Abstract
Caveolin-1 (Cav-1) is a major structural protein of caveolae, specialized plasma membrane invaginations that are involved in a cell-specific fashion in diverse cell activities such as molecular transport, cell adhesion, and signal transduction. In normal adult mammals, Cav-1 expression is abundant in mesenchyme-derived cells but relatively low in epithelial parenchyma. However, epithelial Cav-1 overexpression is associated with development and/or progression of many carcinomas. In this study, we generated and characterized a transgenic mouse model of Cav-1 overexpression under the control of a mouse mammary tumor virus (MMTV) long terminal repeat promoter, which is predominantly expressed in specific epithelial cells. The MMTVcav-1+ transgenic mice were fertile, and females bore litters of normal-size with no obvious developmental abnormalities. However, by age 11 months, the MMTVcav-1+ mice demonstrated overtly different phenotypes in multiple exocrine organs when compared with their nontransgenic MMTVcav-1− littermates. Cav-1 overexpression in MMTVcav-1+ mice produced organ-specific abnormalities, including hypotrophy of mammary glandular epithelia, bronchiolar epithelial hyperplasia and atypia, mucous-cell hyperplasia in salivary glands, elongated hair follicles and dermal thickening in the skin, and reduced accumulation of enzymogen granules in pancreatic acinar cells. In addition, the MMTVcav-1+ transgenic mice tended to have a greater incidence of malignant tumors, including lung and liver carcinomas and lymphoma, than their MMTVcav-1− littermates. Our results indicate that Cav-1 overexpression causes organ-specific, age-related epithelial disorders and suggest the potential for increased susceptibility to carcinogenesis.
Keywords: MMTV-promoter, Cav-1 overexpression, parenchymal epithelia, exocrine organs
Introduction
Caveolin-1 (Cav-1) is a 22-kD protein that functions as a principal structural component of caveolae in most mammalian cells. Cav-1 is involved in a cell- and context-specific fashion in diverse cell activities, such as molecular transport; cell adhesion; vesicular trafficking; cellular cholesterol, fatty acid and triglyceride homeostasis and signal transduction (Razani and Lisanti, 2001; Shaul and Anderson, 1998). In normal adult mammals, Cav-1 is highly expressed in mesenchyme-derived cells including smooth muscle cells, vascular endothelial cells, adipocytes, mammary gland myoepithelial cells, and myofibroblasts in the prostatic (Razani and Lisanti, 2001; Yang et al., 2008).
Early experiments that focused on the role of Cav-1 in growth control showed that Cav-1 levels are reduced in oncogene-transformed NIH-3T3 cells (Koleske et al., 1995), that targeted down-regulation of Cav-1 induces transformation of NIH-3T3 cells (Galbiati et al., 1998), and that enforced expression of Cav-1 suppresses the growth of fibroblasts and specific human breast cancer cell lines with myoepithelial cell features in vitro (Lee et al., 1998). To analyze the in vivo activities of Cav-1, multiple laboratories generated Cav-1 gene homozygous knockout mice (Cao et al., 2003; Drab et al., 2001; Razani et al., 2001; Razani et al., 2002). In general, Cav-1−/− mice are viable and fertile but have a shorter life span than Cav-1+/+ mice have (Cao et al., 2003; Yang et al., 2008) . These mice develop vascular dysfunction and thickened alveolar septa due to proliferation of endothelial cells and fibrosis (Cao et al., 2003; Drab et al., 2001; Razani et al., 2001). Male Cav-1−/− mice also developed hypercalciuria and urinary bladder stones (Cao et al., 2003). In the mammary gland, Cav-1−/− mice may develop epithelial hyperplasic lesions, potentially as a consequence of stromal cell abnormalities (Razani et al., 2001; Yang et al., 2008). Although the absence of Cav-1 has not been reported to increase the incidence of spontaneous malignancies, more hyperplastic lesions and tumors were observed in the skin of Cav-1−/− mice than in that of wild-type mice after application of dimethylbenzanthracene (Capozza et al., 2003). Further studies showed that loss of Cav-1 gene expression can accelerate the development of hyperplastic and dysplastic mammary lesions and enhance tumorigenesis and metastasis in cancer-prone genetically engineered mice (Williams et al., 2003; Williams et al., 2004). These and other study results showing down-regulation of Cav-1 in human malignancies led to the notion that Cav-1 is a tumor-suppressor gene (Williams and Lisanti, 2005).
In contrast to studies that reported potential tumor-suppressor activities of Cav-1 , a recent study showed that Cav-1−/−;TRAMP (transgenic mouse prostate) mice demonstrate significantly fewer primary tumors and lesions than Cav-1+/+;TRAMP mice (Williams et al., 2005). An additional study reported that transgenic mice with targeted overexpression of Cav-1 in prostatic epithelial cells using the short probasin (PB) promoter (i.e., PBcav-1 mice) resulted in prostatic hyperplasia and atypia associated with pro-tumorigenic alterations in the local and metastatic tumor microenvironments (Watanabe et al., 2009). Further, Cav-1 secreted by prostatic epithelial cells in PBcav-1 mice created a local microenvironment that permitted tumor growth and increased serum Cav-1, which was associated with increased experimental prostate cancer lung metastatic activities. These results are consistent with those of numerous studies that have documented overexpression of Cav-1 in prostate cancer and other malignant tissues (Shatz and Liscovitch, 2008; Thompson et al., 2009; Williams and Lisanti, 2005). Thus, the role of Cav-1 in tumorigenesis is complex and depends on the cell type and biological context. Our purposes in conducting this study were to generate a transgenic mouse model that overexpresses Cav-1 under the regulation of the MMTV long terminal–repeat promoter (MMTV-LTR), which is expressed in specific epithelial cells (Choi et al., 1987), and to characterize the pathologic effects of constitutive Cav-1 overexpression in such cells. Our histopathologic phenotyping demonstrated that MMTVcav-1+ transgenic mice had defective parenchymal epithelia in multiple exocrine organs and appeared to be more susceptible than their MMTVcav-1− littermates to the development of malignant tumors. These findings provided evidence of direct involvement of Cav-1 in the regulation of normal epithelial growth, differentiation, and function.
Materials and methods
MMTVcav-1 mice
The mouse Cav-1 (mcav-1) cDNA open reading frame was amplified by using PCR with the following oligonucleotides: a sense primer, 5′ GGGAAACCTCCTCAGAGCCT 3′, and an antisense primer, 5′ GATCAAGACAAACCATTCAT 3′. The mcav-1 fragment was then inserted into a TA-cloning vector. The restriction endonuclease EcoRI (New England Biolabs, Inc., Ipswich, MA, USA) was used to cut out the full-length mcav-1 cDNA from the TA-cloning vector, and it was then inserted into the MMTV-KbpA vector between the rabbit β-globin splice and the bovine growth hormone polyadenylation sequences (Fig. 1A). Expression of mCav-1 was driven by a 2.4-kb fragment of the MMTV LTR. The construct was linearized by application of NotI (New England Biolabs) and Asp718 (Roche). The fragment containing the MMTV promoter mcav-1 cDNA expression cassette was purified from low melting gel, and transgene DNA was then injected into fertilized oocytes from FVB mice.
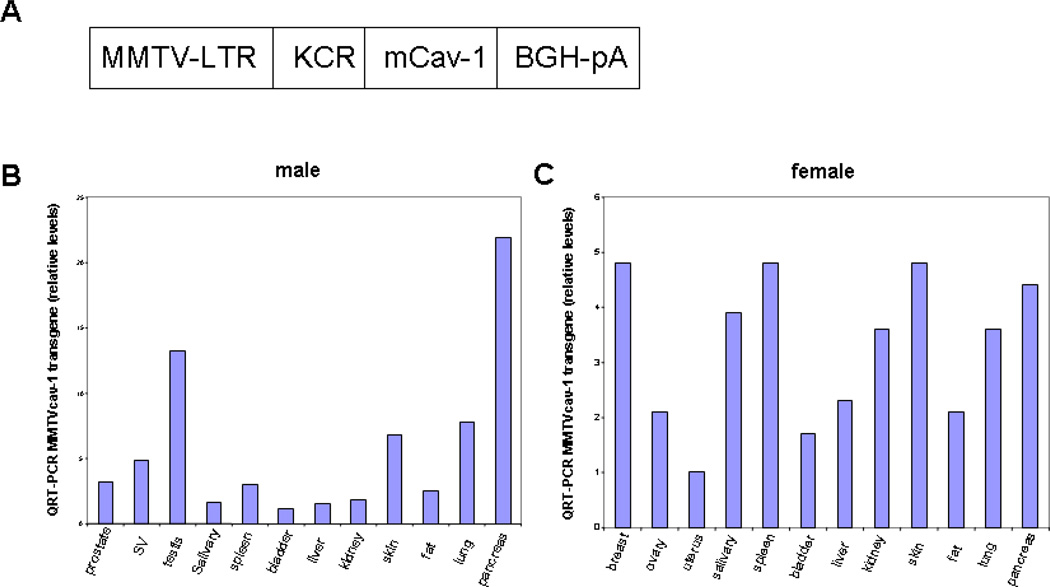
Fig. 1.
