Abstract
Bisphenol-A (BPA) is an endocrine disrupting chemical used in numerous consumer products, resulting in universal exposure in the United States. Prenatal exposure to BPA is associated with numerous reproductive and developmental effects in animals. However, little is known about human fetal exposure or metabolism of BPA during mid-gestation. In the present study, we present a new liquid chromatography-tandem mass spectrometry method to directly measure concentrations of BPA and two predominant metabolic conjugates – BPA glucuronide and BPA sulfate – in umbilical cord serum collected from elective 2nd trimester pregnancy terminations. We detected at least one form of BPA in all umbilical cord serum samples: BPA (GM 0.16; range <LOD-52.26 ng/mL), BPA glucuronide (GM 0.14; range <LOD-5.41 ng/mL) and BPA sulfate (GM 0.32; range <LOD-12.65 ng/mL). Levels of BPA ranged from less than 1/100th to over 400 times higher than levels of BPA in conjugated form. Although levels of BPA in conjugated form exceeded BPA levels in about 3/4 of the samples, BPA levels were higher in samples with Total BPA above the median. Our findings suggest universal fetal exposure to BPA in our study population, with some at relatively high levels, and we provide the first evidence of detectable BPA sulfate in mid-gestation fetuses.
INTRODUCTION
Bisphenol A (BPA) is a high production volume chemical used in a variety of applications, including epoxy resins that line food and beverage cans; polycarbonate plastics such as microwavable food storage containers, medical devices, baby bottles and toys; and carbonless copy and thermal receipt papers [1-3]. In 2009, the U.S. annual production of BPA was more than 2.3 billion pounds, an average of nearly 8 pounds per capita [4]. The wide use of BPA has made human exposure ubiquitous: In 2003-2004, over 90% of the U.S. population had measurable levels of BPA in their bodies [5-6]. Several studies have also found near universal detection of BPA in maternal serum, placenta, amniotic fluid, umbilical cord serum and fetal liver collected either during early, mid or late pregnancy, or at delivery [7-17], Further, animal studies demonstrate that BPA rapidly crosses the placenta and distributes in both placental and fetal tissues, such that fetal levels in certain tissues surpass maternal serum levels shortly after dosage [18-23].
BPA is an endocrine disrupting chemical, specifically a selective estrogen receptor modulator [19], although other endocrine disrupting pathways have also been identified [24-25]. Perinatal exposure to BPA at doses at or below that experienced by the U.S. population (estimated geometric mean daily intake is 46.8 ng/kg-day (95% CI 42.6 – 51.4) based on urinary concentrations, geometric mean BPA concentration 2.6 ng/mL) [26], has been associated with increased risk of a variety of harmful effects in pubertal or adult rodent offspring, including neural and behavioral problems [20, 27], male reproductive effects [28-30] and female reproductive tract abnormalities [31-33], with animal studies indicating that susceptibility to BPA is greatest during prenatal development [34-35].
Animal and human data raise concern that the prenatal period may be a time of higher exposure to BPA. In adults, the majority (~80%) of BPA is metabolized via the Uridine 5′-diphospho-glucuronosyltransferase (UGT) system to BPA glucuronide, which does not bind to the estrogen receptor [24, 36-38]. However, studies of fetal expression of UGT enzymes responsible for BPA glucuronidation detected no to low enzyme activity during the prenatal period [39]. It is unknown whether cytosolic sulfotransferase, which plays a minor role in BPA metabolism in adults (converting BPA to BPA sulfate), compensates for the lack of UGT activity during the prenatal period. The human fetus may also face higher exposure to BPA if, as has been observed in Sprague-Dawley rats [40], BPA glucuronide is deconjugated and thus converted back to BPA in the placenta, and BPA sulfate has also been observed to have deconjugating activities based on in vitro studies [41].
Efforts to characterize human in utero exposure to BPA, BPA glucuronide and BPA sulfate have been hampered by limitations in current analytic methods, which include immunoassays, high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC-MS) and, less frequently, liquid chromatography-tandem mass spectrometry (LC-MS/MS) [42]. Immunoassays and HPLC are good assays to screen for BPA, but their specificity for accurate quantitative analysis is not as reliable as GC-MS or LC-MS/MS, especially for complicated biological matrices, such as serum and amniotic fluid. Due to their structural similarities, steroids present in umbilical cord serum and amniotic fluid may cross-react with immunoassay antibodies targeting BPA and consequently cause interference or elevated detection of false positives in BPA measurements. Steroids and endogenous phenolic compounds in umbilical cord serum and amniotic fluid may also contribute to or interfere with absorbance measurements at the wavelength by which BPA is measured in HPLC, which can produce overestimates or underestimates of BPA exposure. Though offering significantly better specificity, studies using GC-MS and some LC-MS/MS methods may have underestimated levels of BPA and conjugated BPA due to their use of a derivatization step prior to analysis, since 100% conversion of the target analyte cannot be consistently ensured. Lastly, while some studies have evaluated some aspects of BPA contamination in study collection and processing materials, there has not been a systematical documentation of all materials associated with collection, processing and analytic equipment and their potential to contaminate biological specimens with BPA [43]. Establishing the purity of biological specimens is of utmost importance due to the high prevalence of BPA in consumer, medical and industrial products.
Given the broad range of adverse effects reported from studies of developmental exposures in experimental mammals, the lack of accurate data on human in utero exposure to BPA and its metabolites is a critical research gap that hampers our ability to assess the developmental risks posed by this chemical. Therefore, we developed a new analytical approach that allows direct measurement of BPA (referred to as free, unconjugated, parent or aglycone BPA in other studies), BPA glucuronide and BPA sulfate. We applied this method to the measurement of human umbilical cord serum collected during the 2nd trimester of pregnancy. As a preliminary exploration of fetal metabolic capacity, we examined the profile of BPA, BPA glucuronide and BPA sulfate, as well as their relationships with gestational age. In addition, to evaluate the potential for BPA contamination during the collection and analysis of umbilical cord serum, we also conducted a systematic evaluation of all biospecimen collection and extraction materials and BPA quantitation equipment.
