Abstract
We determined the frequency of multidrug resistant (MDR) infections with Shigella spp. and Vibrio cholerae O1 at an urban (Dhaka) and rural (Matlab) hospital in Bangladesh. We also compared sociodemographic and clinical features of patients with MDR infections to those with antibiotic-susceptible infections at both sites. Analyses were conducted using surveillance data from the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), for the years 2000–2012. Compared to patients with antibiotic-susceptible for Shigella infections, those in Dhaka with MDR shigellosis were more likely to experience diarrhea for >24 hours, while, in Matlab, they were more likely to stay inhospital >24 hours. For MDR shigellosis, Dhaka patients were more likely than those in Matlab to have dehydration, stool frequency >10/day, and diarrheal duration >24 hours. Patients with MDR Vibrio cholerae O1 infections in Dhaka were more likely than those in Matlab to experience dehydration and stool frequency >10/day. Thus, patients with MDR shigellosis and Vibrio cholerae O1 infection exhibited features suggesting more severe illness than those with antibiotic-susceptible infections. Moreover, Dhaka patients with MDR shigellosis and Vibrio cholerae O1 infections exhibited features indicating more severe illness than patients in Matlab.
1. Introduction
Shigella and Vibrio cholerae O1 are widely recognized causes of dysentery and acute watery diarrhea, respectively [1, 2]. Both have been responsible for producing epidemics [3] and often require antibiotic treatment to mitigate the severity of disease [4–6]. For Shigella infections, in particular, increasing antibiotic resistance has led to fewer antibiotics capable of producing bacteriostatic or bactericidal minimum inhibitory concentrations (MICs) [5, 7]. The challenge is compounded by the fact that while the regional prevalence of infection may be similar, rates of antibiotic resistance may differ substantially from one nation to another [5, 8].
Since 1979 and 2000, the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), has tested a systematic random sample of patients seeking care at an urban Dhaka Hospital and rural Matlab Treatment Centre for a spectrum of diarrhea-causing pathogens, including Shigella and Vibrio cholerae O1 [9]. Antimicrobial susceptibility patterns for these two pathogens are determined to inform clinicians about appropriate antibiotic treatment options [10]. Underscoring the need for antimicrobial sensitivity testing is the emergence of multidrug resistance (MDR), defined here as isolates resistant to ≥3 drugs [11–13]. Infections due to MDR strains are important not only because they are more difficult to treat but also because they may lead to higher fatality rates [14, 15]. Resource constraints in developing nations such as Bangladesh have limited the amount of information about the clinical features of MDR Shigella and Vibrio cholerae O1 infections. To address this gap, we determined the proportion of patients exhibiting MDR Shigella or Vibrio cholerae O1 infections at icddr,b from 2000 to 2012. We also studied sociodemographic and clinical features of MDR infections compared to antibiotic-susceptible infections, and compared these features in patients with MDR infections treated at the urban (Dhaka) and rural (Matlab) hospitals.
2. Materials and Methods
2.1. Study Sites
2.1.1. Dhaka Hospital
Dhaka Hospital is located in the capital of Bangladesh. The hospital was established in 1962 by icddr,b, and currently provides free care and treatment to around 140,000 patients each year. The Diarrheal Disease Surveillance System (DDSS), approved by the Research Review Committee and Ethical Review Committee, has operated at icddr,b since 1979 to collect data on patient populations. From 1979 to 1995, microbiologic tests for a spectrum of diarrheal etiologies were conducted on a systematic sample of 4% of patients who attended icddr,b, whereas, since 1996, 2% of patients have been sampled to account for a near-doubling in the number of patients seeking care at icddr,b. A structured questionnaire is used to collect information on clinical, epidemiological, and demographic characteristics of patients, the feeding practices of infants and young children, and the use of drug and fluid therapy at home.
2.1.2. Matlab Hospital
Since 1963, icddr,b has maintained a facility in rural Matlab, located about 55 km from Dhaka, for treating patients with diarrhea in the region. Each year, the facility provides free treatment to 20,000 patients with diarrhea. At Matlab, unlike at the Dhaka facility, every patient with diarrhea is screened for the spectrum of diarrheal pathogens assessed as part of the DDSS.
