Abstract
Caveolin-1 (cav-1) and the cancer-promoting growth factors vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGF-β1), and fibroblast growth factor 2 (FGF2) are often found to be up-regulated in advanced prostate cancer and other malignancies. However, the relationship between cav-1 overexpresson and growth factor up-regulation remains unclear. This report presents the first evidence to our knowledge that in prostate cancer cells, a positive autoregulatory feedback loop is established in which VEGF, TGF-β1, and FGF2 up-regulate cav-1, and cav-1 expression, in turn, leads to increased levels of VEGF, TGF-β1, and FGF2 mRNA and protein, resulting in enhanced invasive activities of prostate cancer cells, i.e., migration and motility. Our results further demonstrate that cav-1–enhanced mRNA stability is a major mechanism underlying the up-regulation of these cancer-promoting growth factors. PI3-K-Akt signaling is required for forming this positive autoregulatory feedback loop.
Keywords: caveolin-1, Akt, cancer promoting growth factors, mRNA stability
Introduction
Prostate cancer is one of the most common types of cancers and the second leading cause of cancer-related death in American men (1). In most cases, death from prostate cancer results from metastatic disease. Understanding the mechanisms underlying the progression of prostate cancer will facilitate the development of biomarkers and novel therapeutic strategies to control this devastating malignancy.
Caveolin-1 (cav-1) is a major structural component of caveolae, specialized plasma membrane invaginations that are involved in molecular transport, endocytosis, cell adhesion, and signal transduction (2, 3). The role of cav-1 in cancer is complex and remains somewhat controversial [reviewed in (4–7)]. We previously found that elevated expression of cav-1 is associated with human prostate cancer, correlated with tumor angiogenesis, and may function as a valuable prognostic marker (8–12). In alignment with a growing body of clinical data that showed up-regulation of cav-1 expression in various types of malignancies and multi–drug resistant tumor cells [reviewed in (4, 6, 7)], experimental results showed that suppression of cav-1 expression reverses androgen insensitivity in metastatic androgen-insensitive mouse prostate cancer cells (13) and that genetic ablation of cav-1 attenuates the development and progression of prostate tumors in TRAMP mice (14). We previously showed that cav-1 was secreted by mouse and human prostate cancer cell lines and that secreted cav-1 promoted cancer cell survival and clonal growth in vitro (15–17). We further demonstrated that tumor cell–secreted cav-1 promotes proangiogenic activities in prostate cancer through the PI3-K-Akt-eNOS signaling module (18). With regard to the underlying mechanism(s) responsible for cav-1–mediated oncogenic activities, we demonstrated that cav-1 maintains activated Akt in prostate cancer cells through binding to and inhibition of the serine/threonine protein phosphatases PP1 and PP2A (15).
Of note, multiple growth factors (GFs) with tumor-promoting activities, including vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGF-β1), and fibroblast growth factors (FGFs), are also up-regulated in advanced cancer (19–22). VEGF is one of key mediators of angiogenesis that is produced at high levels in many types of tumors; it promotes proliferation, survival, and migration of endothelial cells and is essential to blood vessel formation and neovascularization (23, 24). TGF-β1 is a potent regulator of cell proliferation and extracellular matrix remodeling, with tumor-suppressor functions in the normal prostate gland and tumor-promoter functions in malignant and metastatic prostate cancer (25, 26). FGF2 is expressed at increased levels in human prostate, bladder, renal, and testicular cancers and play an important role in the neovascularization process that occurs in inflammation, angioproliferative diseases, and tumor growth (27, 28). The intricate balance of these GFs is crucial to the regulation of normal cell growth. Disruption of normal GF homeostasis in malignancy is often associated with apoptotic evasion, uncontrolled proliferation, and increased invasive potential. Various mechanisms are reported to be involved in deregulation of these GFs in cancer cells, including transcriptional regulation (29–31) and alteration of mRNA stability (32–35). The potential association of cav-1 and cancer-promoting growth factors in malignant progression prompted us to investigate whether cav-1 and expression of specific cancer-promoting GFs are functionally and mechanistically linked in prostate cancer progression. In this study, we found that multiple GFs stimulate cav-1 expression in prostate cancer cells and that cav-1 expression leads to increased levels of VEGF, TGF-β1, and FGF2 and enhances cancer cell migration and motility in an Akt-dependent manner. Furthermore, we found that cav-1–enhanced mRNA stability is a major mechanism for the up-regulation of these cancer-promoting GFs.
Results
Induction of Cav-1 Expression and Secretion by GFs
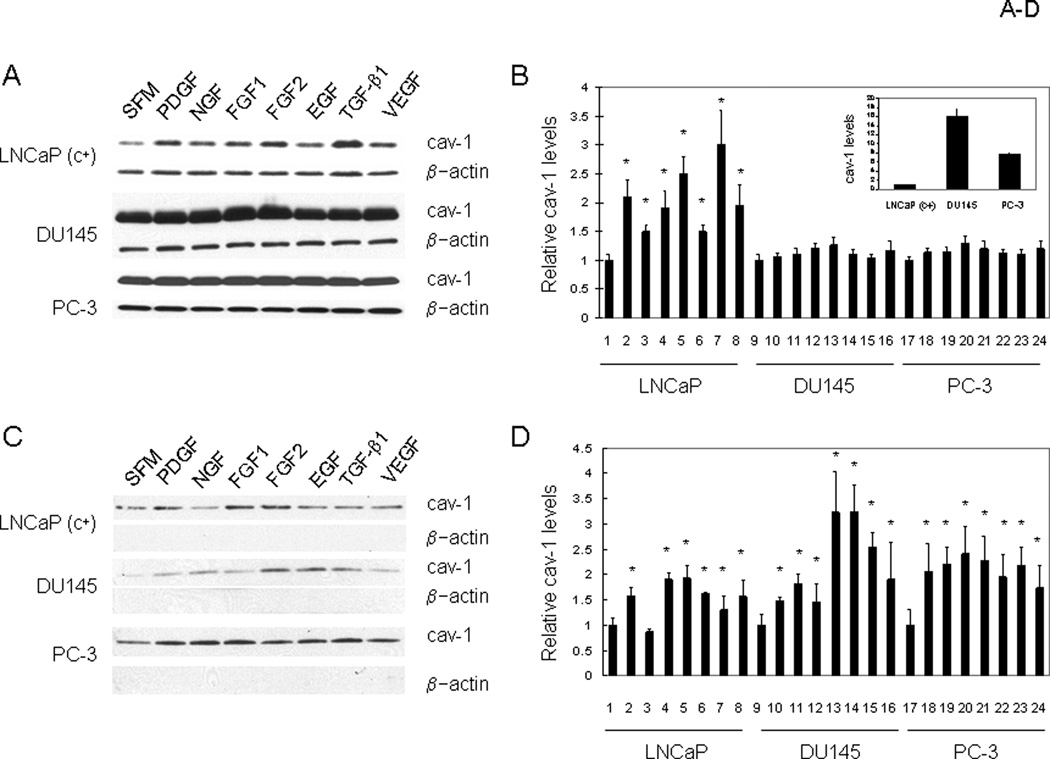
Since overexpression of specific cancer-promoting GFs occurs in parallel with cav-1 overexpression in many types of malignancies, including prostate cancer, we sought to determine whether there was any potential correlation between cav-1 and these GFs. We first examined the ability of selected GFs to stimulate cav-1 expression. Since our early observations suggested that GF treatment does not increase cav-1 expression in early passage (cav-1 negative) LNCaP cells, a cav-1 expressing LNCaP variant, LNCaP (c+), which expresses low-moderate levels of cav-1, and DU145 and PC-3, which express high levels of cav-1 were used for this part of study (see Fig. 1A and the insert in Fig. 1B for the relative cav-1 levels in these three cell lines). Treatment with multiple GFs, including PDGF, NGF, FGF1, FGF2, EGF, TGF-β1, and VEGF, led to significantly increased cav-1 protein levels in LNCaP (c+) cells (1.5- to 3-fold) and slightly increased cav-1 protein levels in DU145 and PC-3 cells (Fig. 1A and 1B). Similar to the low cav-1 LNCaP (c+), a cav-1 antisense stable clone ABAC3 (13) also demonstrated induction of cav-1 expression and secrestion by GFs (Supplemental Fig. 1)
Figure 1.