Caveolin-1 (Cav-1) expression in MMTVcav-1+ transgenic mice. (A) Schematic diagram of the MMTVcav-1+ transgene construct. (B, C) Expression of MMTVcav-1 transgene in various organs of 11-month-old male (B) and female (C).
To identify transgenic founder animals among the offspring, we screened DNA obtained from ear-punch tissue specimens by using PCR to amplify a 983-bp fragment with upstream 5′ GGATCCTGAGAACTTCAG 3′ and downstream 5′ ATCGTAGACAACAAGCGGTA 3′ primers specific for the transgene but not endogenous Cav-1. This resulted in confirmation of the transgenic expression of four independent transgenic lines. However, only one line, #5374, with relatively high transgene expression was chosen and propagated for the study in this report. 11–13 months old progeny of founder #5374 MMTVcav-1+ mice and its wild-type littermates were used for histologic analysis or RNA extraction.
Mice were maintained under specific pathogen–free conditions in facilities accredited by the American Association of Accreditation of Laboratory Animal Care. All animal experimental procedures were conducted in accordance with the principles and procedures outlined in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Quantitative real-time RT-PCR
Total RNA was isolated from organs of MMTVcav-1+ and MMTVcav-1− mice by using a RiboPure RNA extraction kit (Ambion). RT reactions were carried out with a high-capacity cDNA archive kit (Applied Biosystem) according to the manufacturer’s protocol. PCR was performed as previously described (Ren et al., 2004; Ren et al., 2006) using the following Taqman probes and primers (Applied Biosystems): part #4352341E for mouse β-actin and transgene specific Cav-1 probe and primers: forward primer, 5′ TGGTTGTTGTGCTGTCTCATCA 3’; reverse primer, 5′ TGCAGGCTCTGAGGAGGTTT 3’; the probe, 5′ TTTGGCAAAGAATTC 3’. Real-time PCR was performed with a Step-One real-time PCR system (Applied Biosystems) according to the manufacturer’s instructions. The relative quantity of Cav-1 mRNA was determined by the ΔΔCT method as described by the manufacturer and normalized to β-actin RNA in the same cDNA preparation.
Protein extraction and western blot analysis
Formalin fixed, paraffin embedded tissue sections were used to extract protein with Qproteome FFPE tissue kit according to manufacturer protocol (QIAGEN GmbH, Germany). The protein concentration was measured using Micro BCA Protein assay kit (Thermo Scientific, USA). The proteins were separated using standard SDS-PAGE procedures. Western blot analysis of Cav-1 protein expression in the tissues of interest was performed using 1:100 dilution of Cav-1 antibody (# sc-894, Santa Cruz, Biotechnology). The levels of β-actin expression were used as a loading control.
Histopathologic and immunohistochemical analyses
At necropsy a careful observation was made for gross changes, then selected organs were removed by dissection and weighed. Tissue samples were fresh frozen in OCT (Optimal Cutting Temperature compound; Tissue-Tek, Sakura Finetek) or fixed in 10% buffered formalin and embedded in paraffin for sectioning. Sections (4–5 µm) were stained with hematoxylin and eosin (H&E) according to standard protocols and evaluated histologically.
Immunohistochemical analysis using standard ABC detection was done essentially as previously described (Yang et al., 1999). Antibodies used included rabbit polyclonal anti–caveolin-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and proliferative cell nuclear antigen (PCNA) (PC-10, Dako, Carpinteria, CA, USA). Some sections which were incubated in normal rabbit or mouse serum, replacing the primary antibodies, were used as controls. The TUNEL technique as previously described was used to label apoptotic cells (Yang et al., 1997).
Morphologic parameters of various organs were assessed and immunohistochemical quantitation of PCNA-positive and apoptotic bodies were conducted on randomly selected fields (measuring 0.198 µm2 each) for each specimen according to morphometric criteria using the Eclipse 90i automated image analysis system (Nikon Instruments, Inc., Melville, NY, USA) with NIS-Elements software (version AR 3.0; Nikon).
Statistical analyses
All values are expressed as means ± SEM. Statistical analyses for body weight and organ wet weights were carried out using the two-tailed Student’s unpaired t test. Differences were considered statistically significant when P < 0.05. Comparisons in tumor incidence rates were performed using Chi-square test. All analyses were performed using Statview 5.0 software (SAS Institute, Cary, NC, USA).
Results
Generation of MMTVcav-1+ transgenic mice
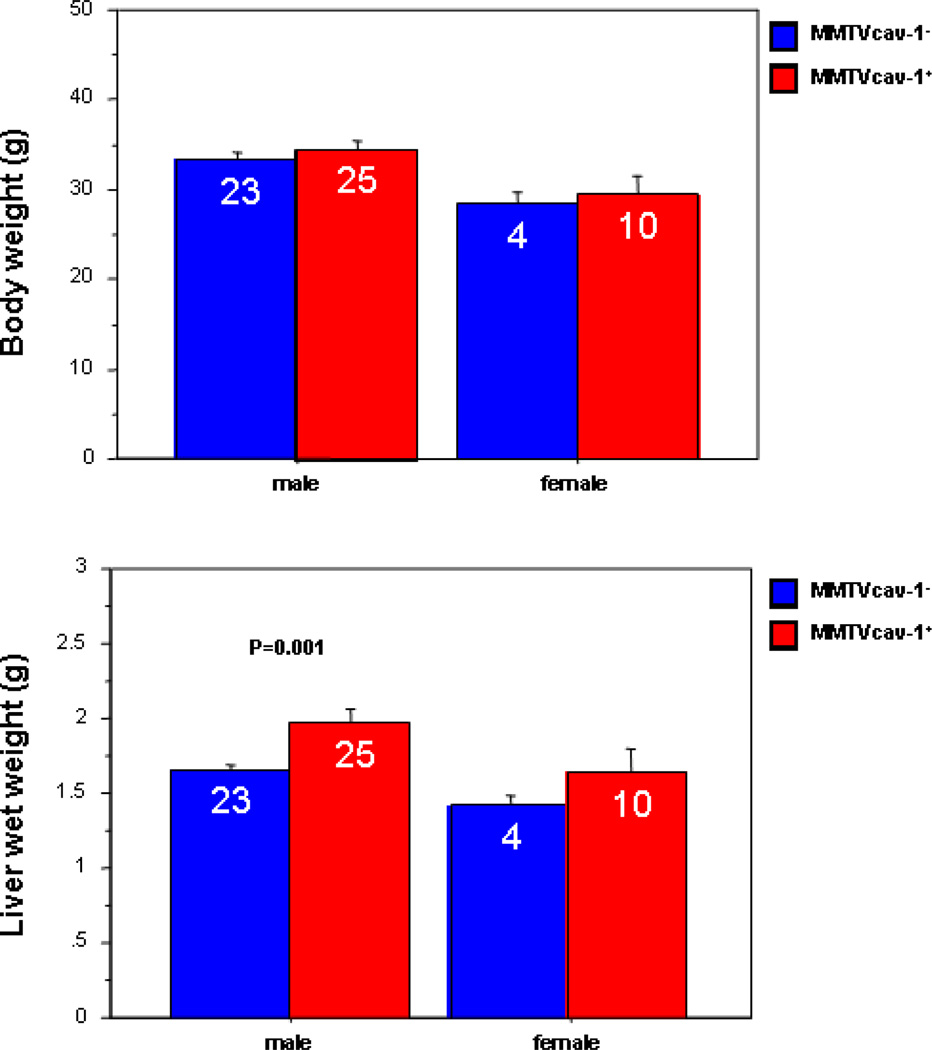
A construct encoding mcav-1 cDNA under the control of the MMTV-LTR (Fig. 1A) was microinjected into fertilized oocytes from FVB mice. The confirmation of the transgenic expression resulted in four founder lines. RNA from various organs was isolated from each of the four lines and was subjected to the quantitative RT-PCR analysis with the Cav-1 transgene–specific primers. Among the 4 transgenic founder lines of mice (#5362, #5374, #5381, #5383), only one line, #5374, robustly expressed the Cav-1 transgene in all the organs analyzed and thus was chosen for further analyses (Figs. 1B, C). From the #5374 founder, a cohort of transgenic mice (MMTVcav-1+) and their nontransgenic Cav-1 wild-type (MMTVcav-1−) littermates were generated. The transgenic MMTVcav-1+ mice appeared normal, and their growth pattern and body weight were indistinguishable from those of their nontransgenic littermates. Reproductive activities, including the number of pups per litter and lactation, appeared normal. An interesting observation was that the average wet weights of the liver in male MMTVcav-1+ mice were statistically significantly higher than those of their nontransgenic counterparts (P = 0.001, Student’s t test) although their average body weights were similar at adult age (Fig. 2).