METHODS
Study Population
We recruited pregnant women seeking elective second trimester pregnancy terminations from the UCSF Women’s Options Center (WOC) at San Francisco General Hospital. The WOC is an outpatient clinic that serves the San Francisco Bay Area community and accepts referrals from clinics throughout Northern and Central California. The WOC serves a diverse, primarily low-income patient population. We recruited English and Spanish-speaking patients, 18 years and older, whose pregnancies were between 13 and 24 weeks. Women were excluded from the study if they were obtaining a pregnancy termination due to fetal anomaly or demise because these conditions could influence BPA metabolism, and the primary focus of the study was exposure assessment in healthy pregnancies. Eligible study participants were identified by reviewing patients’ medical records only after they had 1) consulted with a trained counselor for an elective second trimester termination procedure and 2) consented to the procedure as documentation of their intent to proceed with the elective pregnancy termination. Information on pregnancy and demographic characteristics of enrolled participants was abstracted from medical records. Study protocols were approved by the University of California, San Francisco Committee on Human Research.
Sample Collection and Preparation
We collected umbilical cord blood samples from 85 study participants between 2010 and 2012. Umbilical cord blood collection was performed by WOC medical staff and assisted by our study team. To reduce the likelihood of specimen contamination, all collection, processing and storage containers were made of glass or polypropylene. Umbilical cord blood was drained directly into red-top collection tubes during the procedure to avoid contact with medical devices and the environment. However, minimal contamination from maternal serum on the umbilical cord could not be precluded. Umbilical cord blood samples were centrifuged at 3000 RPM~1300 RCF for 10 minutes at 4°C; serum was transferred via polypropylene pipette into Corning cryovial polypropylene tubes and stored at −80°C until analysis. [44]. To prevent deconjugation of BPA glucuronide and BPA sulfate [45], samples were stored on ice during the collection process and specimens frozen at −80°C within 8 hours of collection.
Chemical Analysis
Direct simultaneous analysis of BPA, BPA glucuronide and BPA sulfate in umbilical cord serum was done by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using Agilent LC 1260- AB Sciex 5500 (binary pump) in the laboratory of Drs. Alan Wu and Roy Gerona at UCSF. Each analyte was ionized using electrospray ionization in the negative mode and monitored by multiple reaction monitoring using two transitions for each analyte and BPA-d16 as an internal standard (Table 1). The sources of standards and other chemicals used in the analysis are described in the Supporting Information, Section S1.
Table 1.
Transitions monitored for each analyte in the LC-MS/MS analysis of BPA levels in mid-gestation umbilical cord serum, Northern and Central California, 2010-2012
| Analyte | Quantifier Transition | Qualifier Transition |
|---|---|---|
| BPA | 227.0- 133.1 | 227.0- 212.1 |
| BPA glucuronide | 402.9- 112.9 | 402.9- 226.9 |
| BPA sulfate | 307.0-227.0 | 307.0-212.1 |
| BPA-d16 | 241.0- 142.2 | 241.0- 222.1 |
We prepared umbilical cord serum for LC-MS/MS analysis by solid phase extraction using Waters Oasis HLB cartridge (10 mg, 1 cc). Each cartridge was washed with 5 column volumes of methanol to eliminate reported BPA contamination before activation with water for loading of umbilical cord serum (250 uL) [46]. The column was washed with 1 mL 5% methanol before each analyte was eluted by 1 mL methanol. Ideal eluting solution for each analyte was determined by methods listed in the Supporting Information, Section S1. The eluates were evaporated under a stream of nitrogen gas after which they were reconstituted in 10% methanol for column injection.
A 25 μL aliquot of the extract was used for each replicate injection of the sample. Our analytic column was Agilent Extend-C18 (2.1×100 mm, 1.8μm), maintained at 50°C. Chromatographic separation of the analytes was achieved by gradient elution using water with 0.05% ammonium acetetate (pH=7.8) as mobile phase A and methanol with 0.05% ammonium acetate (pH=7.8) as mobile phase B. The elution gradient employed was- 0-0.5 min= 30%B; 1 min= 75%B; 4 min= 100%B; 4-6 min= 100%B; and 6.01-12 min= 30% B. All three analytes have LOQs of 0.1 ng/mL (part per billion). The limit of detection (LOD) was 0.05 ng/mL for BPA and BPA glucuronide, and 0.025 ng/mL for BPA sulfate. LOQ and LOD determination along with validation methods are listed in the Supporting Information, Section S1. During the process of method development, we initially measured BPA disulfate as well but did not find significant level in human serum (data not shown).
To accept the data obtained for each analytical run, procedural quality control (QC) materials were run within a batch of samples along with the procedural blank and calibration curve at the start, middle and end of each batch run. Two QC materials were used at low (0.5 ng/mL) and high (20 ng/mL) concentrations. To accept the results of a batch run, QC materials measurements and within run and between run precision, were set to be within 20% of their target values.
Environmental chemical data analysis was done using AB Sciex Analyst 1.6 and AB Sciex MultiQuant 2.1 software packages. Identification and confirmation of each analyte in the sample was based on its retention time and the peak area ratio between its two transitions. Quantification of each analyte was done by isotope dilution method using BPA-d16 as internal standard. No internal standards for BPA glucuronide and BPA sulfate were available at the time the analyses were done (standards are now available [47]); hence, BPA-d16 was used as their surrogate internal standard. Chemical analysis method validation was assessed in terms of precision, linearity and recovery. (Detailed validation methods: Supporting Information, S1).
Specimen BPA Contamination Investigation
We conducted field blank testing of all sample collection, processing and storage containers and all chemical analysis materials throughout the study to ensure that lot-to-lot variation in any material would not contribute BPA contamination to measured analyte levels [44]. Field blank testing was performed by simulating the sample collection, extraction and analytical run using double-charcoal stripped serum. No BPA was detected ≥ LOD in any of the sample collection and extraction materials or in any of the instruments used in the analysis. The testing was repeated using synthetic human serum and generated the same results. In addition, we conducted an extensive evaluation of possible BPA contamination from the IV equipment (IV bag, needles, and tubing) and medical equipment (such as gloves) used during the termination procedure, since previous studies suggest that medical equipment may contain BPA (Supporting Information S1, Table S1) [48].