Induction of cav-1 expression and secretion by growth factors. A. Representative western blot showing cellular levels of cav-1 in LNCaP (c+), DU145 and PC-3 cells after incubation of cells in SFM with and without the indicated GFs for 48 h. B. Quantitative analysis of cellular levels of cav-1 in GF treated LNCaP (c+), DU145 and PC-3 cells. The insert in B shows relative cav-1 levels in these 3 cell lines. C. Representative western blot showing secreted cav-1 in conditioned medium after incubation of LNCaP (c+), DU145 and PC-3 cells in SFM with and without indicated GFs for 24 h. The equivalents of conditioned medium produced from 5.0 × 105 cells were loaded on gel for the determination of secreted cav-1 levels. D. Quantitative analysis of levels of secreted cav-1 in conditioned medium derived from GF-treated LNCaP (c+), DU145 and PC-3 cells. In B and D, data are average of 3 independent experiments. Error bars stand for standard deviation and the symbol * represents statistically significant P< 0.05. Lanes 1, 9, and 17: SFM; lanes 2,10, and 18: PDGF; lanes 3, 11, and 19: NGF; lanes 4, 12, and 20: FGF1; lanes 5, 13, and 21: FGF2; lanes 6, 14, and 22: EGF; lanes 7,15, and 23: TGF-β1; lanes 8, 16, and 24: VEGF.
We previously found that tumor cell–secreted cav-1 stimulates cell survival and clonal growth and contributes to angiogenesis and metastasis (17, 18), so it was also of interest to determine whether these GFs can also increase cav-1 secretion. As shown in Figure 1C and 1D, with the exception of NGF, treatment with GFs also led to increased levels of secreted cav-1 in LNCaP (c+) cells (1.5- to 2.0-fold). Although GFs had only minor effects on the cav-1 expression in high cav-1 prostate cancer cells DU145 and PC-3, they significantly increased cav-1 secretion from these two cell lines (1.5–3.2-fold, Fig. 1C and 1D).
Cav-1 Expression Stimulates Expression of VEGF, TGF-β1, and FGF2
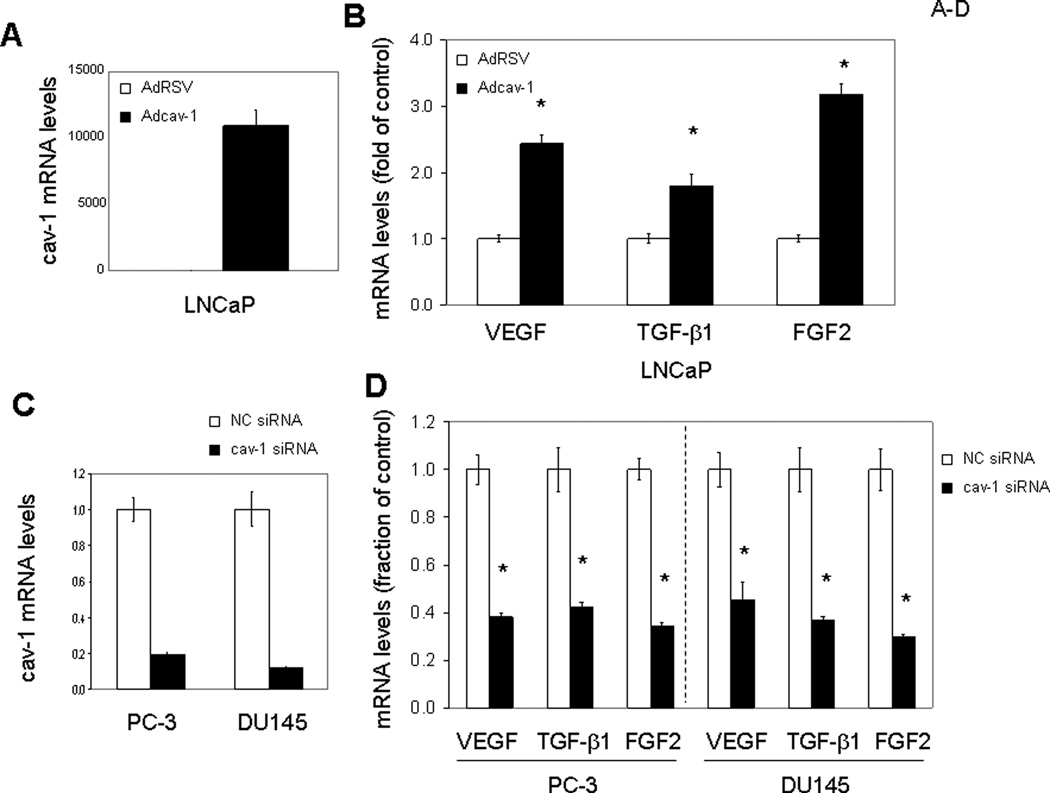
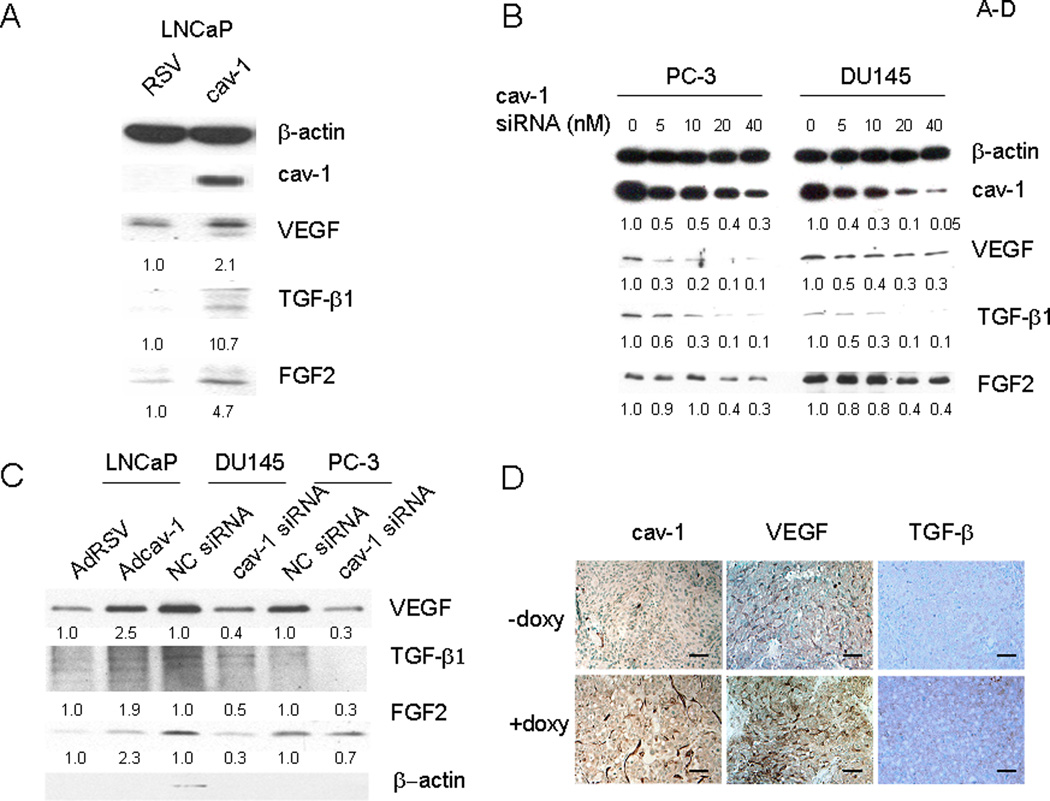
Since multiple GFs are capable of inducing cav-1 expression and cav-1 up-regulation occurs in focal areas of prostate cancer (10), we considered the possibility that cav-1 generates a positive-feedback, autoregulatory loop involving cav-1–stimulated GFs. We selected VEGF, TGF-β1, and FGF2 from the panel of GFs that stimulate cav-1 expression on the basis of their previously established importance in prostate cancer progression and focused on these specific GFs in subsequent experiments. As shown in Figure 2, overexpression of cav-1 in cav-1–negative, low-passage LNCaP prostate cancer cells using adenoviral vector–mediated gene transduction (Fig. 2A) led to significantly increased levels of VEGF, TGF-β1, and FGF2 mRNA (Fig. 2B) and protein (Fig. 3A). In contrast, when endogenous cav-1 in high–cav-1 PC-3 and DU145 prostate cancer cell lines was knocked down by cav-1 siRNA (Fig. 2C), FGF2, TGF-β1, and VEGF mRNA (Fig. 2D) and protein levels (Fig. 3B) were remarkably reduced. In addition to its stimulatory effect on the cellular levels of VEGF, TGF-β1, and FGF2, cav-1 promoted the secretion of these GFs into the medium (Fig. 3C), indicating that cav-1 and these cav-1–stimulated GFs can generate a positive autocrine loop.
