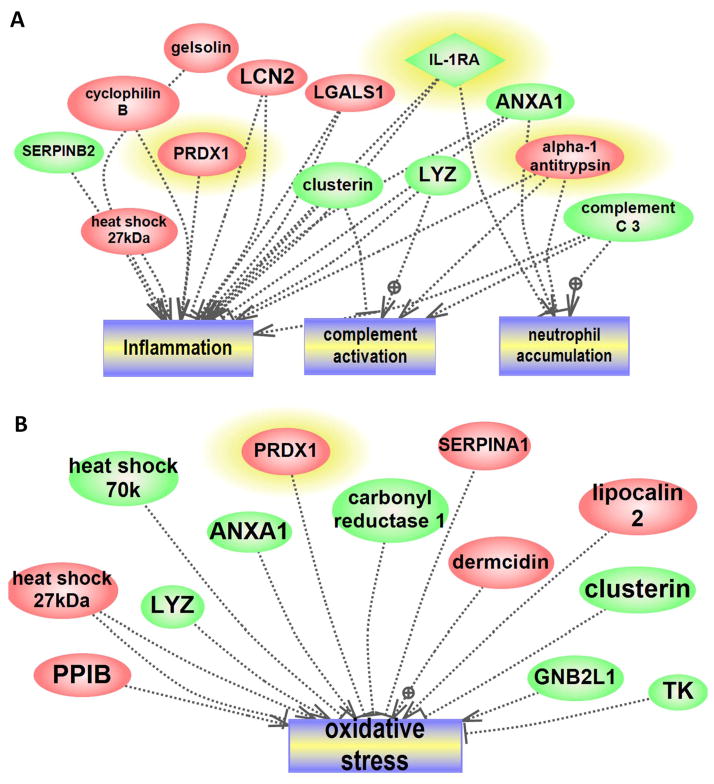
A bioinformatics analysis explored if our 2D based findings would pinpoint specific pathways or classes of proteins altered in NEC. This resulted in 80 assignments for biologic processes, 38 assignments for molecular function and these were sorted into 6 and 12 categories, respectively (Figure 1A, B). The percentages listed are calculated as the number of proteins associated with a particular functional block normalized to the total number of proteins. Altered proteins belonging to different structural and functional families are regulating different biological processes in the development of NEC. The network mapped altered cell processed and pathways regulated by the identified (increased and decreased) proteins. Several processes central to the pathogenesis of NEC were identified, including inflammation, oxidative stress, cell migration, and apoptosis (Figure 2). Of the identified proteins, 13 were shown to regulate the inflammation-related processes (complement activation, neutrophil accumulation and inflammation activation, Figure 3A), among these proteins are the three we subsequently validated by Western blot (peroxiredoxin 1, IL-1RA, and alpha 1 antitrypsin). NEC associated oxidative stress response related to 13 proteins (7 increased and 6 decreased) (Figure 3B).
Figure 1. Molecular function, Biological Process and Cellular localization of Altered NEC Protein Data Set.
(A) Gene Ontology analysis of the NEC altered protein data set was performed to further characterize these proteins. Thirty-eight assignments were obtained for molecular function. (B) Biological process.
Figure 2. Global Analysis of altered Pathways and Networks in the NEC samples.
Several pathways were identified based on the altered proteome; these pathways are believed to be central to the pathogenesis of NEC. These processes include inflammation, cell death, oxidative stress, cell migration and apoptosis. The red color shows up-regulated proteins prior to NEC onset. The green color shows proteins that are down-regulated. The shape of a given protein is indicative of its functional class as shown in the legend. Also included in the legend is the definition of the lines connecting 2 proteins.
Figure 3. Sub-Networks Enriched Analysis of the NEC Altered Proteins.
Enriched sub-networks of altered proteins using the identifiers “Cellular Process/Regulation” filters and downstream directionality identified individual pathway-protein components. These proteins were mapped to inflammation, complement activation and neutrophil activation (A). Similarly, oxidative stress response was among the altered pathways implicated in NEC either by activation and/or inhibition (B). Highlighted proteins indicate differential expression validation by Western blot.