Data Analysis
Statistical analysis was performed using STATA version 12 [49]. Samples with analyte concentrations below the LOD were imputed with LOD/√2. For analyses that compared concentrations of BPA, BPA glucuronide and BPA sulfate within samples, we converted the concentrations of BPA glucuronide and BPA sulfate to the concentrations of BPA in glucuronide and sulfate forms, respectively. Specifically, we multiplied the concentrations of BPA glucuronide by the ratio of the molecular weight of BPA to that of BPA glucuronide (0.5645), and the concentrations of BPA sulfate by the ratio of the molecular weight of BPA to that of BPA sulfate (0.7404). We also summed BPA, BPA in glucuronide form and BPA in sulfate form to calculate Total BPA and the percent of Total BPA in original, glucuronide and sulfate form. In addition to permitting a comparison of the relative levels of the analytes within a sample, BPA in glucuronide form, BPA in sulfate form and Total BPA are the best metrics for comparing concentrations in this study to all previous studies of BPA exposure in umbilical cord serum, which made indirect measurements of conjugated BPA.
We log-transformed analyte concentrations due to their right-skewed distributions; only BPA sulfate and Total BPA levels were approximately normal after transformation and therefore we used non-parametric tests (Kruskal-Wallis, Spearman’s, Wilcoxon signed rank) for univariate analyses. To compare concentrations of analytes within samples, we defined three groups based on the dominant analyte in the sample, compared levels of Total BPA between these groups and, using Pearson’s χ2 test, evaluated the association between these groups and having Total BPA levels above the median. Sex differences in BPA metabolic pathways have been reported in the literature on adult humans; therefore we examined sex differences in analyte levels and in dominant BPA analyte using Fisher’s Exact test. Lastly, we evaluated the association between gestational age and analyte levels through correlation and regression models, adjusting for sex. Due to the frequencies of non-detect BPA and BPA glucuronide, for these outcomes we used tobit regression [50-51], which treats <LOD values as left censored and has been shown to be a technically sound approach [52] that approximates linear regression as detection frequency increases. We used linear regression to model the relationship between BPA sulfate and gestational age. We used log-transformed analyte levels for all plots and multivariate models. Level of significance was set at p<0.1 for our smaller sample size.
RESULTS
Chemical Analysis Method Development and Validation
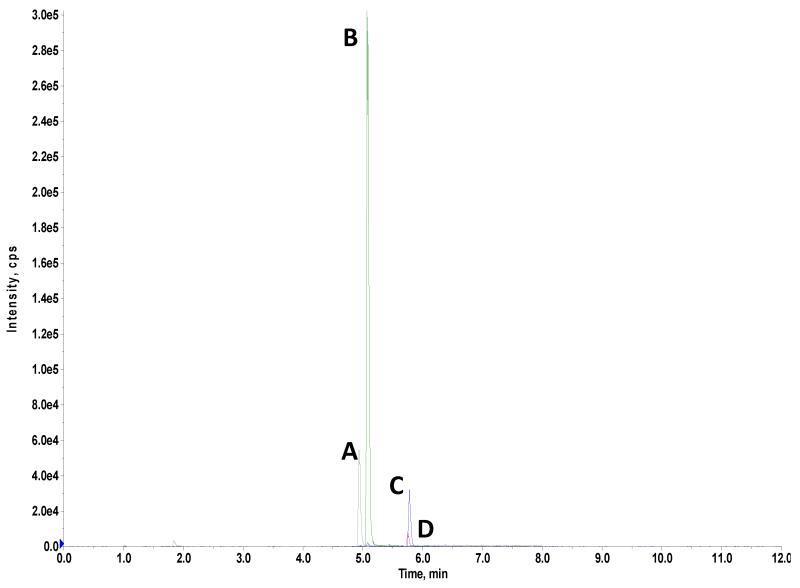
A typical chromatogram obtained from running the analytes is shown in Figure 1. The graph shown in Fig 1 is also typical for extracted ion chromatograms obtained for cord serum samples. We achieved baseline separation of all three analytes at lower concentrations (5 ng/mL and below). We preserved specificity for each analyte as each analyte is quantified by different pairs of transitions with no overlapping mass/charge ratios, even though there is no complete baseline separation of BPA glucuronide and BPA sulfate at concentrations higher than 5 ng/mL. We found shifting retention times for BPA glucuronide and BPA sulfate below pH 7.6 during method development, indicating that keeping the pH of the mobile phase between 7.6 and 8.0 is critical for the stability of the analytes’ retention times.
Figure 1.
Extracted ion chromatogram obtained from 5ng/mL standard mix spiked into synthetic human serum showing peaks for BPA (C), BPA-d16 (D), BPA glucuronide (A) and BPA sulfate (B).
We demonstrated the linearity of the instrument and method using a 10-point calibration curve spanning the concentrations of 0.1 to 80 ng/mL for each analyte. We reproducibly obtained linear regression coefficients of 0.99 or better for each analyte with a 1/x weighting in five separate trials done on separate days. This linearity was maintained for each analyte throughout the time the actual umbilical cord serum samples were analyzed (about six months). The method precision and recoveries are presented in Tables 2 and 3, respectively. We had a within run precision for each analyte with coefficient of variations (CV) of 1.6-3.4%, 2.9-5.4% and 3.0-5.0% for BPA, BPA glucuronide and BPA sulfate over three different analyte levels which are all within the prescribed precision for validated methods (CV≤20%) [53]. We had a slightly larger between run precision for each analyte, but CVs remained less than 10% for all analytes-BPA: 3.2-6.9%; BPA glucuronide: 8.1-9.4%; and, BPA sulfate: 6.0-8.5%. Method recoveries for each analyte were reproducibly over 85% within and between run (Table 3). The range of recoveries obtained for BPA, BPA glucuronide and BPA sulfate are 92.5-98.5%, 86.5-92.5% and 90-95.5%, respectively. For each of the analytes, the recoveries were maintained at a narrow range which ensures that analytical variability would not contribute significantly to measured levels of the analyte in the samples.