Fig. 2.
Body weight and liver wet weight comparisons of the MMTVcav-1+ transgenic mice and their nontransgenic littermates. Values within the bars indicate the numbers of animals examined. Error bars represent the standard error.
Cav-1 overexpression leads to benign histopathologic phenotypes in transgenic MMTVcav-1+ mice
Quantitative real-time RT-PCR (qRT-PCR) analysis of multiple organs demonstrated that MMTVcav-1 transgene expression in testis and pancreas was highest when it was normalized to transgene levels in the bladder, which were the lowest levels found in the panel of organs analyzed in male mouse (Fig. 1B). In female mouse, the mammary gland, spleen, skin, pancreas, and salivary gland expressed relatively high MMTVcav-1 transgene levels when they were normalized to those in the uterus, which demonstrated the lowest transgene levels among the organs analyzed (Fig. 1C). Histopathologic analyses revealed defective morphologic features in multiple organs, including the mammary glands, salivary glands, pancreas, skin, and lungs of the MMTVcav-1+ mice.
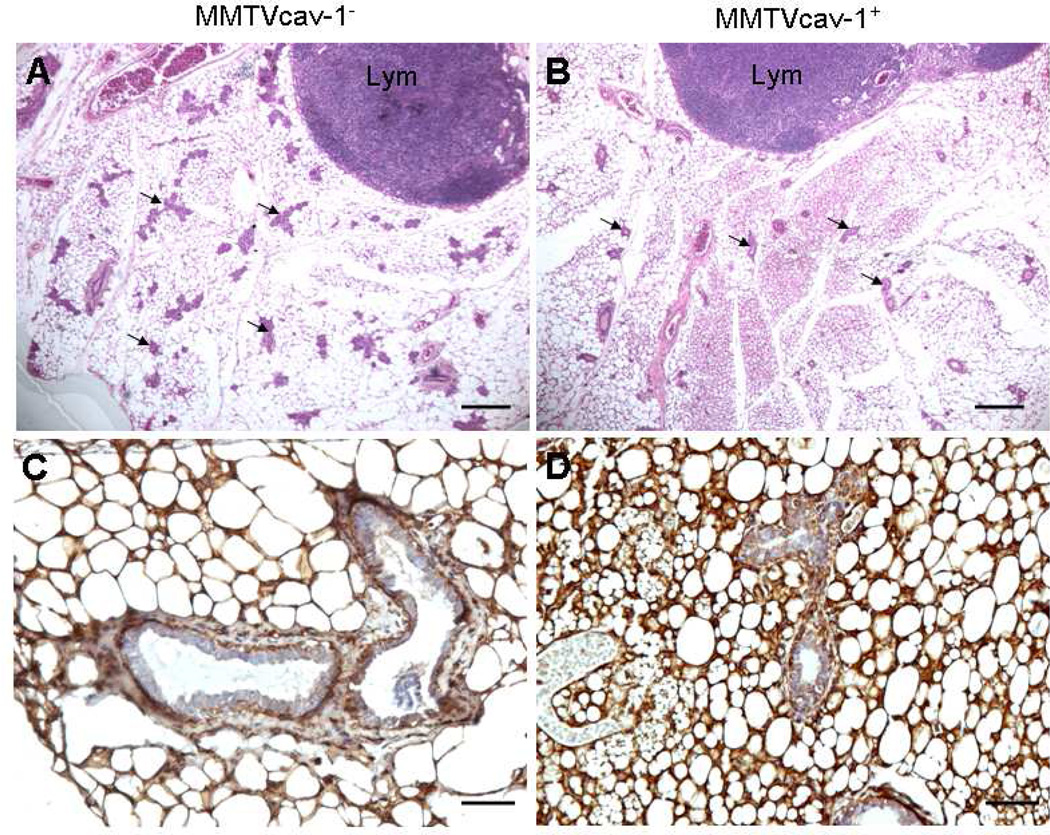
In the mammary glands of 11-month-old virgin female transgenic mice, epithelial ductal tree and ductal side-branching activities and the number of glandular epithelial components were significantly reduced relative to those in the nontransgenic littermates (Figs. 3A, B). On immunostaining, Cav-1 was present predominantly in the adipocytes and myoepithelial cells of the ductal and glandular epithelia in the mammary glands of both transgenic and nontransgenic mice (Figs. 3C, D). However, the Cav-1 immunoreactivity in the cytoplasm of ductal and glandular epithelial cells was greater in the MMTVcav-1+ mice than in the MMTVcav-1− mice. Cellular proliferation and apoptotic activities in the ductal and glandular mammary epithelia were similar in the MMTVcav-1+ and MMTVcav-1− mice, as analyzed by PCNA immunostaining and TUNEL staining, respectively (data not shown).
Fig. 3.
Mammary glands in 11-month-old virgin female MMTVcav-1− (A, C) and MMTVcav-1+ (B, D) mice on H&E-stained (A, B) and Cav-1 immunostained (C, D) sections. The numbers of the ductal and/or glandular components (some of them indicated by arrows) were considerably lower in the transgenic mice (B) than in their nontransgenic littermate controls (A). Lym: lymphoid nodule. Scale bars: 500 µm (A, B), 100 µm (C, D).