Western Blot Analysis
To confirm our findings from first part of study, Western blot were preformed for the same 3 target proteins on time point samples of an additional 10 NEC and 10 matched controls. IL-1RA showed a trend to be lower in NEC infants compared to controls within 2 to 3 weeks prior to the onset of NEC, P=0.08 (Figure 4A). However, no differences were seen in samples 1 week prior to the onset of NEC (data not shown). Alpha-1antitrypsin also showed a trend to be lower in the NEC infants compared to controls 1 week, (P=0.06, Figure 4B) but not 2 to 3 weeks prior to the onset of the disease. Peroxiredoxin-1 did not show promising results in 1week (Figure 4C, P=0.48) and 2 to 3 weeks (data not shown) prior to NEC.
Figure 4. Phase 2 validation study: Western blot.
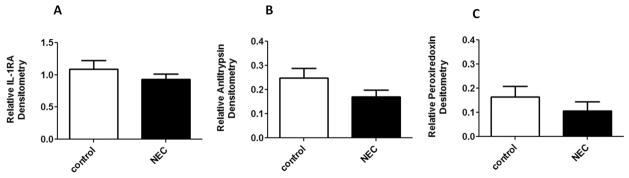
IL-1RA within 2 to 3 weeks before NEC (A) and Alpha-1 antitrypsin 1 week before NEC (B) were decreased in cases compared to control (P=0.08, n=10 and P=0.06, n=8; respectively). Peroxiredoxin 1 week before NEC (C) was not promising (P=0.48, n=6).
DISCUSSION
The ability to predict a high propensity for the development of NEC could be lifesaving in preterm infants. We collected samples in a non-invasive manner and subjected them to proteomics analysis. There were several proteins that differed prior to the onset of NEC could potentially serve as predictive biomarkers. By using a systems biology approach, we evaluated molecular and biologic functions and cellular localization of identified proteins, and then created a map identifying the networks and pathways of proteins of interest. Finally, to evaluate the validity of our approach and confirm proteomic results, we did Western blots on 3 proteins that were our best targets as putative biomarkers of NEC, inculding peroxiredoxin 1, IL-1RA, and alpha-1-antitrypsin. Peroxiredoxin is an antioxidant that reduces hydroxyperoxides and peroxynitrites [22]. Alpha -1-antitrypsin is a protease inhibitor that protects tissues from enzymes of the inflammatory cells [23]. IL-1RA binds the cell surface IL-1 receptor, preventing IL-1 from sending a proinflammatory signal to that cell. IL-1β is especially germane to the pathophysiology of NEC because it causes an increase in intestinal epithelia tight junction permeability [24], which is thought to be an antecedent to NEC. Furthermore, IL-1β has been found associated with NEC lesions in a piglet model [25]. IL-1RA was decreased prior to the onset of NEC in NEC group when compared to controls in our preliminary study of pooled samples and it showed a trend to be lower in the NECs compared to controls in the validation part of our study within 2 to 3 weeks prior to the development of NEC, suggesting that low levels of this particular protein may play an active role in NEC and may serve as a candidate biomarker for additional validation for the prediction of infants at risk for NEC. However, peroxiredoxin 1 and alpha- 1- antitrypsin did not show significant changes between NEC and controls.
It has been suggested that the development of non-invasive biomarkers to help predict and prevent disease in neonates represents a significant need [6]. Using a proteomic approach, a recent study suggested the novel ApoSAA score to help differentiate and diagnose NEC and sepsis [13]. But the study was limited to diagnosis at the time of NEC or sepsis presentation and did not distinguish NEC from sepsis. In our study, 5 out of 10 NEC patients also had positive blood cultures but the diagnosis of sepsis was either made several days later after the diagnosis of NEC or from blood cultures drawn at the moment of the diagnosis of NEC; 80% had negative blood cultures before NEC. In another study [11], a non-invasive approach used urine samples from infants at risk for the development of NEC. This study identified urinary I-FABP, claudin-3, and calprotectin as potential diagnostic markers, but not as predictive biomarkers of NEC. In our current study, we tested a non-invasive method of swabbing the buccal epithelium for proteomic analysis. Obtaining biologic material by swabbing the buccal epithelium in at-risk neonates provides a novel way to investigate the complex physiology likely occurring prior to the onset of NEC. Being non-invasive, this collection method should allow investigators to evaluate the intestine (since the buccal epithelium and intestinal epithelium are from the similar embryologic origins).