2.2. Study Sample
Clinical and epidemiologic details were abstracted from the electronic data archive of DDSS for patients with diarrhea treated at icddr,b facilities in Dhaka and Matlab from 2000 to 2012. We compared sociodemographic and clinical features of patients from whom MDR Shigella spp. or Vibrio cholerae O1 was recovered with patients whose diarrheal stools yielded susceptible strains of the respective pathogens. Figure 1 illustrates the sampling frame for the study.
Figure 1.
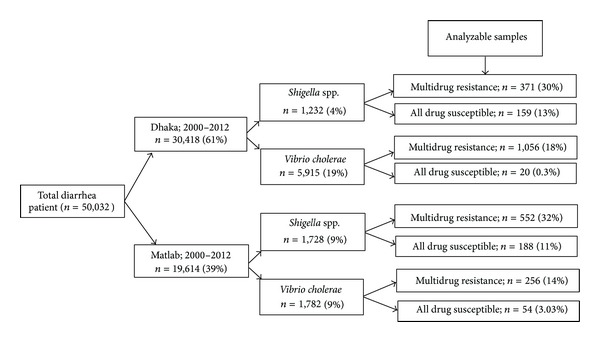
Sampling frame, testing of patients with diarrheal disease for multidrug resistant infections due to Shigella spp. or Vibrio cholerae O1, International Centre for Diarrhoeal Disease Research (icddr,b), Bangladesh, 2000–2012.
2.3. Laboratory Methodology
Fresh whole stool specimens collected from patients were examined at either the central icddr,b laboratory in Dhaka or at the Matlab clinical laboratory. Using standard laboratory methods described elsewhere [16, 17], each specimen was screened for common enteric pathogens, including Shigella spp. and Vibrio cholerae O1.
Bacterial susceptibility to antimicrobial agents was determined by the disk diffusion method as recommended by the Clinical Laboratory Standards Institute (CLSI 2010, June update) with commercial antimicrobial discs (Oxoid, Basingstoke, UK) [18]. The antibiotic discs used in this study for Shigella spp. included ampicillin (10 μg), mecillinam (25 μg), nalidixic acid (30 μg), trimethoprim-sulfamethoxazole, (25 μg), and ciprofloxacin (5 μg); and for V. cholerae O1: tetracycline (30 μg), trimethoprim-sulfamethoxazole (25 μg), erythromycin (15 μg), ciprofloxacin (5 μg), and azithromycin (15 μg) (Dhaka only) [18].
2.4. Data Analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) Windows (Version 15.2; Chicago, IL) and Epi Info (Version 6.0, USD, Stone Mountain, GA). We compared differences in proportions using the Chi-square test. A probability value (P value) of <0.05 was considered to confer statistical significance. Magnitudes of association were determined by estimating odds ratios (OR) and 95% confidence intervals (CI). To identify MDR strains, we determined the frequency with which isolates exhibited resistance to ≥3 antibiotics. We first compared sociodemographic features of patients with MDR infections versus those with susceptible infections. Next, focusing solely on MDR infections, we determined whether patients treated in Dhaka differed from those in Matlab in terms of sociodemographic or clinical features. All statistically significant differences ascertained by univariate analysis were entered into a logistic regression model to calculate adjusted odds ratios, P values, and 95% confidence intervals.
3. Results
3.1. Shigella spp
From 2000 to 2012, the number of patients who yielded MDR Shigella spp. in Dhaka and Matlab was 371/1,232 (30%) and 552/1,728 (32%), respectively (Figure 1). S. flexneri was recovered from a greater proportion of patients in Matlab compared to Dhaka, whereas S. boydii was recovered from a greater proportion of patients in Dhaka versus Matlab (Table 1). Resistance to ampicillin + nalidixic acid + trimethoprim-sulfamethoxazole was the most common resistance pattern for patients treated at both Dhaka and Matlab (Table 2). In Dhaka, the percentages of isolates resistant to individual antibiotics were trimethoprim-sulfamethoxazole (94%), ampicillin (85%), nalidixic acid (91%), mecillinam (25%), and ciprofloxacin (31%). In Matlab, the percentages of isolates resistant to individual antibiotics were nalidixic acid (96%), trimethoprim-sulfamethoxazole (92%), ampicillin (87%), ciprofloxacin (32%), and mecillinam (12%).