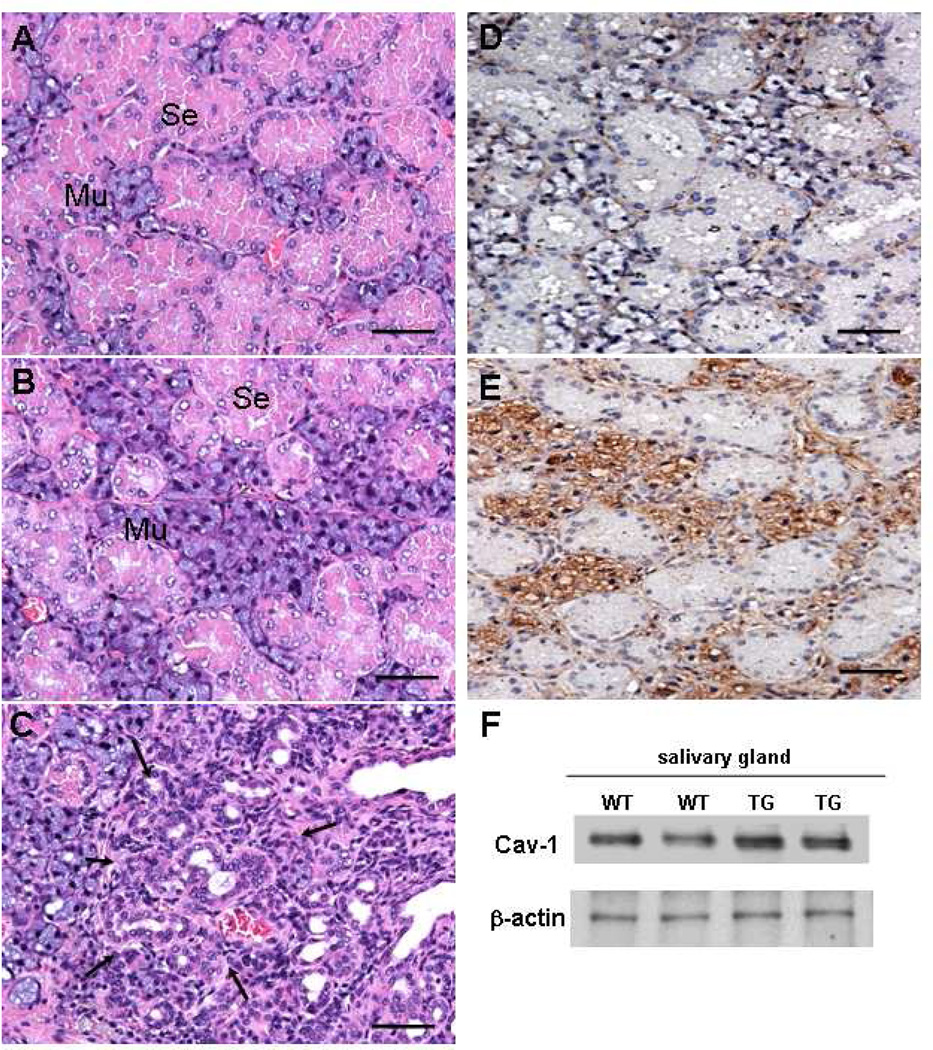
Salivary glands from MMTVcav-1+ mice also exhibited more morphologic abnormalities than did those from MMTVcav-1− mice. In the submandibular glands of MMTVcav-1+ mice, mucous epithelial cells demonstrated hyperplasic or hypertrophic changes. As a result, the mucous acini appeared proportionally larger in the MMTVcav-1+ than in the MMTVcav-1− mice (Figs. 4B and A, respectively). An interesting observation was that these abnormalities were more evident in female mice. Ductal epithelial hyperplasia and/or atypia in the submandibular glands was apparent in 4 of the 11 (36%) MMTVcav-1+ females analyzed but in none of the 10 males analyzed (Fig. 4C). In addition, the mucous epithelial cells in MMTVcav-1+ mice exhibited substantially greater Cav-1 immunostaining than did those in MMTVcav-1− mice, in which Cav-1 immunostaining was mainly localized in the stromal cells (Figs. 4D, E). In the sublingual glands, the morphologic features appeared similar in the MMTVcav-1+ and MMTVcav-1− mice (data not shown). Total Cav-1 protein levels in the salivary glands of the transgenic mice were higher than those in their nontransgenic littermates, as shown by western blotting (Fig. 4F).
Fig. 4.
H&E-stained sections from the submandibular salivary glands of 12-month-old female MMTVcav-1− (A) and MMTVcav-1+ mice (B, C). Cav-1 immunostaining in MMTVcav-1− (D) and MMTVcav-1+ (E). Cav-1 western blotting of salivary glands from MMTVcav-1+ and MMTVcav-1− mice (F). Each lane represents individual mouse. Scale bars: 100 µm. Se :serous ,Mu :mucous glandular cells. Arrows in C outline the area with ductal hyperplasia and/or atypia.
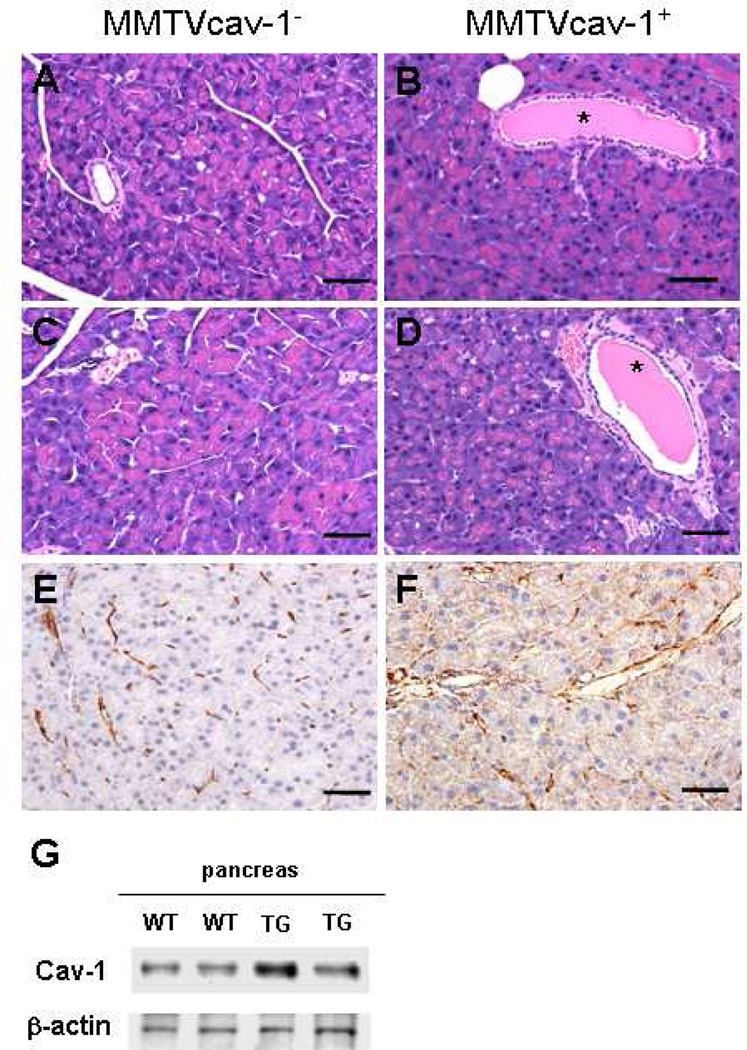
In the exocrine pancreas of MMTVcav-1+ mice, the acinar cells had less accumulation of eosinophilic zymogen granules in their apical cytoplasm and greater basophilic basal cytoplasm than their MMTVcav-1− littermates had (Figs. 5A–D). Moreover, the pancreatic ductules in MMTVcav-1+ mice were dilated and filled with an eosinophilic secretion. Further, punctuate Cav-1 immunostaining was apparent in the cytoplasm of the acinar cells and in the epithelia of pancreatic ductules of MMTVcav-1+ mice (Fig. 5F). But in MMTVcav-1− mice Cav-1 immunostaining was mainly localized to stromal and endothelial cells (Fig. 5E). These phenotypic differences were observed in both sexes (Fig. 5). Additionally, the size and number of the endocrine islets labeled by cav-1 immunostaining of the pancreatic tissues were similar in MMTVcav-1+ and MMTVcav-1− mice (data not shown). Finally, immunoblotting analysis showed that the total Cav-1 protein levels in pancreatic tissues of MMTVcav-1+ transgenic mice were higher than those in their nontransgenic littermates (Fig. 5G).
Fig. 5.
Histologic features of the pancreas of 12-month-old MMTVcav-1− (A, C, E) and MMTVcav-1+ (B, D, F): male (A, B) and female (C, D) mice on H&E-stained sections. The MMTVcav-1+ exocrine pancreas comprised acini with relatively decreased accumulation of eosinophilic zymogen granules in the apical cytoplasm of acinar cells. Greater Cav-1 immunostaining was found in the cytoplasm of pancreatic acinar cells of MMTVcav-1+ mice (F) than in that of the MMTVcav-1− mice (E), in which Cav-1 immunostaining was mainly localized in the stromal and endothelial cells. Scale bars: 100 µm. (G). Cav-1 western blot of pancreatic tissues from MMTVcav-1+ and MMTVcav-1− mice. Each lane represents individual mouse. * denote dilated intralobular ducts full of secretion.