Figure 2.
Cav-1 up-regulates mRNA levels of VEGF, TGF-β1, and FGF2. Quantitative RT-PCR analysis for mRNA levels of cav-1, VEGF, TGF-β1, and FGF2 in cav-1–manipulated prostate cancer cells. Error bars indicate SD. *: statistically significant, P< 0.05. A. mRNA levels of cav-1 in Adcav-1– and AdRSV-infected LNCaP cells. B. mRNA levels of VEGF, TGF-β1, and FGF2 in Adcav-1– and AdRSV-infected LNCaP cells. C. mRNA levels of cav-1 in cav-1 siRNA– or NC siRNA–transfected PC-3 and DU145 cells. D. mRNA levels of VEGF, TGF-β1, and FGF2 in cav-1 siRNA– or NC siRNA–transfected PC-3 and DU145 cells.
Figure 3.
Cav-1 up-regulates protein levels of VEGF, TGF-β1, and FGF2. A–C, Western blot analysis for protein levels of cav-1, VEGF, TGF-β1, and FGF2 in cav-1–manipulated prostate cancer cells. The numbers below protein bands are relative protein levels compared to corresponding controls. A. Adcav-1– or AdRSV-infected LNCaP cells. B. PC-3 and DU145 cells transfected with various concentrations of cav-1 siRNA or NC siRNA. C. Secreted VEGF, TGF-β1, and FGF2 in the conditioned medium of Adcav-1– and AdRSV-infected LNCaP cells and in that of cav-1 siRNA– and NC siRNA–transfected PC-3 and DU145 cells. The equivalents of conditioned medium produced from 5.0 × 105 cells were loaded onto the gel. D. Immunochemical analysis for cav-1, VEGF and TGF-β1 levels in doxycycline-induced or -uninduced LNTB25cav tumors. Bars in the figures= 40 µM.
To confirm the relationship between cav-1 and the expression of these cancer-promoting GFs in vivo, we used an LNCaP tet-on stable clone (LNTB25cav) to establish LNTB25cav subcutaneous xenografts as previously described (18). Immunochemical analysis of tumor tissues from the doxycycline + sucrose– and sucrose only–treated mice showed that the induction of cav-1 in the LNTB25cav xenografts resulted in increased expression of VEGF and TGF-β1 (Fig. 3D). Thus, our in vivo data validated our in vitro results that show increased VEGF and TGF-β1 levels in cav-1–overexpressing cancer cells. We did not include FGF2 immunochemical analysis of tumor tissues owing to low FGF2 protein level in the LNTB25cav tumors.
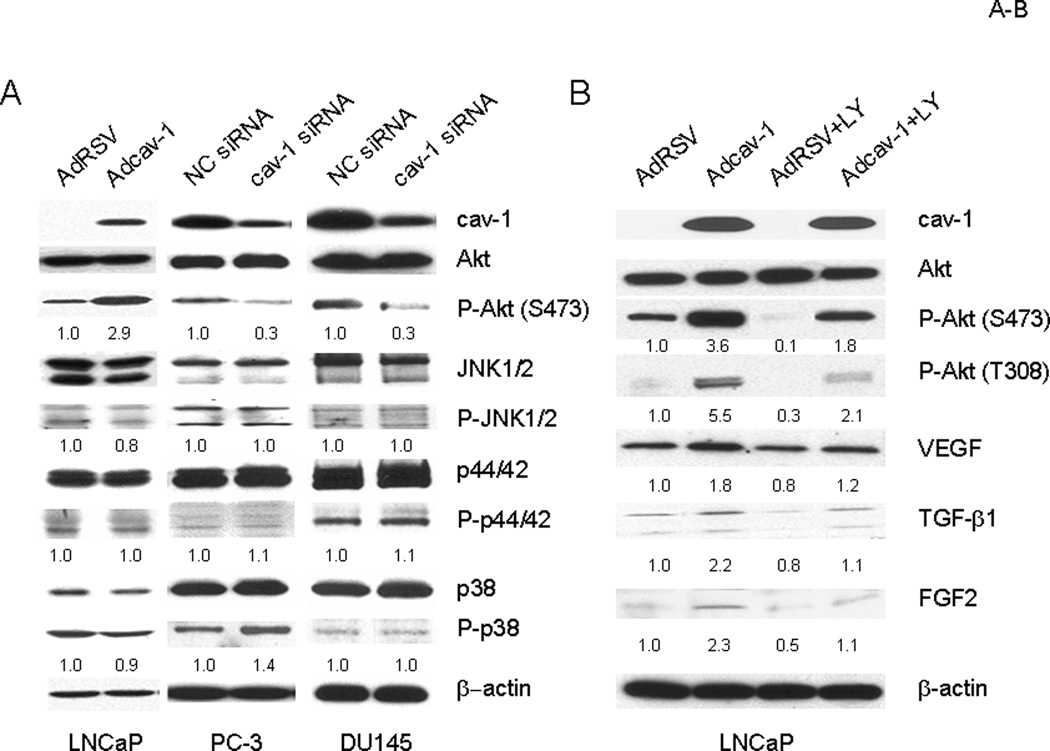
Akt Activation Is Involved in Cav-1–Mediated Up-Regulation of VEGF, TGF-β1, and FGF2
We reported previously that overexpression of cav-1 in cav-1–negative LNCaP cells through adenoviral vector–mediated gene transduction led to significantly increased levels of phosphorylated Akt, which in turn led to enhanced cancer cell survival (15). We wondered whether cav-1–mediated Akt activities are also associated with the expression of cancer-promoting GFs in prostate cancer cells. As we reported previously, overexpression of cav-1 in cav-1–negative LNCaP cells resulted in significantly increased levels of phosphorylated Akt, whereas the phosphorylation status of the JNK, p44/p42, and p38 pathways remained relatively unchanged (Fig. 4A). In contrast, suppression of endogenous cav-1 using cav-1–specific siRNA in the high–cav-1 prostate cancer cell lines PC-3 and DU145 reduced the levels of phosphorylated Akt whereas the activities of JNK, p44/42, and p38 were still relatively unchanged (Fig. 4B). To address whether Akt is involved in cav-1–mediated up-regulation of VEGF, TGF-β1, and FGF2, we suppressed Akt activities in LNCaP cells using the PI3-K inhibitor LY 3 h after the cells were transduced with cav-1–expressing adenoviral vector. The results demonstrated that the treatment with LY effectively inhibited cav-1–mediated Akt activation and largely if not completely eliminated cav-1–mediated up-regulation of VEGF, TGF-β1, and FGF2 (Fig. 4B). Note that LY also suppressed activity of endogenous Akt, leading to lower levels of VEGF, TGF-β1, and FGF2 (Fig. 4B, compared AdRSV+LY to AdRSV).