Table 1.
Multidrug resistant (MDR) Shigella species recovered from patients in Dhaka and Matlab, International Centre for Diarrhoeal Disease Research (icddr,b), Bangladesh, 2000–2012.
| Shigella spp. | Dhaka; n = 371 (%) | Matlab, n = 552 (%) | OR (95% CI) P value |
|---|---|---|---|
| Shigella flexneri | 257 (69) | 495 (90) | 0.26 (0.18, 0.37) <0.001 |
| Shigella boydii | 68 (18) | 33 (6) | 0.28 (0.18, 0.45) <0.001 |
| Shigella sonnei | 22 (6) | 17 (3) | 1.98 (0.99, 3.97) 0.051 |
| Shigella dysenteriae 1 | 4 (1) | 1 (0.2) | 6.01 (0.63, 141.61) 0.016 |
| Shigella dysenteriae | 20 (5) | 6 (1) | 5.19 (1.95, 14.56) <0.001 |
Table 2.
Multidrug resistant Shigella spp. and Vibrio cholerae O1 recovered from patients with diarrhea, Dhaka and Matlab, International Centre for Diarrhoeal Research Bangladesh (icddr,b), 2000–2012.
| Antimicrobials | Shigella spp. | Vibrio cholerae | ||||
|---|---|---|---|---|---|---|
| Dhaka; n = 371 (%) | Matlab; n = 552 (%) | OR (95% CI) P | Dhaka; n = 1056 (%) |
Matlab; n = 256 (%) |
OR (95% CI) P | |
| AMP + NAL + TMST | 203 (55) | 301 (56) | 0.94 (0.72, 1.24) 0.715 | — | — | — |
| AMP + NAL + MEC | 4 (1) | 19 (3) | 0.31 (0.09, 0.96) 0.040 | — | — | — |
| AMP + NAL + CIP | 1 (0.3) | 20 (4) | 0.07 (0.00, 0.51) <0.001 | — | — | — |
| AMP + TMST + MEC | — | 21 (4) | — | — | — | — |
| AMP + TMST + CIP | 18 (5) | 1 (0.2) | 28.10 (3.96, 567.52) <0.001 | — | — | — |
| AMP + CIP + MEC | 24 (7) | — | — | — | — | — |
| AMP + TMST + AZI | 5 (1) | — | — | — | — | — |
| AMP + CIP + AZI | 2 (0.5) | — | — | — | — | — |
| AMP + MEC + AZI | 2 (0.5) | — | — | — | — | — |
| TMST + CIP + AZI | 3 (1) | — | — | — | — | — |
| TMST + MEC + AZI | 2 (0.3) | — | — | — | — | — |
| CIP + MEC + AZI | 1 (0.3) | — | — | — | — | — |
| NAL + CIP + MEC | — | 20 (4) | — | — | — | — |
| NAL + TMST + MEC | 4 (1) | 1 (0.2) | 6.01 (0.63, 141.61) 0.163 | — | — | — |
| NAL + TMST + CIP | 22 (6) | 67 (12) | 0.48 (0.28, 0.82) 0.005 | — | — | — |
| TMST + CIP + MEC | 3 (1) | 3 (1) | 1.49 (0.24, 9.27) 0.689 | — | — | — |
| TET + TMST + FUR | — | — | — | 103 (10) | 89 (35) | 0.20 (0.14, 0.29) <0.001 |
| TET + FUR + ERY | — | — | — | — | 1 (0.4) | — |
| TMST + FUR + ERY | — | — | — | 7 (1) | 17 (7) | 0.09 (0.03, 0.24) <0.001 |
| TMST + FUR + CIP | — | — | — | — | — | — |
| TET + TMST + ERY | — | — | — | 38 (4) | 148 (58) | 0.03 (0.02, 0.04) <0.001 |
| AMP + TMST + CIP + AZI | 7 (2) | — | — | — | — | — |
| AMP + TMST + MEC + AZI | 2 (1) | — | — | — | — | — |
| AMP + CIP + MEC + AZI | 1 (0.3) | — | — | — | — | — |
| AMP + CIP + NAL + AZI | 1 (0.3) | — | — | — | — | — |
| AMP + NAL + TMST + CIP | 19 (5) | 67 (12) | 0.39 (0.22, 0.68) <0.001 | — | — | — |
| AMP + NAL + TMST + MEC | 3 (1) | — | — | — | — | — |
| AMP + NAL + CIP + MEC | 3 (1) | 4 (1) | 1.12 (0.20, 5.92) 1.000 | — | — | — |
| AMP + CIP + TMST + MEC | 9 (3) | — | — | — | — | — |
| TMST + NAL + CIP + MEC | 1 (0.3) | 3 (1) | 0.49 (0.02, 5.32) 0.652 | — | — | — |