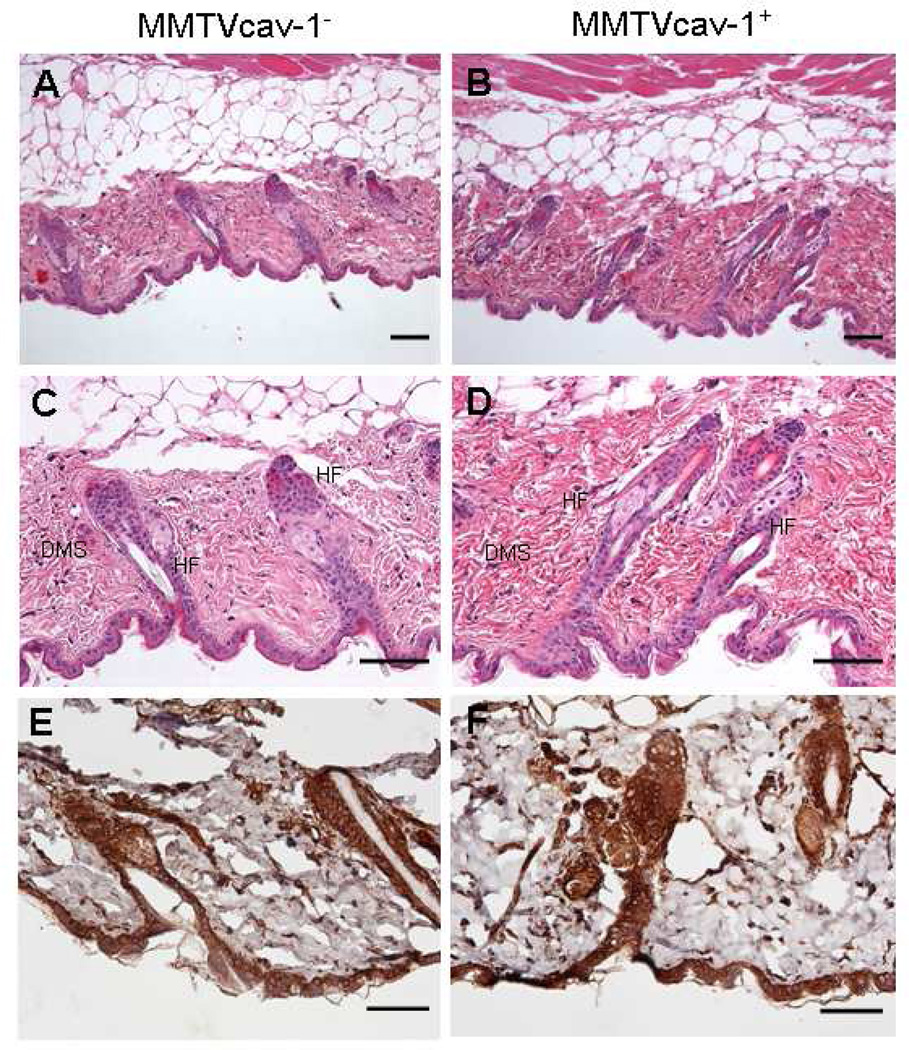
The general structural organization of skin tissues in the MMTVcav-1+ and MMTVcav-1− mice appeared similar, and there were no obvious differences in the epidermis and sebaceous glands. However, when hematoxylin and eosin stained skin sections obtained from the same anatomical area and cut transversely at a similar plane were analyzed, length of the hair follicles in the telogen phase, and thickness of the dermis in the MMTVcav-1+ mice were greater than those in MMTVcav-1− mice (Figs. 6A–D). These differences between MMTVcav-1+ and MMTVcav-1− mice appeared to be more prominent in male than in female mice. There were high levels of Cav-1 immunostaining in the epidermis and hair follicles, but there were no obvious differences in Cav-1 immunostaining patterns between the MMTVcav-1+ and MMTVcav-1− mouse skin tissues (Figs. 6E, F).
Fig. 6.
H&E-stained skin from MMTVcav-1− (A and C) or MMTVcav-1+ (B and D) mice. The skin samples with hair follicles at the telogen phase were obtained from the same anatomic areas and cut transversely at a similar body plane for morphological comparisons. MMTVcav-1+ mouse skin (B, D) had longer hair follicles (HF) and a thicker dermis (Dms) than those in MMTVcav-1− mouse skin (A, C). The epithelia of the epidermis and hair follicles and the fibroblasts and smooth muscle cells of the dermis of both MMTVcav-1− (E) and MMTVcav-1+ (F) mice were strongly labeled by Cav-1 antibody. Scale bars: 100 µm.
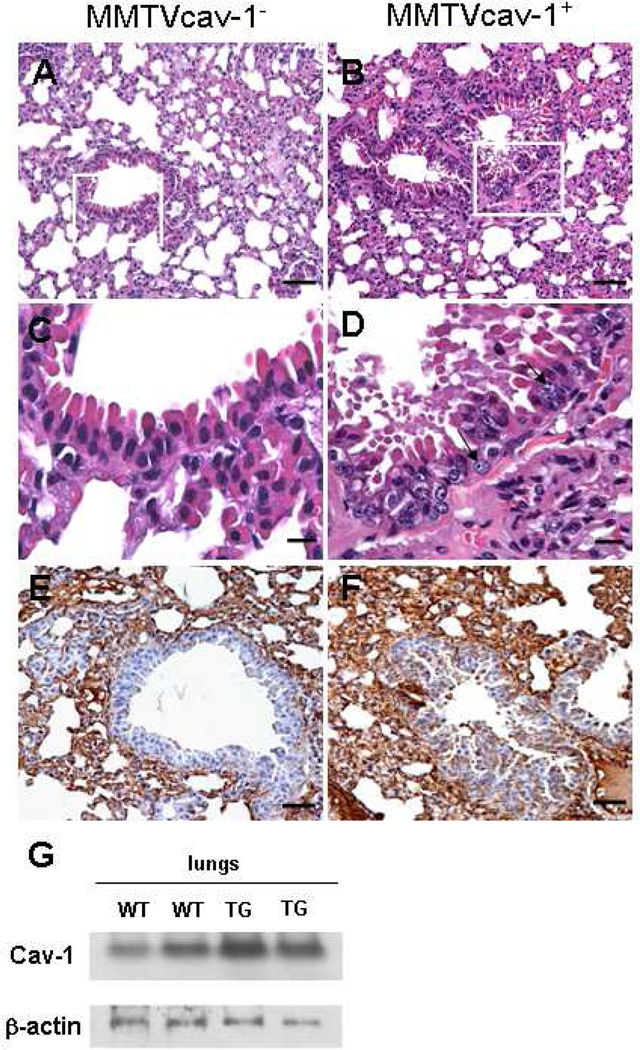
Finally, in the lung tissues, atypia and hyperplastic epithelial lesions were observed in the intrapulmonary bronchi and bronchioles in 7 of 14 (50%) female and 2 of 13 (15%) of the MMTVcav-1+ male mice, whereas such lesions were seen in only 1 of 18 (6%) of the MMTVcav-1− mice of both sexes. The pulmonary epithelial atypia and/or hyperplasia in MMTVcav-1+ mice were characterized by increased infoldings and the presence of cells with nuclear irregularity, such as enlarged, pale nuclei with prominent nucleoli (Fig. 7). In lung tissues of both MMTVcav-1+ and MMTVcav-1− mice, Cav-1 was highly expressed in the interalveolar septa, as demonstrated by immunostaining; however, the bronchiolar epithelia appeared to have greater Cav-1 immunostaining in MMTVcav-1+ mice than that in the MMTVcav-1− mice (Figs. 7F and E, respectively). Moreover, the total Cav-1 protein levels in the lungs of the transgenic mice were higher than those in their nontransgenic littermates, as shown by western blotting (Fig. 7G).
Fig. 7.
Histologic features of the lung from 12-month-old male MMTVcav-1− (A) and MMTVcav-1+ mice (B). C and D are the magnified regions outlined by the frames in A and B, respectively. Arrows in D indicate atypical nuclei. Cav-1 immunostaining was predominantly localized to alveolar septa in both MMTVcav-1− (E) and MMTVcav-1+ mice (F). The bronchiolar epithelia of MMTVcav-1+ mice (F) had greater Cav-1 immunostaining than the MMTVcav-1− mice (E) had. Scale bars: 100 µm (A, B, E, F), 30 µm (C, D). (G). Cav-1 western blot of lung tissues from MMTVcav-1+ and MMTVcav-1− mice. Each lane represents individual mouse.