Table 2.
Validation of method precision for direct analysis of BPA and its primary metabolic conjugates in mid-gestation umbilical cord serum using LC-MS/MS, Northern and Central California, 2010-2012.
| Analyte | Precision (% CV) | |||||
|---|---|---|---|---|---|---|
| Within Run (n=5) | Between Run (n=15) | |||||
| Low 0.5ng/mL |
Med 5ng/mL |
High 10ng/mL |
Low 0.5ng/mL |
Med 5ng/mL |
High 10ng/mL |
|
| BPA | 3.4 | 2.8 | 1.6 | 6.9 | 5.5 | 3.2 |
| BPA glucuronide |
2.9 | 3.5 | 5.4 | 8.1 | 9.4 | 8.1 |
| BPA sulfate | 5.0 | 4.4 | 3.0 | 8.5 | 5.5 | 6.0 |
Table 3.
Validation of method recoveries for direct analysis of BPA and its primary metabolic conjugates in mid-gestation umbilical cord serum using LC-MS/MS, Northern and Central California, 2010-2012.
| Analyte | Recovery (%) | |||||
|---|---|---|---|---|---|---|
| Within Run (n=5) | Between Run (n=15) | |||||
| Low 0.5ng/mL |
Med 5ng/mL |
High 10ng/mL |
Low 0.5ng/mL |
Med 5ng/mL |
High 10ng/mL |
|
| BPA | 92.5 | 98.5 | 96.5 | 93.0 | 96.5 | 95.0 |
| BPA glucuronide | 90.5 | 92.5 | 89.5 | 86.5 | 88.0 | 90.5 |
| BPA sulfate | 90.5 | 90.5 | 95.5 | 90.0 | 91.5 | 92.3 |
BPA Levels in Umbililcal Cord Serum
Our study population consisted of pregnant women aged 18 – 45 (median 23), with similar percentages of Latinas, African Americans and Caucasians and a smaller percentage of unknown race/ethnicity (Table 4). Distribution of gestational age was right skewed, with a median of 21.3 gestational weeks (range 13.3 – 24). Most fetuses were female.
Table 4.
Mid-Gestation Maternal and Fetal Characteristics, Northern and Central California, 2010-2012 (n=85)
| Maternal Characteristics | Median (Min - Max) |
|---|---|
| Age a | 23 (18-45) |
| Body Mass Index (BMI) a | 27 (19-54) |
| Parity | 1 (0-6) |
| Ethnicity | n (%) |
| Latina | 23 (28%) |
| African-American | 24 (29 %) |
| White | 21 (25%) |
| Asian/PI/Other | 14 (16%) |
| Missing | 3 (4%) |
| Fetal Characteristics | Median (Range) |
|
| |
| Gestational Age | 21.3 weeks (13.3 - 24 weeks) |
| Fetal Sex | n (%) |
| Female | 44 (52%) |
| Male | 24 (28%) |
| Unknown | 17 (20%) |
Data missing for maternal age (n=1) and BMI (n=1)
We found detectable levels of BPA, BPA glucuronide and BPA sulfate in 47, 76 and 96% of the 85 umbilical cord serum samples, respectively (Table 5). We found higher variability in concentrations of BPA (range <LOD – 52.26 ng/mL) and BPA in sulfate form (range <LOD – 9.37 ng/mL) compared to BPA in glucuronide form (<LOD – 3.05 ng/mL) (Table 5 and Supporting Information, Figure S1). A moderate but statistically significant correlation was evident between BPA glucuronide and BPA sulfate (ρ = 0.45, p-value < 0.0001), but no other significant correlation between analytes was observed.
Table 5.
Levels of BPA analytes (ng/mL) in mid-gestation umbilical cord serum, Northern and Central California, 2010-2012 (n=85)
| BPA Analyte | LOD (ng/mL) |
% > LOD |
Geometric Mean, (GSD) |
min | 5th | 25th | Median | 75th | 95th | max |
|---|---|---|---|---|---|---|---|---|---|---|
| BPA | 0.05 | 47% | 0.16 (7.22) | <LOD | <LOD | <LOD | <LOD | 0.06 | 9.91 | 52.26 |
| BPA glucuronide | 0.05 | 76% | 0.14 (3.30) | <LOD | <LOD | .05 | 0.12 | 0.28 | 0.96 | 5.41 |
| BPA in glucuronide forma | --- | --- | 0.08 (1.19) | <LOD | 0.07 | 0.54 | 3.05 | |||
| BPA sulfate | 0.025 | 96% | 0.32 (4.89) | <LOD | 0.04 | 0.08 | 0.24 | 0.95 | 8.71 | 12.65 |
| BPA in sulfate forma | --- | --- | 0.24 (1.61) | <LOD | 0.18 | 6.45 | 9.37 | |||
| Total BPAb | --- | --- | 0.79 (1.54) | 0.08 | 0.11 | 0.20 | 0.80 | 1.64 | 10.81 | 62.77 |
| Conjugated BPA-BPA Ratioc | --- | --- | 0.42 (2.26) | 0.004 | 0.02 | 0.13 | 0.31 | 1.62 | 14.41 | 412.54 |
BPA in glucuronide form=BPA glucuronide*0.5614. BPA in sulfate form=BPA sulfate*0.7404. The factors 0.5614 and 0.7404 are the ratios of the molecular weight of BPA to the molecular weights of BPA glucuronide and BPA sulfate, respectively.
Total BPA=BPA + BPA in glucuronide form + BPA in sulfate form.
Conjugated BPA=BPA in Glucuronide Form + BPA in Sulfate Form.