| TMST + TET + ERY + FUR | — | — | — | 906 (86) | — | — |
| TMST + ERY + CIP + FUR | — | — | — | 1 (0.1) | — | — |
| TMST + TET + ERY + AZI | — | — | — | 1 (0.1) | — | — |
| AMP + TMST + NAL + CIP + MEC | 24 (7) | 16 (3) | 2.32 (1.16, 4.64) 0.014 | — | — | — |
| AMP + TMST + CIP + MEC + AZI | 7 (1) | — | — | — | — | — |
| FUR + ERY + CIP + TMST + TET | — | — | — | — | 1 (0.4) | — |
AMP: Ampicillin; AZI: azithromycin; CIP: ciprofloxacin; ERY: erythromycin; FUR: furazolidin; MEC: mecillinam; NAL: nalidixic acid; TET: tetracycline; TMST: trimethoprim-sulfamethoxazole.
In Dhaka, compared to patients with antibiotic-susceptible Shigella infections, those with MDR infections were more likely be male and to experience diarrhea for >24 hours (Table 3). In Matlab, patients with MDR Shigella infections were more likely than those with susceptible infections to stay in hospital >24 hours and to report having used antimicrobials at home prior to coming to hospital (Table 4). For MDR Shigella infections, patients in Dhaka were more likely than those in Matlab to be male and to have dehydration, frequency of stools >10/day, and duration of diarrhea >24 hours (Table 5).
Table 3.
Sociodemographic and clinical factors among patients with shigellosis, by drug resistance status, Dhaka, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), 2000–2012.
| Indicators | Multidrug resistant n = 371 (%) | Susceptible n = 159 (%) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Male sex | 225 (61) | 111 (70) | 6.72 (3.25, 14.23)* | 1.58 (1.02, 2.43)* |
| Monthly family income ≥100 USD | 345 (93) | 147 (93) | 1.08 (0.50, 2.31) | — |
| Slum residence | 33 (9) | 19 (12) | 0.72 (0.38, 1.37) | — |
| Nonsanitary latrine | 133 (36) | 61 (38) | 0.90 (0.60, 1.34) | — |
| Not treated water | 249 (67) | 103 (65) | 1.11 (0.74, 1.67) | — |
| Vomiting | 243 (66) | 120 (76) | 0.62 (0.40, 0.96)* | 0.84 (0.52, 1.35) |
| Abdominal pain | 206 (56) | 76 (48) | 1.36 (0.92, 2.01) | — |
| Fever (≥38°C) | 40 (11) | 7 (4) | 2.62 (1.10, 6.57)* | 2.18 (0.93, 5.12) |
| Bloody or mucoid stool | 165 (45) | 63 (40) | 1.22 (0.82, 1.81) | — |
| Frequency of stool (>10/day) | 189 (51) | 75 (47) | 1.16 (0.79, 1.72) | — |
| Duration of diarrhea (>24 hours) | 262 (71) | 87 (55) | 1.99 (1.33, 2.97)* | 1.73 (1.11, 2.69)* |
| Duration of stay in hospital >24 hrs | 134 (37) | 54 (35) | 1.09 (0.72, 1.64) | — |
| Some or severe dehydration | 211 (57) | 84 (53) | 1.18 (0.80, 1.74) | — |
| Use of intravenous saline for rehydration | 77 (21) | 27 (17) | 1.28 (0.77, 2.14) | — |
| Use of antimicrobials at home | 251 (68) | 91 (57) | 1.56 (1.05, 2.33)* | 1.20 (0.78, 1.86) |
| Red blood cell (1 to >50) | 241 (69) | 76 (52) | 2.07 (1.37, 3.13)* | 0.77 (0.33, 1.83) |
| Faecal leukocyte (11 to >50) | 272 (78) | 88 (60) | 2.34 (1.51, 3.62)* | 1.62 (0.81, 3.24) |
| Macrophage (1 to 10) | 219 (63) | 62 (42) | 2.29 (1.52, 3.46)* | 0.92, 4.08) |
*P < 0.05.