Figure 4.
Akt is required for the cav-1–induced up-regulation of VEGF, TGF-β1, and FGF2. A. Western blot analysis showing the increase of Akt phosphorylation in Adcav-1–infected LNCaP cells and the decrease of Akt phosphorylation in cav-1 siRNA–transfected PC-3 and DU145 cells. Note that JNK, p44/42, and p38 phosphorylation were relatively unchanged. B. PI3-K inhibitor LY294002 (LY) effectively blocked Akt activation and up-regulation of VEGF, TGF-β1, and FGF2 in Adcav-1–infected LNCaP cells. The numbers below protein bands are relative protein levels compared to corresponding controls.
Cav-1 Enhances Cancer Cell Motility in an Akt-Dependent Manner
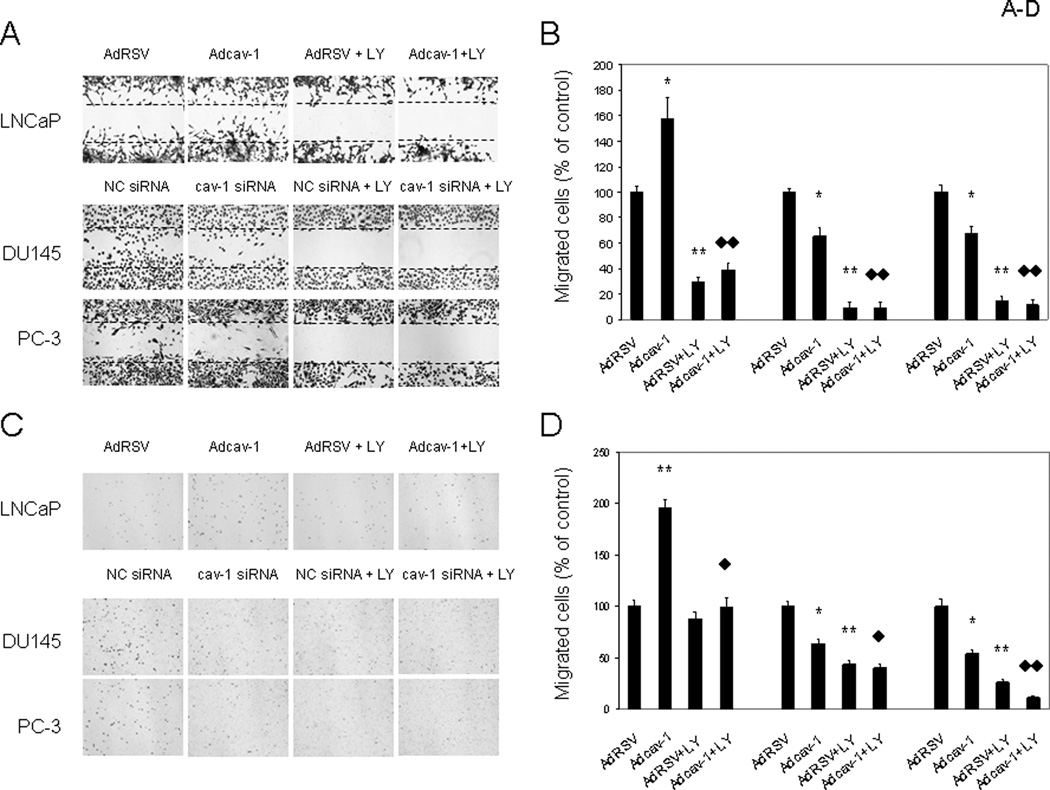
Cell survival and invasion are two important components of cancer progression. Previously we reported that endogenously expressed and/or secreted cav-1 enhances prostate cancer cell survival and promotes angiogenic activities of endothelial cells (15–18). In this study, we tested the effects of cav-1 on cancer cell motility. Using a wound-healing assay, we showed that Adcav-1–mediated overexpression of cav-1 in cav-1–negative LNCaP cells increased the number of cells that migrated into the cleared area ~60% compared with the number of AdRSV-infected LNCaP cells (Fig. 5A and 5B). Conversely, suppression of endogenous cav-1 with cav-1 siRNA in high–cav-1 DU145 and PC-3 prostate cancer cells reduced cell migration 30–40% compared with that in corresponding control cells that were transfected with NC siRNA (Fig. 5A and 5B). Treatment with LY effectively blocked cancer cell migration in Adcav-1–transduced LNCaP cells and in NC siRNA–transfected DU145 and PC-3 cells (Fig. 5A and 5B), demonstrating that the PI3-K-Akt pathway plays an important role in cav-1–mediated cancer cell migration (potentially through VEGF, TGF-β1, and FGF2).
Figure 5.
Cav-1 promotes cancer cell migration/motility. A and B. Wound healing assay. A. Representative images showing increased cell migration to the wounded area (clear area, defined by dashed lines) in Adcav-1–infected LNCaP cells and decreased cell migration to the wounded area in cav-1 siRNA–transfected DU145 and PC-3 cells. PI3-K-Akt inhibitor LY effectively blocked cancer cell migration. B. Quantitative analysis of triplicate wound healing assay experiments. C and D, Transwell chamber assays. C. Representative images showing increased transwell cell migration in Adcav-1–infected LNCaP cells and decreased migration in cav-1 siRNA–transfected DU145 and PC-3 cells. LY significantly reduced transwell cell migration. D. Quantitative analysis of triplicate Transwell migration assay experiments. In b and d, error bars indicate SD, * is used for the comparison to control AdRSV or NC siRNA, and ♦ is used for the comparison of Adcav-1 + LY with Adcav-1. * or ♦ stands for statistically significant (P < 0.05), and ** or ♦♦ stands for statistically very significant (P < 0.0001).
To extend our results on the role of cav-1 in prostate cancer cell migration, we performed Transwell chamber assays. The results showed that enforced expression of cav-1 in cav-1–negative LNCaP cells nearly doubled cancer cell migration through the chamber membrane relative to that of the empty-vector control cells (Fig 5C and 5D). In contrast, suppression of endogenous cav-1 in DU145 and PC-3 cells using cav-1 siRNA reduced cancer cell migration through the chamber membrane ~40–50% relative to that of the NC siRNA control cells (Fig. 5C and 5D). As it did in the wound-healing assay, treatment with LY effectively blocked cancer cell migration through the chamber membranes (Fig. 5C and 5D).