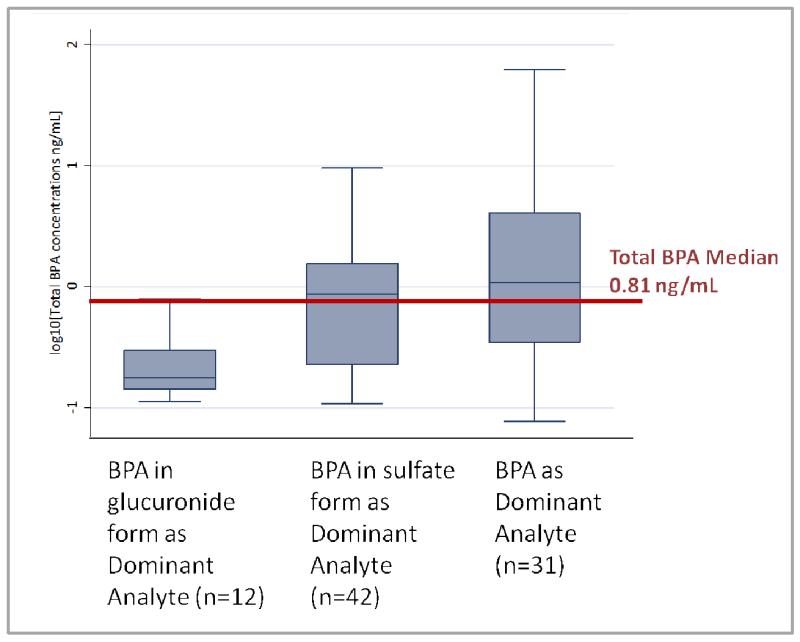
BPA was the dominant analyte in 31 specimens, while BPA in sulfate form dominated in 42 specimens and BPA in glucuronide form in 12 specimens (Figure 2). We found that, on average, Total BPA was comprised of 36% BPA (range 4-100%), 45% BPA sulfate (range 2-96%) and 19% BPA glucuronide (range 0.1-82%). Total BPA levels differed depending on the dominant analyte (Kruskal-Wallis p-value<0.01); specimens with BPA as the dominant analyte were more likely to have higher than median levels of Total BPA (1.03 ng/mL, χ2 p-value <0.1), and specimens with BPA in glucuronide form as the dominant analyte were more likely to have lower than median levels of Total BPA (Fisher’s Exact p-value <0.01). Concentrations of both BPA and BPA in sulfate form were higher than levels of BPA in glucuronide form (p-values 0.02 and <0.01, respectively), while BPA levels surpassed levels of BPA in sulfate form only at the upper end of the distribution (95th percentile 9.91 ng/mL versus 6.45 ng/mL, respectively; Table 5). Levels of BPA ranged from less than 1/100th to over 400 times higher than levels of BPA in conjugated form.
Figure 2.
Total BPA concentrationsa (ng/mL) by dominant form of BPA measured in mid-gestation umbilical cord serum, Northern and Central California, 2010-2012 (n=85). (Kruskal-Wallis p-value <0.01)
Gestational age was not significantly correlated with any BPA analyte, including BPA, BPA glucuronide, BPA sulfate or Total BPA. Tobit and linear regression analyses, controlling for sex, yielded similar results. However, among the subgroup of samples with Total BPA levels above the median, gestational age was significantly and inversely associated with log(BPA) levels (β= −0.27, p <0.05). Sex was not associated with levels of any BPA analyte (Supporting Information, Table S2).
Specimen BPA Contamination Investigation
We found all laboratory and specimen collection materials and equipment and IV equipment to be free of BPA, BPA glucuronide and BPA sulfate (Supporting Information, Table S1). We collected IV usage information for 88% of study participants (n=75). Length of IV use ranged from 19 to 257 minutes with a median of 62 minutes. We found a negative association between length of IV use and both BPA and BPA glucuronide levels using Spearman’s correlation test (ρ=−0.20 and −0.21, respectively, p-values 0.08), whereas no association was observed in other statistical tests (Pearson’s correlation, linear regression and tobit regression).
DISCUSSION
We report the first direct measurements of BPA, BPA glucuronide and BPA sulfate in human umbilical cord serum from mid-gestation fetuses using LC MS-MS, which is considered the most sensitive and specific method for measuring levels of BPA and other environmental chemicals in biological samples [42]. Our results demonstrate measurable levels of at least one form of BPA in all umbilical cord serum samples, with higher levels of BPA and BPA in sulfate form than BPA in glucuronide form. We did not detect any evidence of BPA contamination by IV use or by specimen collection, processing or analysis materials and equipment.
The LC-MS/MS quantitation method used in the present study is a sound approach for quantification of environmental chemicals such as BPA, BPA glucuronide and BPA sulfate. Further, LC-MS/MS allows for more accurate and specific analyte measurement than methods used in most previous studies (immunoassay, HPLC, and GC-MS), and our method for directly measuring BPA, BPA glucuronide and BPA sulfate eliminates the potential loss of analyte incurred by derivatization. Both method precision and recovery for each analyte did not deviate by more than 15% of the target values (within the standard criteria limit of 20%). Excellent linearities were consistently achieved for the calibration curves of each analyte with regression coefficients ≥ 0.99. Lastly, the method detection limits achieved for all analytes are significantly lower than the mean values reported for BPA and conjugated BPA by published biomonitoring studies to date (Table 6).
Table 6.