aOR: adjusted odds ratio.
Table 4.
Sociodemographic and clinical factors among patients with shigellosis, by drug resistance status, Matlab, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), 2000–2012.
| Indicators | Multidrug resistant n = 552 (%) | Susceptible n = 188 (%) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Male sex | 297 (54) | 95 (51) | 1.14 (0.81, 1.61) | — |
| Monthly family income ≥100 USD | 257 (47) | 79 (42) | 1.20 (0.85, 1.70) | — |
| Nonsanitary latrine | 486 (88) | 165 (88) | 1.03 (0.60, 1.75) | — |
| Not treated water | 544 (99) | 186 (99) | 0.73 (0.11, 3.75) | — |
| Vomiting | 273 (50) | 99 (53) | 0.88 (0.62, 1.24) | — |
| Abdominal pain | 370 (67) | 130 (69) | 0.91 (0.62, 1.32) | — |
| Fever (≥38°C) | 132 (24) | 51 (27) | 0.84 (0.57, 1.25) | — |
| Bloody or mucoid stool | 425 (77) | 126 (67) | 1.65 (1.13, 2.40)* | 1.23 (0.80, 1.87) |
| Frequency of stool (>10/day) | 230 (42) | 77 (41) | 1.03 (0.73, 1.46) | — |
| Duration of diarrhea (>24 hours) | 373 (68) | 107 (57) | 1.58 (1.11, 2.25)* | 1.35 (0.92, 1.99) |
| Duration of stay in hospital >24 hrs | 216 (40) | 57 (31) | 1.51 (1.04, 2.18)* | 1.79 (1.23, 2.62)* |
| Some or severe dehydration | 108 (20) | 53 (28) | 0.62 (0.42, 0.92)* | 0.69 (0.45, 1.06) |
| Use of intravenous saline for rehydration | 24 (4) | 9 (5) | 0.90 (0.39, 2.14) | — |
| Use of antimicrobials at home | 33 (60) | 88 (47) | 1.73 (1.22, 2.45)* | 1.48 (1.03, 2.12)* |
| Red blood cell (1 to >50) | 509 (93) | 154 (82) | 2.80 (1.65, 4.73)* | 2.12 (0.85, 5.43) |
| Faecal leukocyte (11 to >50) | 527 (96) | 166 (89) | 3.17 (1.62, 6.22)* | 1.78 (0.66, 4.85) |
| Macrophage (1 to 10) | 451 (82) | 136 (73) | 1.74 (1.16, 2.62)* | 0.92 (0.51, 1.65) |
*P < 0.05.
aOR: adjusted odds ratio.
Table 5.
Sociodemographic and clinical features of patients with multidrug resistant Shigella infections in Dhaka compared to those in Matlab, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), 2000–2012.