Incidence of malignancies in the MMTVcav-1+ transgenic mice
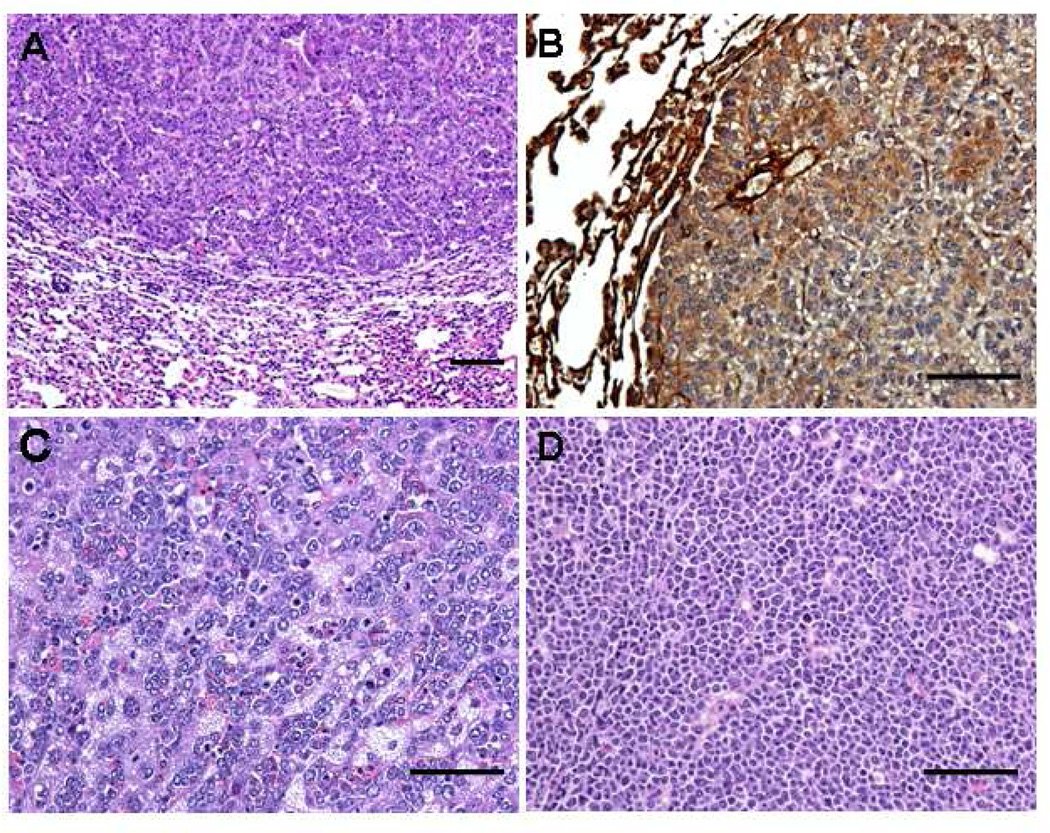
Cohorts of 47 MMTVcav-1+ mice and 29 MMTVcav-1− littermates, 11–13 months old, were evaluated for the occurrence of malignant tumors. Eight of the 47 (17%) MMTVcav-1+ mice developed malignancies (Table 1). The spectrum of tumors (Fig. 8) included 4 lung carcinomas, 3 lymphomas, and 1 liver carcinoma. In contrast, only 1 of the 29 (3%) MMTVcav-1− mice developed a malignancy (lymphoma). Thus, the incidence of malignancy in the transgenic mice was about 5 times higher than that in the MMTVcav-1+ mice (17% vs. 3%). This difference approached but did not achieve the level of statistical significance (P = 0.0752, X2 test). In 2 of the 4 lung carcinomas, Cav-1 immunostaining labeled the cancer cells (Fig. 8B).
Table 1.
Malignant tumors arising in transgenic micea
| MMTVcav-1− | MMTVcav-1+ | |||
|---|---|---|---|---|
| Type | n=29 | % | n=47 | % |
| Lung carcinoma | 0 | 4 | 8.5 | |
| Lymphoma | 1 | 3.4 | 3 | 7.9 |
| Liver carcinoma | 0 | 1 | 1.8 | |
| total | 1 | 3.4 | 8 | 17.0 |
MMTVcav-1+ mice and their MMTVcav-1− littermates aged 11–13 months were examined for malignancies. n denotes the number of animals. The percentage indicates tumor incidence rate.
Fig. 8.
Histologic features of malignant tumors that developed in MMTVcav-1+ mice. (A) Lung carcinoma. (B) Cav-1 immunostaining of lung carcinoma. (C) Liver carcinoma. (D) Lymphoma. Scale bars: 100 µm.
Discussion
In this study, we successfully generated a transgenic mouse model based on the Cav-1 overexpression driven by the MMTV-LTR promoter and used it to characterize the pathologic effects of constitutive Cav-1 overexpression on specific epithelial cells. Those effects were overt benign phenotypic changes: hypotrophy of the glandular epithelia of mammary glands, mucous cell hyperplasia in salivary glands, reduced accumulation of zymogen granules in pancreatic acinar cells, elongated hair follicles and thicker skin dermis, and epithelial atypia and hyperplasia of the pulmonary bronchioles. This broad range of lesions in multiple cell types and organs may reflect the diverse functions of Cav-1 (Liu et al., 2002).
Although Cav-1 is an integral membrane protein, it is also present in the secretory pathway of many exocrine cells (Liu et al., 1999). Our results showed that its overexpression induces disorders in specific exocrine cells (i.e. glandular epithelial cells of the mammary gland, mucous cells in the submandibular gland, and acinar cells in the exocrine pancreas). Secretion of Cav-1 from pancreatic acinar cells has been found in a complex with lipids (Thomas et al., 2004). Our observations are thus consistent with a role for Cav-1 in regulation of exocytosis (Liu et al., 1999).
Some of the phenotypic changes we observed, such as hypotrophy of the glandular epithelia of mammary glands, may be related to the aging process. The transgenic female mice appeared to conceive normally, deliver healthy, normal pups, and have normal lactation. However, 11-month-old virgin transgenic females had fewer epithelial cells in the mammary gland than the nontransgenic female mice had. This hypotrophic effect is in sharp contrast to but still somewhat consistent with the occurrence of hyperplastic mammary lesions observed in Cav-1−/− mice (Razani et al., 2001; Williams et al., 2003; Williams et al., 2005; Yang et al., 2008). The mechanism(s) that underlie mammary gland hypotrophy remain(s) to be elucidated. It has been reported that elevated Cav-1 expression may induce cell senescence in cultured mouse embryonic fibroblasts (Volonte et al., 2002). Additionally, degenerating neurons in Alzheimer’s diseases had higher Cav-1 levels than normal cells (Gaudreault et al., 2004), and nucleus pulposus cells from degenerated spinal disks in humans exhibited elevated Cav-1 levels and a positive correlation between Cav-1 expression and p16INK4a, a cell-senescence marker (Heathfield et al., 2008). Although Cav-1 overexpression has not been shown to contribute directly to the degenerative processes, it is possible that Cav-1 overexpression accelerates degeneration changes associated with the aging process in the transgenic mammary gland.
We also found differences between the sexes for some of the phenotypic alterations. Significantly higher liver wet weights and skin phenotypic changes were found predominantly in males; and the proportion of mucous acini in the submandibular glands, and atypia and hyperplastic epithelial lesions in the intrapulmonary bronchi and bronchioles were found preferentially in females. These sexual differences may reflect, in part, the effects of sex hormone activities that have been shown under some conditions to be related to Cav-1 expression (Li et al., 2003; Lu et al., 2001; Mercier et al., 2009). Alternatively, the differences may result from different levels of transgenic activation through the MMTV promoter, which is regulated by steroid sex hormones (Otten et al., 1988).
Our analysis also revealed relatively elongated hair follicles and thickened dermis in the transgenic mice. These results are intriguing in light of the finding that Cav-1 expression in the human skin is preferentially localized to the bulge region of hair follicles (Selleri et al., 2005), where the stem cells reside (Cotsarelis et al., 1990). It has been proposed that Cav-1 is involved in regulation of follicle growth (Selleri et al., 2005) and has been reported that increased Cav-1 expression in skin may lead to increased procollagen synthesis in the dermis (Kim et al., 2008). Thus, it is conceivable that Cav-1 overexpression contributes to longer hair follicles and thickened dermis but further experiments are needed to confirm a possible mechanistic role for Cav-1 in hair growth.