| Authors | Year Published |
Location | Gestation | N | Method c | Quantification Method |
LOD (ng/mL) |
Unconjugated BPA (ng/mL) | Range (ng/mL) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Median | Mean | |||||||||
| UCSF Study | 2013 | USA |
mid-
gestation |
85 | Direct | LC-MS/MS | 0.05 | 0.16 | <LOD | 2.18 ± 8.10 |
<LOD –
52.62 |
| Zhang et al. a | 2013 | China | delivery | 30 | indirect | HPLC-MS/MS | 0.10 (LOQ) |
0.08 | <LOQ | 0.13 ± 0.12 | <LOQ – 0.79 |
| Fenichel et al. | 2012 | France | delivery | 106 | indirect | radioimmunoassay | 0.08 | --- | 0.9 | 1.12 ± 0.86 | 0.14 – 4.76 |
| Kosarac et al. a | 2012 | Canada | delivery | 12 | indirect | GC/EI-MS/MS | 0.087 (LOQ) |
--- | 1.82 | --- | <LOQ – 2.57 |
| Chou et al. | 2011 | Taiwan | delivery | 97 | indirect | HPLC | 0.13 | 0.5 | --- | 1.1 ± 2.2 | 0.3 - 18.5 |
| Wan et al. | 2010 | South Korea |
delivery | 25 | indirect | LC-MS/MS | 0.1 (LOQ) |
--- | --- | <0.6 | <0.6 – 0.7 |
| Lee et al.a | 2008 | South Korea |
delivery | 300 | indirect | HPLC | 0.625 | 0.65 | <LOD | 1.13±1.43 | <LOD – 8.86 |
| Kuroda et al. | 2003 | Japan | delivery | 9 | indirect | HPLC | 0.04 | --- | 0.64 | 0.62 ± 0.13 | 0.45 – 0.76 |
| Schonfelder et al. |
2002 | Germany | delivery | 37 | indirect | GC-MS | 0.01 | --- | 2.3 | 2.9 ± 2.5 | 0.2 – 9.2 |
| Todaka et al. | 2002 | Japan | delivery | 9 | indirect | ---b | --- | 1.94 | 4.43 ± 5.04 | 0.35 – 15.24 |
|
| Ikezuki et al. | 2001 | Japan | delivery | 32 | indirect | ELISA | 0.5 | --- | --- | 2.2 ± 1.8 | n/a |
Study by Zhang et al., Kosarac et al. and Lee et al. only measured Total BPA
Study by Todaka published BPA quantitation methods in a non-English (Japanese) journal
Indirect method – enzymatic deconjugation of Total BPA
Several observations from our study suggest that the immaturity of the UGT system and saturation of metabolic pathways may both act to increase fetal exposure to BPA during early to mid-gestation. First, levels of BPA and BPA in sulfate form were 2-3 times higher than levels of BPA in glucuronide form in our population and concentrations of BPA in glucuronide form surpassed those of BPA and BPA in sulfate form in only 12 (14%) specimens. These findings are consistent with animal, in vitro, and gene expression studies that have found lower UGT activity and higher sulfotransferase activity in human placenta and fetal liver compared to adults [54-57].
Second, we observed that the subgroup of umbilical cord sera with the higher Total BPA levels also had higher levels of BPA compared to BPA in glucuronide and sulfate forms (Supporting Information, Figure S2). This could reflect saturation of BPA metabolism due to the immature metabolic capacity of the mid-gestation fetus. This has been shown in in vitro studies using adult-rat hepatocytes – high levels of BPA glucuronide were found using lower doses of substrate BPA, but levels of BPA sulfate and other secondary hydroxylated metabolites were detected, in addition to BPA glucuronide, when using higher BPA substrate levels – though there is far less data to evaluate the extent to which saturation of the sulfation pathway occurs [58-59]. In agreement with these studies, the umbilical cord sera that have BPA glucuronide as the dominant BPA form also had the lowest Total BPA levels. Higher levels of BPA in the fetus may also result from deconjugation of BPA glucuronide by the placenta [40] and the greater ability of BPA to pass through the placenta (compared to BPA glucuronide and BPA sulfate) [23]. However, the placental BPA deconjugation and preferred placental transfer hypotheses in humans remain controversial and our data do not allow us to discriminate between these possibilities; clearly a better understanding of the metabolic role of the fetal liver and the placenta during different stages of gestation is needed, as there may be species specific and life-stage differences in BPA metabolism [60].
We did not observe any decline in analyte levels with gestational age, as reported by Ikezuki et al. for samples collected mid-gestation and at birth [7]. However, the gestational age range in our study is relatively narrow, right skewed (13-24 weeks with GM 21.3) and limited to the second trimester, when increases in fetal blood volume could potentially dilute BPA levels [61]. Nevertheless, our findings confirm a relatively inactive UGT system during the second trimester of human pregnancy compared to adulthood.
Although this is the only study to report levels of BPA in umbilical cord serum at mid-gestation, several studies have reported BPA levels in umbilical cord serum from term infants (gestational ages 32 to 41 weeks; Table 6). Our median BPA levels are similar to those measured in term umbilical cord serum from larger studies, the concentrations of BPA in our study include the highest levels reported to date (52.26 ng/mL; Table 5), with measured levels in 3 fetuses above 18 ng/mL (the highest level reported in the other studies, Table 6). Thus, our data suggest that a significant subset of mid-gestation fetuses is exposed to relatively high levels of BPA. Experimental studies in pregnant rats have shown parallel concentration-time curves for maternal blood and fetal BPA levels post maternal administration of BPA [62-63], suggesting a high correlation between circulating BPA levels in mother and fetus. Thus, the high levels of BPA observed in our study may also be direct reflections of high BPA exposure experienced by the mother. A recent animal study reported high bioavailability of BPA in serum post sublingual exposure to BPA, and hence high levels of BPA exposure from maternal serum is likely [64]. Our study population is primarily low-income and more ethnically diverse than the general U.S. population and hence may experience higher exposure to BPA than populations in other studies [65-66].
Differences in analytic chemistry methods could also contribute to the higher levels of Total BPA found in our study. BPA quantification methods used by other studies are listed in Table 6. Six studies used either immunoassays or HPLC methods, which are good assays to screen for BPA, but their specificity for accurate quantitative analysis is not as reliable as GC-MS or LC-MS/MS, especially for complicated biological matrices like umbilical cord serum. Further, all studies used a derivatization step prior to quantification, which introduces the possibility of some loss of analyte, since 100% conversion of the target analyte cannot be consistently ensured. Thus, higher levels of conjugates (and thus Total BPA) in our study could be a reflection of the improved ability of our methodology to reliably and completely capture BPA and its conjugates.