Cav-1 Up-Regulates VEGF, TGF-β1, and FGF2 through Akt-Mediated Maintenance of mRNA Stability
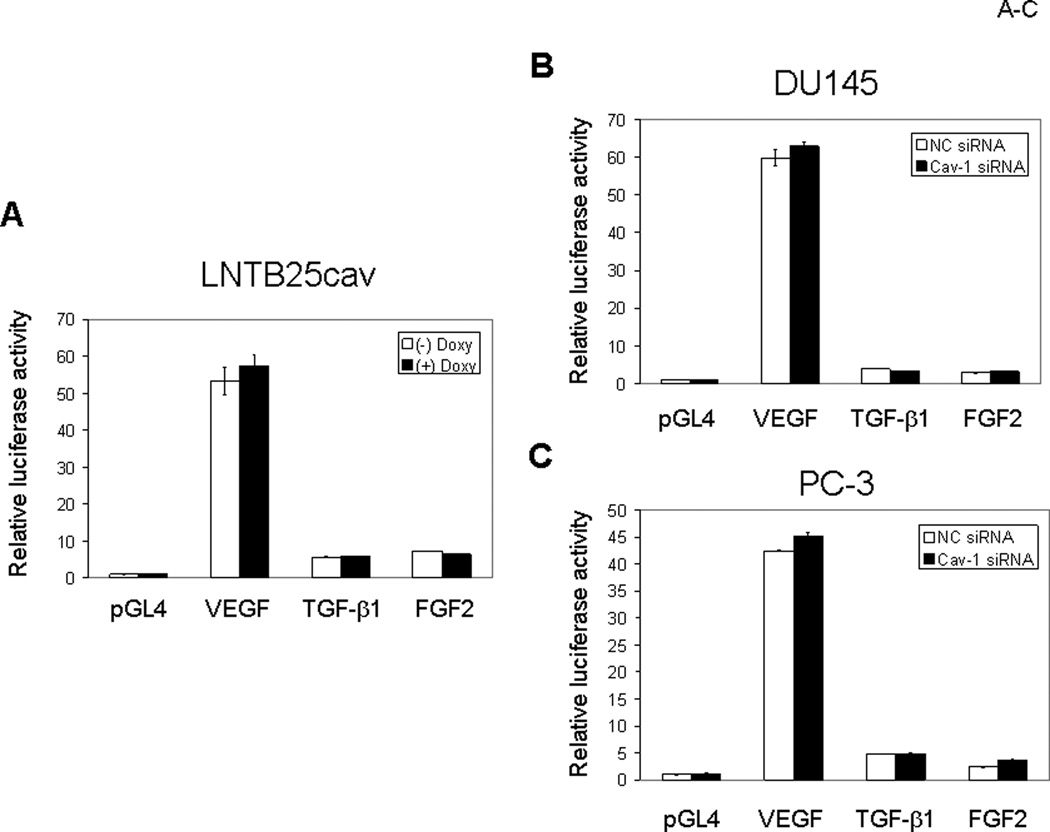
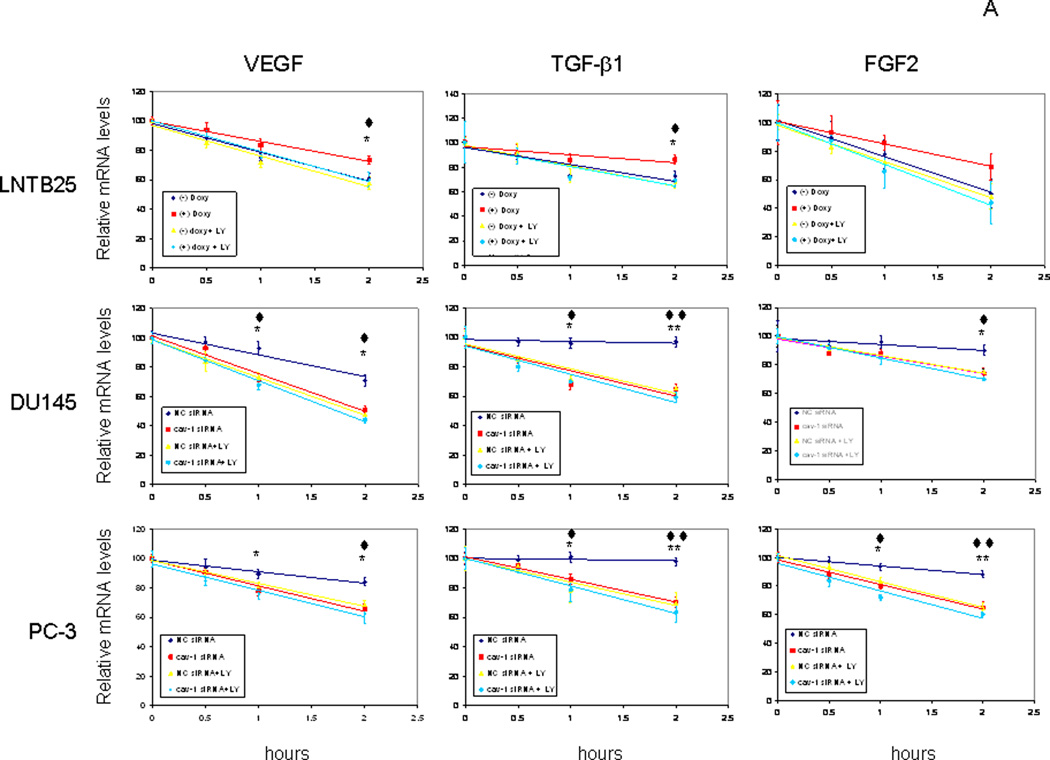
Regulation of GF expression is complex because it is controlled by steroid hormones, oncogenes, extracellular stresses, transcriptional factors, and GFs themselves. Mechanistically, GF regulation may occur at the transcriptional or posttranscriptional level. To gain insight into cav-1 stimulation of tumor-promoting GFs, we first examined the effect of cav-1 expression on VEGF, TGF-β1, and FGF2 promoter activities in cav-1–manipulated prostate cancer cells using a dual luciferase reporter assay system. We found, unexpectedly, that cav-1 did not alter the promoter activities of VEGF, TGF-β1, and FGF2, either in LNTB25cav cells when cav-1 expression was induced by doxycycline (Fig. 6A) or in high–cav-1 DU145 (Fig. 6B) and PC-3 (Fig. 6C) cells when cav-1 was suppressed by cav-1 siRNA. Given these results, we considered the possibility of cav-1 effects on mRNA stability of these GFs. A time-course study following the incubation of cells with the transcriptional inhibitor actinomycin revealed that induction of cav-1 expression in LNTB25cav cells significantly increased mRNA stabilities of VEGF and TGF-β1 and showed a clear trend of increased mRNA stability of FGF2 (Fig. 7A, upper panels). In contrast, suppression of cav-1 expression in high–cav-1 DU145 and PC-3 prostate cancer cells using cav-1 siRNA significantly reduced mRNA stability of VEGF, TGF-β1, and FGF2 (Fig. 7A, middle and lower panels). Thus, our data demonstrate that cav-1–mediated, enhanced mRNA stability is a major mechanism for the up-regulation of these cancer-promoting GFs. Importantly, our results also indicate that PI3-K-Akt signaling is required for cav-1–mediated, enhanced VEGF, TGF-β1, and FGF2 mRNA stability, since the PI3-K inhibitor LY abolished these cav-1-induced, or sustained activities in LNTB25cav cells (Fig. 7A, upper panels) or in high cav-1 DU145 and PC-3 (Fig. 7A middle and lower panels).
Figure 6.
Luciferase reporter assays for VEGF, TGF-β1, and FGF2 promoter activities. The GF-luc readings were normalized with Renilla luciferase readings generated from a cotransfected vector (pGL4.73) and expressed as fold of control (pGLA4, –doxy). A. LNTB25cav. B. DU145. C. PC-3. Error bars indicate SD.
Figure 7.
Cav-1 enhances mRNA stabilities of VEGF, TGF-β1, and FGF2. A. cav-1–manipulated LNTB25cav, DU145, and PC-3 cells were treated with 10 µg/mL of actinomycin in the presence and absence of LY for the indicated times, followed by quantitative RT-PCR analysis for mRNA levels of VEGF, TGF-β1, and FGF2. Error bars indicate SD. The data were fitted with linear trend lines, and the data from actinomycin treatment for 1 and 2 h were subjected to statistical analysis. * is used for the comparison to control (–) doxycycline (doxy) or NC siRNA, and ♦ is used for the comparison of (+) doxy + LY with (+) doxy or NC siRNA+LY with NC siRNA. * or ♦ indicates statistically significant (P < 0.05), and ** or ♦♦ indicates statistically very significant (P < 0.0001). B. diagram summarizes cav-1–GF autoregulatory loop. T = testosterone.
Together, our in vitro and in vivo data provide the first evidence to our knowledge that cav-1 increases levels of cancer-promoting GFs and enhances cancer progression through PI3-K-Akt–mediated maintenance of mRNA stability.