Using a proteomic approach, we characterized several biomarker candidates from buccal swabs of very low birthweight neonates. We utilized 2-DIGE results to map global interactions that identified a number of NEC-relevant pathways (inflammation, colorectal cancer, oxidative stress, and chemotaxis etc). Two major pathways (inflammation, and oxidative stress) involved in NEC pathogenesis. A sub-network enriched analysis showed that there are 13 proteins involved in the inflammatory process (Fig3A) which was mapped to inflammation, complement activation and neutrophil accumulation. Similarly, 13 proteins were shown to be regulating the dynamics of oxidative stress response either by activation and/or inhibition which has a major implication on cell death process (Fig 3B).
In conclusion, using a non-invasive method and a proteomic approach, we identified several proteins that are altered prior to the onset of NEC in susceptible neonates. Thorough analysis of these proteins revealed their molecular functions, biologic functions, and we were able to map the pathways in which these proteins are involved. IL1-RA, according to these preliminary studies, appears to be a promising candidate for additional studies. Currently, we are enrolling patients into a larger study that will provide us with additional samples for validation of these biomarkers.
Acknowledgments
This work was partially supported by National Institutes of Health grants RO1 HD059143 and 1UL1TR000064.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RC, Stol lBJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin PW, Stoll BJ. Necrotising enterocolitis. The Lancet. 2006;368:1271–83. [Google Scholar]
- 4.Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–6. doi: 10.1016/j.jpedsurg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Martin CR, Dammann O, Allred E, Patel S, O’Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr. 2010;157:751–6. e1. doi: 10.1016/j.jpeds.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res. 2009;65:91R–7R. doi: 10.1203/PDR.0b013e31819dba7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–8. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 10.Claud ECLL, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci U S A. 2004;101:7404–8. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–80. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94:267–71. doi: 10.1159/000151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng PC, Ang IL, Chiu RW, Li K, Lam HS, Wong RP, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest. 2010;120:2989–3000. doi: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157:757–61. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low JM, Chauhan AK, Gibson DS, Zhu M, Chen S, Rooney ME, et al. Proteomic analysis of circulating immune complexes in juvenile idiopathic arthritis reveals disease-associated proteins. Proteomics Clin Appl. 2009;3:829–40. doi: 10.1002/prca.200800073. [DOI] [PubMed] [Google Scholar]
- 16.Kobeissy FH, Warren MW, Ottens AK, Sadasivan S, Zhang Z, Gold MS, et al. Psychoproteomic analysis of rat cortex following acute methamphetamine exposure. J Proteome Res. 2008;7:1971–83. doi: 10.1021/pr800029h. [DOI] [PubMed] [Google Scholar]
- 17.Jiang P, Sangild PT, Siggers RH, Sit WH, Lee CL, Wan JM. Bacterial colonization affects the intestinal proteome of preterm pigs susceptible to necrotizing enterocolitis. Neonatology. 2011;99:280–8. doi: 10.1159/000317807. [DOI] [PubMed] [Google Scholar]
- 18.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis:Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–6. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 20.Mi H, Guo N, Kejariwal A, Thomas PD. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic acids research. 2007;35:D247–52. doi: 10.1093/nar/gkl869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–4. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–52. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Crystal RG. The alpha 1-antitrypsin gene and its deficiency states. Trends Genet. 1089;5:411–7. doi: 10.1016/0168-9525(89)90200-x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–61. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Haver ER, Sangild PT, Oste M, Siggers JL, Weyn sAL, Van Ginneken CJ. Diet-dependent mucosal colonization and interleukin-1beta responses in preterm pigs susceptible to necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2009;49:90–8. doi: 10.1097/MPG.0b013e31818de393. [DOI] [PubMed] [Google Scholar]