Abstract
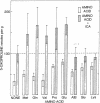
5-Oxoprolinase catalyzes the conversion of 5-oxo-L-proline (L-pyroglutamate, L-2-pyrrolidone-5-carboxylate) to L-glutamate with concomitant stoichiometric cleavage of ATP to ADP and inorganic orthophosphate. In this reaction, a step in the γ-glutamyl cycle, 5-oxoproline (formed by the action of γ-glutamylcyclotransferase on γ-glutamyl amino acids, which are in turn formed by transpeptidation of amino acids with glutathione), is made available for glutathione synthesis. When mice are injected with L-2-imidazolidone-4-carboxylate, a competitive inhibitor of 5-oxoprolinase, they accumulate 5-oxoproline in their tissues (kidney, liver, brain, and eye) and excrete it in the urine. Mice given the inhibitor together with one of several L-amino acids accumulate and excrete much more 5-oxoproline than when they are given the inhibitor alone. Such augmentation of 5-oxoproline accumulation offers evidence for the function of the γ-glutamyl cycle in vivo and supports the view that 5-oxoproline is a quantitatively significant metabolite.
Keywords: pyroglutamate, pyrrolidone carboxylate, glutathione, amino-acid transport
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eldjarn L., Jellum E., Stokke O. Pyroglutamic aciduria: studies on the enzymic block and on the metabolic origin of pyroglutamic acid. Clin Chim Acta. 1972 Sep;40(2):461–476. doi: 10.1016/0009-8981(72)90359-2. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Larsson A., Zetterström R. Pyroglutamic aciduria. Studies in an infant with chronic metabolic acidosis. Acta Paediatr Scand. 1974 Jan;63(1):1–8. doi: 10.1111/j.1651-2227.1974.tb04342.x. [DOI] [PubMed] [Google Scholar]
- Jellum E., Kluge T., Börresen H. C., Stokke O., Eldjarn L. Pyroglutamic aciduria--a new inborn error of metabolism. Scand J Clin Lab Invest. 1970 Dec;26(4):327–335. doi: 10.3109/00365517009046241. [DOI] [PubMed] [Google Scholar]
- MEISTER A., BUKENBERGER M. W. Enzymatic conversion of D-glutamic acid to D-pyrrolidone carboxylic acid by mammalian tissues. Nature. 1962 May 12;194:557–559. doi: 10.1038/194557a0. [DOI] [PubMed] [Google Scholar]
- MEISTER A., BUKENBERGER M. W., STRASSBURGER M. THE OPTICALLY-SPECIFIC ENZYMATIC CYCLIZATION OF D-GLUTAMATE. Biochem Z. 1963;338:217–229. [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASCHER G. Die Wasserlöslichen Bestandteile der peripheren Hornschicht (Hautoberfläche); quantitative Analysen. III. Alpha-Pyrrolidoncarbonsäure. Arch Klin Exp Dermatol. 1956;203(3):234–238. [PubMed] [Google Scholar]
- POLGAR P., MEISTER A. A CONVENIENT PROCEDURE FOR THE DETERMINATION OF PYRROLIDONECARBOXYLIC ACID AND RELATED COMPOUNDS. Anal Biochem. 1965 Aug;12:338–343. doi: 10.1016/0003-2697(65)90100-4. [DOI] [PubMed] [Google Scholar]
- Ross L. L., Barber L., Tate S. S., Meister A. Enzymes of the gamma-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2211–2214. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömme J. H., Eldjarn L. The metabolism of L-pyroglutamic acid in fibroblasts from a patient with pyroglutamic aciduria: the demonstration of an L-pyroglutamate hydrolase system. Scand J Clin Lab Invest. 1972 May;29(3):335–342. doi: 10.3109/00365517209080249. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Ross L. L., Meister A. The -glutamyl cycle in the choroid plexus: its possible function in amino acid transport. Proc Natl Acad Sci U S A. 1973 May;70(5):1447–1449. doi: 10.1073/pnas.70.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Stephani R. A., Orlowski M., Meister A. Inhibition of 5-oxoprolinase by 2-imidazolidone-4-carboxylic acid. Proc Natl Acad Sci U S A. 1973 Mar;70(3):759–761. doi: 10.1073/pnas.70.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. The occurrence of free L-pyrrolidone carboxylic acid in body fluids and tissues. FEBS Lett. 1973 Jul 1;33(2):157–160. doi: 10.1016/0014-5793(73)80182-6. [DOI] [PubMed] [Google Scholar]