The 5-fold greater incidence rate of malignant tumors we observed in the transgenic mice was interesting, but the difference was not statistically significant. The roles of Cav-1 in cellular growth, cell transformation, and malignant progression are complex, however, and a substantial body of work now clearly indicates that it can have either growth-suppressive or oncogenic properties, depending on the cell type. In addition to experimental studies using in vitro and in vivo models that show cell type–specific growth-suppressive or oncogenic activities, numerous correlative studies using clinical tissue samples have corroborated this functional dichotomy
Some reports have documented down-regulation of Cav-1 in various malignant human tissues, including osteosarcoma (Cantiani et al., 2007), fibrosarcoma (Wiechen et al., 2001), colon cancer (Bender et al., 2000), follicular thyroid cancer (Aldred et al., 2003), ovarian cancer (Davidson et al., 2001; Wiechen et al., 2001), mucoepidermoid carcinoma of the salivary gland (Shi et al., 2007), lung adenocarcinoma (Kato et al., 2004; Wikman et al., 2004), and relatively small, estrogen receptor–positive breast cancer (Sagara et al., 2004). It is remarkable that many of these malignancies are of stromal cell origin. Overall, those findings concur with the notion that Cav-1 is a tumor-suppressor protein. A recent novel and somewhat surprising observation is relatively reduced concentrations of Cav-1 in human cancer–associated fibroblasts from breast cancers and prostate cancer (Di Vizio et al., 2009; Mercier et al., 2008).
In contrast to those correlative studies that show Cav-1 down-regulation in malignant cells, numerous other studies have documented Cav-1 overexpression in multiple cancers, including prostate cancer (Di Vizio et al., 2008; Goto et al., 2008; Karam et al., 2007; Satoh et al., 2003; Yang et al., 1998; Yang et al., 1999), esophageal squamous carcinoma (Hu et al., 2001; Kato et al., 2002), oral carcinoma (Hung et al., 2003), papillary carcinoma of the thyroid (Ito et al., 2002), pancreatic cancer (Suzuoki et al., 2002; Terris et al., 2002), renal carcinoma (Carrion et al., 2003; Horiguchi et al., 2004; Joo et al., 2004), bladder cancer (Rajjayabun et al., 2001; Sanchez-Carbayo et al., 2002), metastatic lung cancer (Ho et al., 2002), squamous carcinoma of the lung (Yoo et al., 2003), Ewing sarcoma (Tirado et al., 2006), and basal-like breast carcinoma (Elsheikh et al., 2008; Garcia et al., 2007). The results of these studies have been consistent with Cav-1–mediated oncogenic activities in human malignancies.
We find it notable that our results from this study demonstrated both hypotrophy of glandular epithelia of the mammary glands and hyperplasia of lung and salivary gland epithelial cells in MMTVcav-1 transgenic mice, findings that are consistent with both cell type– and context-dependent Cav-1 growth-related activities.
In summary, we generated a transgenic mouse model that constitutively overexpresses Cav-1 under the regulation of MMTV promoter, i.e. MMTVcav-1 transgenic mice. The transgenic mice displayed defective morphologic features, mainly in epithelial cells of multiple exocrine organs. Our results indicate that Cav-1 overexpression causes organ-specific, age-related epithelial disorders and suggest the potential for increased susceptibility to carcinogenesis. MMTVcav-1 transgenic mice should serve as a valuable tool for further characterization of the biologic functions of Cav-1 and its pathologic role in tumor progression.
Acknowledgments
This work was supported by National Cancer Institute grant CA R0168814. We are also grateful for M.D. Anderson Cancer Center DNA Analysis Facility which is funded by National Cancer Institute grant CA 16672 for mouse genotyping. Finally we thank Karen Phillips, ELS, for expert editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aldred MA, Ginn-Pease ME, Morrison CD, Popkie AP, Gimm O, Hoang-Vu C, Krause U, Dralle H, Jhiang SM, Plass C, Eng C. Caveolin-1 and caveolin-2,together with three bone morphogenetic protein-related genes, may encode novel tumor suppressors down-regulated in sporadic follicular thyroid carcinogenesis. Cancer Res. 2003;63:2864–2871. [PubMed] [Google Scholar]
- Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- Cantiani L, Manara MC, Zucchini C, De Sanctis P, Zuntini M, Valvassori L, Serra M, Olivero M, Di Renzo MF, Colombo MP, Picci P, Scotlandi K. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and met signaling. Cancer Res. 2007;67:7675–7685. doi: 10.1158/0008-5472.CAN-06-4697. [DOI] [PubMed] [Google Scholar]
- Cao G, Yang G, Timme TL, Saika T, Truong LD, Satoh T, Goltsov A, Park SH, Men T, Kusaka N, Tian W, Ren C, Wang H, Kadmon D, Cai WW, Chinault AC, Boone TB, Bradley A, Thompson TC. Disruption of the caveolin-1 gene impairs renal calcium reabsorption and leads to hypercalciuria and urolithiasis. Am. J. Pathol. 2003;162:1241–1248. doi: 10.1016/S0002-9440(10)63920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. Absence of Caveolin-1 Sensitizes Mouse Skin to Carcinogen-Induced Epidermal Hyperplasia and Tumor Formation. Am. J. Pathol. 2003;162:2029–2039. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R, Morgan BE, Tannenbaum M, Salup R, Morgan MB. Caveolin expression in adult renal tumors. Urol. Oncol. 2003;21:191–196. doi: 10.1016/s1078-1439(02)00235-1. [DOI] [PubMed] [Google Scholar]
- Choi YW, Henrard D, Lee I, Ross SR. The mouse mammary tumor virus long terminal repeat directs expression in epithelial and lymphoid cells of different tissues in transgenic mice. J. Virol. 1987;61:3013–3019. doi: 10.1128/jvi.61.10.3013-3019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Davidson B, Nesland JM, Goldberg I, Kopolovic J, Gotlieb WH, Bryne M, Ben-Baruch G, Berner A, Reich R. Caveolin-1 expression in advanced-stage ovarian carcinoma--a clinicopathologic study. Gynecol. Oncol. 2001;81:166–171. doi: 10.1006/gyno.2001.6156. [DOI] [PubMed] [Google Scholar]
- Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, Demichelis F, Solomon KR, Loda M, Rubin MA, Lisanti MP, Freeman MR. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8 doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Elsheikh SE, Green AR, Rakha EA, Samaka RM, Ammar AA, Powe D, Reis-Filho JS, Ellis IO. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br. J. Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. Embo. J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Dales JP, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, Andonian C, Lavaut MN, Allasia C, Bonnier P, Charpin C. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum. Pathol. 2007;38:830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Gaudreault SB, Dea D, Poirier J. Increased caveolin-1 expression in Alzheimer's disease brain. Neurobiol. Aging. 2004;25:753–759. doi: 10.1016/j.neurobiolaging.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Goto T, Nguyen BP, Nakano M, Ehara H, Yamamoto N, Deguchi T. Utility of Bcl-2, P53, Ki-67, and caveolin-1 immunostaining in the prediction of biochemical failure after radical prostatectomy in a Japanese population. Urology. 2008;72:167–171. doi: 10.1016/j.urology.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res. Ther. 2008;10:R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am. J. Pathol. 2002;161:1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi A, Asano T, Asakuma J, Sumitomo M, Hayakawa M. Impact of caveolin-1 expression on clinicopathological parameters in renal cell carcinoma. J. Urol. 2004;172:718–722. doi: 10.1097/01.ju.0000130943.23317.08. [DOI] [PubMed] [Google Scholar]
- Hu YC, Lam KY, Law S, Wong J, Srivastava G. Profiling of Differentially Expressed Cancer-related Genes in Esophageal Squamous Cell Carcinoma (ESCC) Using Human Cancer cDNA Arrays: Overexpression of Oncogene MET Correlates with Tumor Differentiation in ESCC. Clin. Cancer Res. 2001;7:3519–3525. [PubMed] [Google Scholar]