Discussion
Overexpression of cav-1 and cancer-promoting GFs is frequently observed in advanced prostate cancer and many other types of malignancies (4, 6, 23, 25, 28, 36, 37). In addition, increased levels of tissue and serum cav-1 and specific GFs have prognostic potential for prostate cancer progression (10, 22, 38–40). Previous studies suggested that cav-1 and cancer-promoting GFs may collaborate in the progression of prostate cancer, although evidence is lacking (12). We demonstrated in this study that multiple GFs, including VEGF, TGF-β1, and FGF2, can up-regulate cav-1 expression and secretion in prostate cancer cells, and cav-1 expression can, in turn, increase cellular levels and secretion of VEGF, TGF-β1, and FGF2. Our in vitro data together with LNTB25cav xenograft data support those of our previous in vivo studies, in which we found that cav-1–expressing tumors had significantly higher tumor weight and significantly increased microvessel density (18). In addition, our results using two complementary assays showed that cav-1 overexpression leads to enhanced prostate cancer cell migration. This new information, together with our previous data (15–18), demonstrates that up-regulated cav-1 expression can promote cancer cell survival, angiogenesis, and invasion, all critical factors for cancer progression.
We noted that there were some controversial observations regarding the effect of GFs on cav-1 expression in the literature. For example, earlier reports showed that VEGF and basic FGF reduced cav-1 expression in ECV304, a human umbilical vein endothelial cell line (41), and that chronic epidermal GF treatment resulted in transcriptional down-regulation of cav-1 in A431, a human epidermoid carcinoma cell line (42). The causes for such diverse observations remain unclear at this stage; however, context-dependent growth factor effects on cav-1 expression, including origin of cells, endogenous cav-1 levels, and concentrations of GFs used, could be a possible explanation.
We demonstrated, mechanistically, that cav-1 mediates up-regulation of VEGF, TGF-β1, and FGF2 and promotes cancer cell migration through Akt signaling. Furthermore, we showed that cav-1 up-regulates VEGF, TGF-β1, and FGF2 through Akt-dependent increased mRNA stability. We believe that these results provide the first evidence to our knowledge that cav-1 and specific cancer-promoting GFs form a positive autoregulatory feedback loop in which GFs up-regulate cav-1 expression and cav-1 expression, in turn, leads to increased mRNA stability of the same GFs, leading to the enhanced survival and invasive activities of prostate cancer cells.
The role of cav-1 in cancer is complex and continues to be somewhat controversial (4–7). However, a growing body of evidence indicates that cav-1 is up-regulated in many types of human cancer and in several multi–drug resistant and metastatic cancer cell lines. In many of these studies, cav-1 was shown to correlate with aggressive disease [reviewed in (4, 6, 7)]. Besides that gained from clinically based studies, substantial insight into the biological functions of cav-1 in prostate cancer progression has been gained from studies of genetically engineered mice. Genetic ablation of cav-1 impedes prostate tumor progression in TRAMP mice (14). Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation (43). Mouse and human prostate cancer cell–secreted cav-1 has proangiogenic activities (17, 18, 44). These findings clearly demonstrate an oncogenic role for cav-1 in prostate cancer.
We previously showed that cav-1 maintains activated Akt in prostate cancer cells through interaction with and inhibition of the serine/threonine protein phosphatases PP1 and PP2A (15). We also reported recently that tumor cell–secreted cav-1 can promote tumor angiogenesis through a PI3-K-Akt-eNOS–mediated mechanism (18). In this study, we found that PI3-K-Akt signaling is required for cav-1–mediated up-regulation of VEGF, TGF-β1, and FGF2 and for cav-1-enhanced prostate cancer cell migration in vitro. Although we did not show that cav-1 inhibition of PP1 and/or PP2A underlies Akt activation in these experiments, this mechanism seems likely. The results of this current study extend the role of cav-1–mediated Akt activation as a contributing pathway to prostate cancer.
The regulation of GFs is complex. In normal cells, the intricate balance of these GFs is controlled by coordinated regulation of hormones, transcriptional factors, and GFs themselves. In cancer and other malignant cells, however, this homeostasis is disrupted through various mechanisms. Inactivation or mutation of tumor suppressor genes (32, 45), activation of oncogenes (33), or overexpression of certain GFs (34) can impair the mRNA decay machinery, leading to aberrant GF mRNA accumulation. Akt, interestingly, may also stabilize AU-rich element (ARE)–containing transcripts by phosphorylation of butyrate response factor 1 (BRF1), preventing it from binding to those ARE-containing transcripts to facilitate deadenylation and rapid degradation of these transcripts (46, 47). We previously reported that cav-1 maintains activated Akt in prostate cancer cells (15). In this current study, we found that cav-1 promotes mRNA stability of VEGF, TGF-β1, and FGF2 and invasive activities of prostate cancer cells in an Akt-dependent manner. Taken together, the results of these studies suggest a cav-1 ⇒ Akt ⇒ GF signaling pathway. We speculate that Akt-mediated BRF1 activities are also involved in cav-1–induced VEGF, TGF-β1, and FGF2 mRNA stability.
We also found that PI3-K-Akt inhibitor LY could not only eliminate cav-1 mediated Akt activation and GF up-regulation, leading to the inhibition of cancer cell migration, but also suppress endogenous Akt in AdRSV transduced LNCaP cells (AdRSV+LY), cav-1 un-induced LNTB25cav, impairing cancer cell migration and reducing GF mRNA stability. In high cav-1 DU145 and PC-3 cells, LY further reduced cancer cell migration and GF mRNA stability in cav-1 siRNA transfected cells. Together, these data suggest: 1) Akt is required for cav-1 mediated maintenance of GF mRNA stability and cav-1 enhanced cancer cell motility; 2) Akt works downstream of cav-1 and therefore can facilitate maintenance of GF mRNA stability and enhance cancer cell motility through other molecular signaling events that activate Akt.
Importantly, the results of this study define a positive autoregulatory feedback mechanism (Fig. 7B) that could lead to sustained cav-1, VEGF, TGF-β1, and FGF2 expression and secretion in prostate cancer cells. Since we previously showed that prostate cancer cell–derived, secreted cav-1 can stimulate angiogenic activities in vitro and in vivo (18), it seems reasonable to assume that induction of VEGF, TGF-β1, and FGF2, which are potent angiogenic factors, would contribute to cav-1–mediated angiogenesis in prostate cancer. Together with cav-1–stimulated prostate cancer cell migration, these cav-1–induced growth and invasive activities likely have a profound effect on the malignant properties of prostate cancer cells. In addition, this cav-1–GF positive feedback loop has implications for identification of aggressive, virulent prostate cancer among a considerable fraction of prostate cancers that will not progress to clinical significance (48). Further, the autonomous nature of this positive feedback loop provides insight into the inexorable nature of prostate cancer progression.
In light of this new information, it becomes important to consider the events that lead to the cav-1–GF positive feedback loop in prostate cancer. Since we previously showed that cav-1 is induced by testosterone in prostate cancer cells (16), it is conceivable that cav-1 expression is initially up-regulated by testosterone. However, this possibility raises important questions, such as at what point in the development of prostate cancer does this occur? and what molecular events facilitate testosterone-mediated cav-1 expression? It is also possible that GF expression initiates cav-1 expression. In our early studies of cav-1, we showed that androgen-insensitive cav-1–positive prostate cancer can arise in the presence of physiologic levels of testosterone in mouse models of prostate cancer (9, 13). These results are consistent with a model that implicates testosterone as the initial stimulatory event, with subsequent cav-1–stimulated GF expression sustaining cav-1 expression (Fig. 7B). The eventual development of the cav-1–GF positive feedback loop suggests a plausible pathway for the development of androgen-insensitive prostate cancer. Since cav-1 expression is very low to undetectable in normal prostate epithelial cells, it seems likely that initiating oncogenic events are required to facilitate cav-1 expression.
Overall, the results of this study demonstrate that the cav-1–induced PI3-K-Akt–mediated increased mRNA stability of VEGF, TGF-β1, and FGF2 is a novel and important molecular mechanism by which cav-1 promotes cancer progression. Our results further define a cav-1–GF positive regulatory loop that could sustain many malignant properties in prostate cancer cells. Further studies are necessary to understand the biologic and clinical implications of this cav-1–mediated oncogenic pathway in prostate cancer.