BPA levels in over one-third of the umbilical cord serum samples in our study are similar to or above those found to be associated with developmental effects in animals. Specifically, BPA levels in 36 percent (n=31) of umbilical cord serum samples are comparable to or greater than 0.23 ng/mL, a dose that stimulates human tissues in vitro [19, 33, 42]. Further, they are also similar to or above levels found in fetal CD-1 mice (0.48 ng/mL) after dosing the dam via subcutaneous injection with 25 ug/kg-body weight BPA – a dose that resulted in adverse developmental effects [18-19, 67-69]. Studies of the toxicity and estrogenicity of conjugated BPA are limited in number and have focused on evaluating estrogen binding and gene expression activity in cultured adult cells [38, 59, 70]. While the data from these studies suggest that, in adults, the toxicity of BPA conjugates is significantly lower than that of BPA, we have no knowledge of whether or how BPA conjugates affect biological pathways during development. Given the level of mid-gestation exposure to BPA sulfate indicated by our findings, the need to understand the biological effects of BPA conjugates, and life stage differences in deconjugation enzymatic activity should be considered paramount in understanding the toxicity of this chemical.
One challenge in measuring BPA is the possibility of contamination, as BPA is a component of many products, including those used for collection and processing of serum. Our study is the first to extensively test all components of our specimen collection process. We also tested the LC and MS equipment using a previously reported, systematic approach [44] and found no evidence of contamination. To rule out potential maternal contamination introduced via IV fluids, we tested both the IV fluid and equipment and did not detect BPA. Neither did we observe any positive association between duration of IV fluid delivery and BPA levels in umbilical cord serum. Our collective analysis of contamination issues shows that neither sample collection, analysis nor IV use are likely to be sources of BPA contamination in this study.
In summary, this study provides the first data on direct measurements of mid-gestation fetal exposure to BPA, BPA glucuronide and BPA sulfate. Our findings demonstrate that all fetuses are exposed to at least one form of BPA during mid-gestation, with a subset having high levels of BPA. Further, we have identified sulfation as an important metabolic pathway during mid-gestation that previously has not been explored in humans. These findings underscore the need for further evaluation of potential effects of BPA sulfate on development. Overall our findings point to the importance of fetal exposure to BPA during development and the need to accurately assess the full range of human exposure during pregnancy.
Supplementary Material
ACKNOWLEDGMENT
We thank the staff and faculty at San Francisco General Hospital Women’s Options Center for assistance in the collection of tissues. We also thank Katie Stevenson, Dylan Atchley and Cynthia Megloza for their assistance in recruitment and data collection. Thank you to Dr. Alan Wu. This project was supported by NIH grants P20 ES018135 (funded jointly by NIEHS and the U.S. Environmental Protection Agency: STAR RD83467801); R21 ES17763; R01 ES013527, and R01HD31544.
ABBREVIATIONS
- BPA
Bisphenol A
- GM
Geometric Mean
- GC-MS
Gas chromatography–mass spectrometry
- HPLC
High-Performance Liquid Chromatography
- MW
Molecular weight
- UGT
Uridine 5′-diphospho-glucuronosyltransferase
- LC/MS-MS
Liquid chromatography-tandem mass spectrometry
- LOD
Limit of Detection
REFERENCES
- 1.Schecter A, et al. Bisphenol A (BPA) in U.S. food. Environmental science & technology. 2010;44(24):9425–30. doi: 10.1021/es102785d. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg LN, et al. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Liao C,KK. Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45(21):9372–9. doi: 10.1021/es202507f. [DOI] [PubMed] [Google Scholar]
- 4.National Toxicology Program . NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. 2008. [PubMed] [Google Scholar]
- 5.Calafat AM, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the US: NHANES 2003-2004. Environmental health perspectives. 2011 doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikezuki Y, et al. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 8.Yamada H, et al. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16(6):735–9. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 9.Nahar MS, et al. Fetal Liver Bisphenol A Concentrations and Biotransformation Gene Expression Reveal Variable Exposure and Altered Capacity for Metabolism in Humans. Journal of Biochemical and Molecular Toxicology. 2012 doi: 10.1002/jbt.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlow AG, et al. Fetal bisphenol A exposure: Concentration of conjugated and unconjugated bisphenol A in amniotic fluid in the second and third trimesters. Reproductive Toxicology. 2012;34(1):1–7. doi: 10.1016/j.reprotox.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Schonfelder G, et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda N, et al. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. Journal of Pharmaceutical and Biomedical Analysis. 2003;30(6):1743–1749. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 13.Lee YJ, et al. Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol. 2008;25(4):413–9. doi: 10.1016/j.reprotox.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 14.Wan Y, et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environmental Science & Technology. 2010;44(13):5233–5239. doi: 10.1021/es1002764. [DOI] [PubMed] [Google Scholar]
- 15.Chou WC, et al. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health. 2011;10:94. doi: 10.1186/1476-069X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.P. Fenichel H.D.c., Harthe C, Gal J, Ferrari P, Pacini P, Wagner-Mahler MPK, Brucker-Davis F. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Human Reproduction. 2012;0(0):1–8. doi: 10.1093/humrep/der451. [DOI] [PubMed] [Google Scholar]
- 17.Todaka E, Mori C. Necessity to establish new risk assessment and risk communication for human fetal exposure to multiple endocrine disruptors in Japan. Congenit Anom (Kyoto) 2002;42(2):87–93. doi: 10.1111/j.1741-4520.2002.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 18.Zalko D, et al. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect. 2003;111(3):309–19. doi: 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6 Suppl):S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 20.National Toxicology Program . NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A. Research Triangle Park, NC: 2007. [DOI] [PubMed] [Google Scholar]
- 21.Doerge DR,TN, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255(3):261–70. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Patterson TA, et al. Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys. Toxicology and applied pharmacology. 2013;267(1):41–8. doi: 10.1016/j.taap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan B, et al. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 202(4):393 e1–7. doi: 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Taylor JA, v.S.F., Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama K,TT, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87(11):5185–90. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 26.Lakind JS, Naiman DQ. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003-2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 27.Richter CA, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aikawa H, et al. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315(1):119–24. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 29.vom Saal FS, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicology and Industrial Health. 1998;14(1-2):239–60. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 30.Prins GS, et al. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008;102(2):134–8. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Susiarjo M, et al. Bisphenol A Exposure In Utero Disrupts Early Oogenesis in the Mouse. PLoS Genet. 2007;3(1):e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varayoud J, et al. Developmental exposure to bisphenol A impairs the uterine response to ovarian steroids in the adult. Endocrinology. 2008 doi: 10.1210/en.2008-0651. [DOI] [PubMed] [Google Scholar]