- Hung KF, Lin SC, Liu CJ, Chang CS, Chang KW, Kao SY. The biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesis. J. Oral Pathol Med. 2003;32:461–467. doi: 10.1034/j.1600-0714.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yoshida H, Nakano K, Kobayashi K, Yokozawa T, Hirai K, Matsuzuka F, Matsuura N, Kakudo K, Kuma K, Miyauchi A. Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br. J. Cancer. 2002;86:912–916. doi: 10.1038/sj.bjc.6600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004;93:291–296. doi: 10.1111/j.1464-410x.2004.04604.x. [DOI] [PubMed] [Google Scholar]
- Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929–933. [PubMed] [Google Scholar]
- Kato T, Miyamoto M, Kato K, Cho Y, Itoh T, Morikawa T, Okushiba S, Kondo S, Ohbuchi T, Katoh H. Difference of caveolin-1 expression pattern in human lung neoplastic tissue. Atypical adenomatous hyperplasia, adenocarcinoma and squamous cell carcinoma. Cancer Lett. 2004;214:121–128. doi: 10.1016/j.canlet.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee Y, Seo JE, Cho KH, Chung JH. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal. 2008;20:1313–1319. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol. Cell. Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat. Cell. Biol. 1999;1:369–375. doi: 10.1038/14067. [DOI] [PubMed] [Google Scholar]
- Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J. Biol. Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J. Biol. Chem. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Minkeu A, Allen KG, Danilo C, Sotgia F, Bonuccelli G, Jasmin JF, Xu H, Bosco E, Aronow B, Witkiewicz A, Pestell RG, Knudsen ES, Lisanti MP. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol. Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier I, Casimiro MC, Zhou J, Wang C, Plymire C, Bryant KG, Daumer KM, Sotgia F, Bonuccelli G, Witkiewicz AK, Lin J, Tran TH, Milliman J, Frank PG, Jasmin JF, Rui H, Pestell RG, Lisanti MP. Genetic ablation of caveolin-1 drives estrogen-hypersensitivity and the development of DCIS-like mammary lesions. Am. J. Pathol. 2009;174:1172–1190. doi: 10.2353/ajpath.2009.080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten AD, Sanders MM, McKnight GS. The MMTV LTR promoter is induced by progesterone and dihydrotestosterone but not by estrogen. Mol. Endocrinol. 1988;2:143–147. doi: 10.1210/mend-2-2-143. [DOI] [PubMed] [Google Scholar]
- Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001;58:811–814. doi: 10.1016/s0090-4295(01)01337-1. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Razani B, Lisanti MP. Caveolin-deficient mice: insights into caveolar function human disease. J. Clin. Invest. 2001;108:1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell. Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Li L, Yang G, Timme TL, Goltsov A, Ren C, Ji X, Addai J, Luo H, Ittmann MM, Thompson TC. RTVP-1, a tumor suppressor inactivated by methylation in prostate cancer. Cancer Res. 2004;64:969–976. doi: 10.1158/0008-5472.can-03-2592. [DOI] [PubMed] [Google Scholar]
- Ren C, Ren CH, Li L, Goltsov AA, Thompson TC. Identification and characterization of RTVP1/GLIPR1-like genes, a novel p53 target gene cluster. Genomics. 2006;88:163–172. doi: 10.1016/j.ygeno.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Mimori K, Yoshinaga K, Tanaka F, Nishida K, Ohno S, Inoue H, Mori M. Clinical significance of Caveolin-1, Caveolin-2 and HER2/neu mRNA expression in human breast cancer. Br. J. Cancer. 2004;91:959–965. doi: 10.1038/sj.bjc.6602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, Cordon-Cardo C. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. [PubMed] [Google Scholar]
- Satoh T, Yang G, Egawa S, Addai J, Frolov A, Kuwao S, Timme TL, Baba S, Thompson TC. Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer. 2003;97:1225–1233. doi: 10.1002/cncr.11198. [DOI] [PubMed] [Google Scholar]
- Selleri S, Arnaboldi F, Palazzo M, Hussein U, Balsari A, Rumio C. Caveolin-1 is expressed on multipotent cells of hair follicles and might be involved in their resistance to chemotherapy. Br. J. Dermatol. 2005;153:506–513. doi: 10.1111/j.1365-2133.2005.06746.x. [DOI] [PubMed] [Google Scholar]
- Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int. J. Radiat. Biol. 2008;84:177–189. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- Shi L, Chen XM, Wang L, Zhang L, Chen Z. Expression of caveolin-1 in mucoepidermoid carcinoma of the salivary glands: correlation with vascular endothelial growth factor, microvessel density, and clinical outcome. Cancer. 2007;109:1523–1531. doi: 10.1002/cncr.22573. [DOI] [PubMed] [Google Scholar]
- Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, Kondo S, Katoh H. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br. J. Cancer. 2002;87:1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris B, Blaveri E, Crnogorac-Jurcevic T, Jones M, Missiaglia E, Ruszniewski P, Sauvanet A, Lemoine NR. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am. J. Pathol. 2002;160:1745–1754. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DD, Krzykowski KJ, Engelke JA, Groblewski GE. Exocrine pancreatic secretion of phospholipid, menaquinone-4, and caveolin-1 in vivo. Biochem. Biophys. Res. Commun. 2004;319:974–979. doi: 10.1016/j.bbrc.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Thompson TC, Tahir SA, Li L, Watanabe M, Naruishi K, Yang G, Kadmon D, Logothetis CJ, Troncoso P, Ren C, Goltsov A, Park S. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2009 doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado OM, Mateo-Lozano S, Villar J, Dettin LE, Llort A, Gallego S, Ban J, Kovar H, Notario V. Caveolin-1 (CAV1) is a target of EWS/FLI-1 and a key determinant of the oncogenic phenotype and tumorigenicity of Ewing's sarcoma cells. Cancer Res. 2006;66:9937–9947. doi: 10.1158/0008-5472.CAN-06-0927. [DOI] [PubMed] [Google Scholar]
- Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol. Biol. Cell. 2002;13:2502–2517. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Yang G, Cao G, Tahir SA, Naruishi K, Tabata K, Fattah EA, Rajagopalan K, Timme TL, Park S, Kurosaka S, Edamura K, Tanimoto R, Demayo FJ, Goltsov AA, Thompson TC. Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Mol. Cancer Res. 2009;7:1446–1455. doi: 10.1158/1541-7786.MCR-09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am. J. Pathol. 2001;158:833–839. doi: 10.1016/S0002-9440(10)64031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman H, Seppanen JK, Sarhadi VK, Kettunen E, Salmenkivi K, Kuosma E, Vainio-Siukola K, Nagy B, Karjalainen A, Sioris T, Salo J, Hollmen J, Knuutila S, Anttila S. Caveolins as tumour markers in lung cancer detected by combined use of cDNA and tissue microarrays. J. Pathol. 2004;203:584–593. doi: 10.1002/path.1552. [DOI] [PubMed] [Google Scholar]
- Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol. Biol. Cell. 2003;14:1027–1042. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Hassan GS, Li J, Cohen AW, Medina FA, Philippe GF, Pestell RG, De Vizio D, Loda M, Lisanti MP. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: Genetic ablation of Cav-1 delays advanced prostate tumor development in TRAMP mice. J. Biol. Chem. 2005;10:1074. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, Scherer PE, Pestell RG, Lisanti MP. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J. Biol. Chem. 2004;279:24745–24756. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- Yang G, Timme TL, Naruishi K, Fujita T, Fattah el MA, Cao G, Rajocopolan K, Troung LD, Thompson TC. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp. Mol. Pathol. 2008;84:131–140. doi: 10.1016/j.yexmp.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yang G, Timme TL, Park SH, Wu X, Wyllie MG, Thompson TC. Transforming growth factor beta 1 transduced mouse prostate reconstitutions: II: Induction of apoptosis by doxazosin. Prostate. 1997;33:157–163. doi: 10.1002/(sici)1097-0045(19971101)33:3<157::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin. Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–5723. [PubMed] [Google Scholar]
- Yoo SH, Park YS, Kim HR, Sung SW, Kim JH, Shim YS, Lee SD, Choi YL, Kim MK, Chung DH. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer. 2003;42:195–202. doi: 10.1016/s0169-5002(03)00287-3. [DOI] [PubMed] [Google Scholar]