Materials and Methods
Cell lines and Cell Culture Conditions
Human prostate cancer cell lines LNCaP, PC-3, DU145 and LNCaP (c+), a cav-1–expressing LNCaP variant that was obtained during propagation of LNCaP, were grown in RPMI 1640 medium with 10% fetal bovine serum. LNTB25cav, a cav-1–inducible clone generated from LNCaP, was grown in RPMI 1640 medium with 9% tet system–approved FBS.
Induction of Cav-1 Expression and Secretion by GFs
LNCaP (c+), DU145 and PC-3 cells were seeded in complete culture medium and grown overnight. After being rinsed once with serum-free medium (SFM), cells were incubated in SFM with or without a specific GF at the following concentrations: human GFs: platelet-derived GF (PDGF)-AB, 10 ng/mL (Invitrogen); never GF (NGF), 10 ng/mL (Prospec); FGF1, 5 ng/mL (Invitrogen); FGF2, 2 ng/mL (Invitrogen); epidermal GF (EGF), 5 ng/mL (Invitrogen); TGF-β1, 1 ng/mL (Invitrogen), and VEGF, 10 ng/mL (Upstate). Mouse GFs: PDGF-BB, 10 ng/mL (Invitrogen); NGF, 10 ng/mL (Invitrogen); FGF1, 5 ng/mL (R & D Systems); FGF2, 2 ng/mL (Invitrogen); EGF, 5 ng/mL (Invitrogen); and VEGF, 10 ng/mL (Invitrogen).
For determining cav-1 expression, cell lysates were prepared 48 h after treatment with the GFs. For determining secreted cav-1 levels, conditioned media were collected 24 h after treatment with the GFs. The conditioned media were then centrifuged once at 130 × g for 5 min to remove floating cells and once at 10,000 × g for 20 min to removed remaining insoluble materials. The resulting supernatants were concentrated 50 to 100 fold using Amicon Ultra-4 with 10-kDa cutoff. The equivalents of conditioned media produced from 5.0 × 105 cells were loaded on gel for the determination of secreted cav-1 levels.
Analysis of mRNA Expression
Total RNA from human prostate cancer cell lines was extracted with TRI Reagent Solution (Ambion). Reverse transcription (RT) was carried out with the High Capacity cDNA Archive Kit (Applied Biosystems). Polymerase chain reactions (PCR) were performed using the following Taqman probes and primers (Applied Biosystems): Hs00184697_m1 for cav-1, Hs99999905_m1 for GAPDH, Hs00173626_m1 for VEGF, Hs00171257_m1 for TGF-β1, and Hs00266645-m1 for FGF2. Real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). The relative quantity of a specific mRNA was determined by the ΔΔCT method and normalized to GAPDH or actin RNA in the same cDNA preparation.
Western Blot Analysis
Proteins in the samples were separated by 4–15% gradient SDS-PAGE and western blotting was performed according to standard procesures. Primary antibodies: rabbit polyclonal antibodies against cav-1, VEGF, TGF-β1, and FGF2 (Santa Cruz); monoclonal antibody to PKBα/Akt (clone 55) (BD Biosciences); rabbit polyclonal antibodies against phospho-Akt (ser473), phospho-Akt (Thr 308), p42/44, phosphor-p42/44, JNK, phospho-JNK, p38, and phosphor-p38 (Cell Signaling); and monoclonal antibody to β-actin (Sigma). Quantitative analysis were performed by measuring the density of each protein bands using a computer-assisted software (Nikon, NIS-Elements AR3.0) and by making calculations according to the following methods: for cell lysates, the protein bands of interest were first normalized by corresponding β–actin and each nomorlized value was then compared to control (control as 1); for conditioned medium, the protein bands of interest were compared to control (SFM).
Small Interfering RNA (siRNA) Transfection and Viral Infection
Cells were seeded at a density of 1.0 × 105 cells/well in 12-well plates or at equivalent cell density in other culture dish formats 1 day prior to transfection. Transfection was performed using siPORTAmine (Ambion) and cav-1–specific siRNA or negative control (NC) siRNA (Ambion). Adenovirus-mediated gene transduction was carried out as described previously (16).
Preparation of Conditioned Media for Analysis of Secreted GFs
The conditioned media for analysis of secreted GFs were prepared and concentrated as described for the preparation of secreted cav-1.
Wound Healing Assay
Twenty-four hours after gene transduction with adenoviral vectors (LNCaP) or transfection with siRNA (PC-3 or DU145), a straight longitudinal scratch was made on the monolayer of cells using a pipet tip. After removal of the existing medium, fresh RPMI 1640 complete medium with or without 20 µM PI3-K inhibitor LY294002 (LY; CalBiochem) was added, and the cells were incubated for 16 h (PC-3 and DU145) or 48 h (LNCaP). Cells were then stained with HEMA3 (Biochemical Sciences), and the numbers of cells migrating into the clear area were counted and imaged with a microscope using NIS-Elements AR2.30 software (Nikon).
Transwell Chamber Assay
Twenty-four hours after gene transduction with adenoviral vectors (LNCaP) or transfection with siRNA (PC-3 or DU145), the cells were trypsinized and single-cell suspensions were prepared. Five thousand cells were seeded into each Falcon cell culture insert (8.0 µm pore size, 24-well format; Becton Dickinson Labware) with 300 µL of serum-free RPMI 1640 medium with or without 20 µM LY and with 700 µL RPMI 1640 complete medium in the outer well. After 16- to 24-h incubation, the medium and cells inside the insert were carefully removed, and the cells that had migrated onto the outer membrane of the insert were stained, counted, and imaged as just described for the wound healing assay.
Generation of LNTB25cav Xenograft and Processing of Tumor Tissue
LNTB25cav xenograft was established as described previously (18), with 10 of the mice given 2 mg/mL of doxycycline in their drinking water containing 5% sucrose and 11 animals given drinking water containing only 5% sucrose as controls. After 21 d, the animals were euthanized, and the tumor tissues were harvested and fixed in 10% neutral formalin. The tissues were then processed for making 5-µm paraffin-embedded sections.
Immunohistochemistry
Immunostaining of paraffinized sections was conducted using polyclonal antibodies to cav-1, TGF-β1, or VEGF and an ABC kit (Vector Laboratories). The specificity of the immunoreactions was verified by using PBS to replace the specific primary antibodies.
Luciferase Reporter Assay
A 1.5 kb VEGF promoter containing Sp1 sites and an HIF-1αsite was obtained from genomic DNA by PCR using forward primer 5′ GGAGAAGTAGCCAAGGGAT 3′ and reverse primer 5′ CCTGTCGCTTTCGCTGCT 3′. The PCR fragment was digested with SacI and inserted into the SacI site of the pGL4 luciferase reporter vector (Promega). A 1,077 bp TGF-β1 promoter (CHR19_M0615_R1) and a 1,012 bp FGF2 promoter (CHR4-P0576-R1) were purchased from SwitchGear Genomics. LNTB25cav cells were seeded (2.5 × 105 cells/well, 12-well plate) and grown in the medium with or without 0.2 µg/mL doxycycline overnight and then were cotransfected with a specific GF-luc reporter vector or control vector pGL4 and a Renilla luciferase reporter vector (pGL4.73, Promega) using FuGENE HD transfection reagent (Roche). DU145 and PC-3 cells were seeded (1.0 × 105 cells/well, 12-well plate) and grown in the complete medium overnight and then were transfected with 50 nM cav-1 siRNA or NC siRNA using siPORT Amine. Three hours after siRNA transfection, cells were cotransfected with a specific GF-luc reporter vector or control vector pGL4 and the normalizer reporter vector as described above. Twenty-four hours after the reporter transfection, luciferase activity was determined using a dual-luciferase reporter assay system (Promega) and a Synergy 2 multi-mode microplate reader (BioTek). The GF-luc readings were normalized with Renilla luciferase readings and expressed relative to the control (pGLA4, doxycycline).
mRNA Stability Assay
Cav-1 expresion in LNTB25cav cells was induced with doxycycline, and DU145 or PC-3 cells were transfected with cav-1 siRNA or NC siRNA as described for the luciferase reporter assay. Twenty-four hours after induction with doxycycline or transfection of siRNA, cells were treated with 10 µg/mL of actinomycin in the presence or absence of the PI3-K inhibitor LY (20 µM) for 0, 0.5, 1 or 2 h. RNAs were prepared, and mRNA levels of cav-1 and each GF were determined by quantitative RT-PCR as described earlier.