- 33.Vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environmental health perspectives. 2005;113(8):926. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamanti-Kandarakis E, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves CR, et al. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environ Toxicol Pharmacol. 2010;30(2):195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Volkel W, et al. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–7. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 37.Teeguarden JG, et al. Evaluation of oral and intravenous route pharmacokinetics, plasma protein binding, and uterine tissue dose metrics of bisphenol A: A physiologically based pharmacokinetic approach. Toxicological Sciences. 2005;85(2):823–838. doi: 10.1093/toxsci/kfi135. [DOI] [PubMed] [Google Scholar]
- 38.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chemical research in toxicology. 2001;14(2):149–57. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 39.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–67. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa M,IH, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118(9):1196–203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stowell CL, et al. A role for sulfation-desulfation in the uptake of bisphenol a into breast tumor cells. Chem Biol. 2006;13(8):891–7. doi: 10.1016/j.chembiol.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberg LN,CI, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;188(8):1055–70. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye X, et al. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect. 2013;121(3):283–6. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerona RR, et al. Short Communication: Identification of a unique source of BPA contamination in blood sample collection. Submitted. [Google Scholar]
- 45.Ye X, et al. Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. J Expo Sci Environ Epidemiol. 2007;17(6):567–72. doi: 10.1038/sj.jes.7500566. [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, et al. Determination of bisphenol A in human serum by high-performance liquid chromatography with multi-electrode electrochemical detection. Journal of chromatography. B, Biomedical sciences and applications. 2000;749(1):17–23. doi: 10.1016/s0378-4347(00)00351-0. [DOI] [PubMed] [Google Scholar]
- 47.Lacroix MZ, et al. Simultaneous quantification of bisphenol A and its glucuronide metabolite (BPA-G) in plasma and urine: applicability to toxicokinetic investigations. Talanta. 2011;85(4):2053–9. doi: 10.1016/j.talanta.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 48.Calafat AM, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environmental Health Perspectives. 2009;117(4):639–44. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.StataCorp. Stata Statistical Software. StataCorp; 2011. Stata Statistical Software: Release 12. College Station, T.S.L. 2011. [Google Scholar]
- 50.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 51.Stata . Stata Base Reference Manual Release 12. 1-4. Vol. 2011. Stata Press; [Google Scholar]
- 52.Gardner M. Improving the interpretation of ‘less than’ values in environmental monitoring. Water and Environment Journal. 2012;26(2):285–290. [Google Scholar]
- 53.Biopharmaceutics Coordinating Committee. U.S. Department of Health. Human Services Food and Drug Administration. C.f.D.E.a.R.C. Center for Veterinary Medicine (CVM) U.S.D.o.H.a.H.S.F.a.D. Administration . Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services Food and Drug Administration; Silver Spring, MD: 2001. [Google Scholar]
- 54.Kang JH, Katayama Y, Kondo F. Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicology. 2006;217(2-3):81–90. doi: 10.1016/j.tox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110(2):193–6. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strassburg CP, et al. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50(2):259–65. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coughtrie MWH, Burchell B, Leakey JEA, Hume R. The inadequacy of perinatal glucuronidation: Immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Molecular Pharmacology. 1988;34(6):729–735. [PubMed] [Google Scholar]
- 58.Pottenger LH, et al. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54(1):3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 59.Elsby R, et al. Comparison of the modulatory effects of human and rat liver microsomal metabolism on the estrogenicity of bisphenol A: implications for extrapolation to humans. J Pharmacol Exp Ther. 2001;297(1):103–13. [PubMed] [Google Scholar]
- 60.Corbel T, et al. Bisphenol a disposition in the sheep maternal-placental-fetal unit: mechanisms determining fetal internal exposure. Biol Reprod. 2013;89(1):11. doi: 10.1095/biolreprod.112.106369. [DOI] [PubMed] [Google Scholar]
- 61.Faupel-Badger JM, et al. Plasma volume expansion in pregnancy: Implications for biomarkers in population studies. Cancer Epidemiology Biomarkers & Prevention. 2007;16(9):1720–1723. doi: 10.1158/1055-9965.EPI-07-0311. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi O, Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108(10):931–5. doi: 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin BS, et al. Maternal-fetal disposition of bisphenol a in pregnant Sprague-Dawley rats. J Toxicol Environ Health A. 2002;65(5-6):395–406. doi: 10.1080/15287390252808064. [DOI] [PubMed] [Google Scholar]
- 64.Gayrard V, et al. High bioavailability of bisphenol a from sublingual exposure. Environ Health Perspect. 2013;121(8):951–6. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calafat AM, et al. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson JW, et al. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environ Health. 2012;11:10. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markey CM, et al. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65(4):1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 68.Cabaton NJ, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environmental Health Perspectives. 2011;119(4):547–52. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunt PA, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13(7):546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu M, et al. Sulfation of bisphenol A abolished its estrogenicity based on proliferation and gene expression in human breast cancer MCF-7 cells. Toxicology in vitro: an international journal published in association with BIBRA. 2002;16(5):549–56. doi: 10.1016/s0887-2333(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 71.Fenichel P, H.D.c., Harthe C, Gal J, Ferrari P, Pacini P, Wagner-Mahler MPK, Brucker-Davis F. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Human Reproduction 2012. 0(0):1–8. doi: 10.1093/humrep/der451. [DOI] [PubMed] [Google Scholar]
- 72.Zhang T, Sun H, Kannan K. Blood and Urinary Bisphenol A Concentrations in Children, Adults, and Pregnant Women from China: Partitioning between Blood and Urine and Maternal and Fetal Cord Blood. Environmental science & technology. 2013;47(9):4686–94. doi: 10.1021/es303808b. [DOI] [PubMed] [Google Scholar]
- 73.Kosarac I, et al. A novel method for the quantitative determination of free and conjugated bisphenol A in human maternal and umbilical cord blood serum using a two-step solid phase extraction and gas chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;898:90–4. doi: 10.1016/j.jchromb.2012.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.