Statistical Analysis
The unpaired t test was used in the experiments in which probability levels were determined. A P value < 0.05 (*) was considered statistically significant, and one < 0.0001 (**) was considered statistically very significant.
Supplementary Material
Acknowledgments
We are grateful to Karen Phillip and Park Sanghee for editorial assistance.
Grant support: This work was supported by grant R01-50588 from the National Cancer Institute and by by grant PC051247 from the Department of Defense.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277(44):41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 3.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 4.Mouraviev V, Li L, Tahir SA, et al. The role of caveolin-1 in androgen insensitive prostate cancer. J Urol. 2002;168(4 Pt 1):1589–1596. doi: 10.1016/S0022-5347(05)64526-0. [DOI] [PubMed] [Google Scholar]
- 5.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288(3):C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 6.Shatz M, Liscovitch M. Caveolin-1: A tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84(3):177–189. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- 7.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Timme TL, Frolov A, Wheeler TM, Thompson TC. Combined c-Myc and caveolin-1 expression in human prostate carcinoma predicts prostate carcinoma progression. Cancer. 2005;103(6):1186–1194. doi: 10.1002/cncr.20905. [DOI] [PubMed] [Google Scholar]
- 9.Yang G, Truong LD, Timme TL, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4(8):1873–1880. [PubMed] [Google Scholar]
- 10.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59(22):5719–5723. [PubMed] [Google Scholar]
- 11.Satoh T, Yang G, Egawa S, et al. Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer. 2003;97(5):1225–1233. doi: 10.1002/cncr.11198. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Addai J, Wheeler TM, et al. Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Hum Pathol. 2007;38(11):1688–1695. doi: 10.1016/j.humpath.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Nasu Y, Timme TL, Yang G, et al. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med. 1998;4(9):1062–1064. doi: 10.1038/2048. [DOI] [PubMed] [Google Scholar]
- 14.Williams TM, Hassan GS, Li J, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280(26):25134–25145. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23(24):9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Yang G, Ebara S, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61(11):4386–4392. [PubMed] [Google Scholar]
- 17.Tahir SA, Yang G, Ebara S, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61(10):3882–3885. [PubMed] [Google Scholar]
- 18.Tahir SA, Yang G, Goltsov AA, et al. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68(3):731–739. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 19.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18(7):1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 20.Landriscina M, Cassano A, Ratto C, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78(6):765–770. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito N, Kawata S, Tamura S, et al. Elevated levels of transforming growth factor beta messenger RNA and its polypeptide in human hepatocellular carcinoma. Cancer Res. 1991;51(15):4080–4083. [PubMed] [Google Scholar]
- 22.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 24.Breen EC. VEGF in biological control. J Cell Biochem. 2007;102(6):1358–1367. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 25.Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur J Cancer. 2005;41(6):846–857. doi: 10.1016/j.ejca.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31(1):61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Cronauer MV, Schulz WA, Seifert HH, Ackermann R, Burchardt M. Fibroblast growth factors and their receptors in urological cancers: basic research and clinical implications. Eur Urol. 2003;43(3):309–319. doi: 10.1016/s0302-2838(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 28.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11(4):709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 29.Josko J, Mazurek M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med Sci Monit. 2004;10(4):RA89–RA98. [PubMed] [Google Scholar]
- 30.Koos RD, Kazi AA, Roberson MS, Jones JM. New insight into the transcriptional regulation of vascular endothelial growth factor expression in the endometrium by estrogen and relaxin. Ann N Y Acad Sci. 2005;1041:233–247. doi: 10.1196/annals.1282.037. [DOI] [PubMed] [Google Scholar]
- 31.Buck MB, Knabbe C. TGF-beta signaling in breast cancer. Ann N Y Acad Sci. 2006;1089:119–126. doi: 10.1196/annals.1386.024. [DOI] [PubMed] [Google Scholar]
- 32.Cash J, Korchnak A, Gorman J, Tandon Y, Fraizer G. VEGF transcription and mRNA stability are altered by WT1 not DDS(R384W) expression in LNCaP cells. Oncol Rep. 2007;17(6):1413–1419. [PubMed] [Google Scholar]
- 33.Kanies CL, Smith JJ, Kis C, et al. Oncogenic Ras and transforming growth factor-beta synergistically regulate AU-rich element-containing mRNAs during epithelial to mesenchymal transition. Mol Cancer Res. 2008;6(7):1124–136. doi: 10.1158/1541-7786.MCR-07-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song QH, Klepeis VE, Nugent MA, Trinkaus-Randall V. TGF-beta1 regulates TGF-beta1 and FGF-2 mRNA expression during fibroblast wound healing. Mol Pathol. 2002;55(3):164–176. doi: 10.1136/mp.55.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touriol C, Morillon A, Gensac MC, Prats H, Prats AC. Expression of human fibroblast growth factor 2 mRNA is post-transcriptionally controlled by a unique destabilizing element present in the 3'-untranslated region between alternative polyadenylation sites. J Biol Chem. 1999;274(30):21402–21408. doi: 10.1074/jbc.274.30.21402. [DOI] [PubMed] [Google Scholar]
- 36.Juhasz M, Chen J, Tulassay Z, Malfertheiner P, Ebert MP. Expression of caveolin-1 in gastrointestinal and extraintestinal cancers. J Cancer Res Clin Oncol. 2003;129(9):493–497. doi: 10.1007/s00432-003-0468-0. [DOI] [PubMed] [Google Scholar]
- 37.Podar K, Anderson KC. Caveolin-1 as a potential new therapeutic target in multiple myeloma. Cancer Lett. 2006;233(1):10–15. doi: 10.1016/j.canlet.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 38.El-Gohary YM, Silverman JF, Olson PR, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127(4):572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 39.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Expression of bFGF/FGFR-1 and vascular proliferation related to clinicopathologic features and tumor progress in localized prostate cancer. Virchows Arch. 2006;448(1):68–74. doi: 10.1007/s00428-005-0075-3. [DOI] [PubMed] [Google Scholar]
- 40.Tahir SA, Frolov A, Hayes TG, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res. 2006;12(16):4872–4875. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Razani B, Tang S, Terman BI, Ware JA, Lisanti MP. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. Angiogenesis inhibitors block vascular endothelial growth factor-induced down-regulation of caveolin-1. J Biol Chem. 1999;274(22):15781–15785. doi: 10.1074/jbc.274.22.15781. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4(6):499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 43.Yang G, Timme TL, Naruishi K, et al. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp Mol Pathol. 2008;84(2):131–140. doi: 10.1016/j.yexmp.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Bartz R, Zhou J, Hsieh JT, Ying Y, Li W, Liu P. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2007 doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 45.Datta K, Mondal S, Sinha S, et al. Role of elongin-binding domain of von Hippel Lindau gene product on HuR-mediated VPF/VEGF mRNA stability in renal cell carcinoma. Oncogene. 2005;24(53):7850–7858. doi: 10.1038/sj.onc.1208912. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin D, Schmidlin M, Min L, Gross B, Moroni C. BRF1 protein turnover and mRNA decay activity are regulated by protein kinase B at the same phosphorylation sites. Mol Cell Biol. 2006;26(24):9497–9507. doi: 10.1128/MCB.01099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidlin M, Lu M, Leuenberger SA, et al. The ARE-dependent mRNA-destabilizing activity of BRF1 is regulated by protein kinase B. Embo J. 2004;23(24):4760–4769. doi: 10.1038/sj.emboj.7600477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dall'Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112(8):1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.