| Indicators | OR (95% CI) | aOR (95% CI) |
|---|---|---|
| Male sex | 1.24 (0.94, 1.64) | 1.52 (1.08, 2.16)* |
| Vomiting | 1.94 (1.47, 2.57)* | 1.26 (0.87, 1.82) |
| Abdominal pain | 0.61 (0.46, 0.81)* | 0.87 (0.59, 1.28) |
| Fever (≥38°C) | 0.38 (0.26, 0.57)* | 0.39 (0.25, 0.65)* |
| Bloody or mucoid stool | 0.24 (0.18, 0.32)* | 0.39 (0.26, 0.62)* |
| Frequency of stool (>10/day) | 1.45 (1.11, 1.91)* | 1.83 (1.28, 2.59)* |
| Duration of diarrhea (>24 hours) | 1.15 (0.86, 1.55) | 1.64 (1.07, 2.52)* |
| Duration of stay in hospital >24 hrs | 0.90 (0.68, 1.19) | 0.84 (0.58, 1.20) |
| Some or severe dehydration | 5.42 (4.00, 7.36)* | 5.61 (3.75, 8.39)* |
| Use of intravenous saline for rehydration | 5.80 (3.51, 9.66)* | 1.53 (0.79, 2.94) |
| Use of antimicrobials at home | 1.38 (1.03, 1.83)* | 1.42 (0.97, 2.08) |
| Red blood cell (1 to >50) | 0.17 (0.11, 0.26)* | 0.21 (0.08, 0.52)* |
| Faecal leukocyte (11 to >50) | 0.14 (0.08, 0.24)* | 0.42 (0.17, 1.07) |
| Macrophage (1 to 10) | 0.36 (0.26, 0.50)* | 1.81 (0.98, 3.35) |
*P < 0.05.
aOR: adjusted odds ratio.
3.2. Vibrio cholerae O1
From 2000 to 2012, the number of patients who yielded MDR Vibrio cholerae O1 in Dhaka and Matlab was 1,056/5,915 (18%) and 256/1,782 (15%), respectively (Figure 1). In Dhaka, resistance to trimethoprim-sulfamethoxazole + tetracycline + erythromycin + furazolidone was the most common resistance pattern, while in Matlab it was trimethoprim-sulfamethoxazole + tetracycline + erythromycin (Table 2). In Dhaka, the proportions of isolates resistant to individual antibiotics were trimethoprim-sulfamethoxazole (99%), furazolidone (99%), erythromycin (85%), tetracycline (98%), ciprofloxacin (5%), and azithromycin (5%). In Matlab, the percentages of isolates resistant to individual antibiotics were trimethoprim-sulfamethoxazole (99%), furazolidone (42%), erythromycin (65%), tetracycline (93%), and ciprofloxacin (<1%).
Patients with MDR Vibrio cholerae O1 infections in Dhaka were more likely than those in Matlab to experience a frequency of stools of >10/day, dehydration, and the presence in stools of 1–10 macrophages per high power field (Table 6).
Table 6.
Sociodemographic and clinical features of patients with multidrug resistant Vibrio cholerae O1 infections in Dhaka compared to those in Matlab, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), 2000–2012.
| Indicators | Dhaka; n = 1,056 (%) | Matlab; n = 256 (%) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Male sex | 607 (56) | 126 (46) | 1.39 (1.05, 1.85)* | 1.40 (0.79, 2.48) |
| Vomiting | 976 (92) | 216 (84) | 2.26 (1.47, 3.46)* | 1.86 (0.71, 4.84) |
| Abdominal pain | 448 (42) | 131 (51) | 0.70 (0.53, 0.93)* | 0.50 (0.28, 0.89)* |
| Fever (≥38°C) | 7 (1) | 16 (6) | 0.10 (0.04, 0.26)* | 0.09 (0.02, 0.57)* |
| Watery stool | 1048 (99) | 245 (96) | 5.88 (2.17, 16.19)* | 0.27 (0.00, 18.62) |
| Frequency of stool (>10/day) | 592 (56) | 237 (93) | 2.31 (1.73, 3.10)* | 3.32 (1.78, 6.17)* |
| Duration of diarrhea (>24 hours) | 369 (35) | 75 (29) | 1.30 (0.95, 1.76)* | 0.98 (0.49, 1.95) |
| Duration of stay in hospital >24 hrs | 391 (39) | 170 (67) | 0.31 (0.23, 0.42)* | 0.39 (0.22, 0.71)* |
| Some or severe dehydration | 998 (94) | 214 (84) | 3.44 (2.20, 5.37)* | 3.07 (1.13, 8.32)* |
| Use of intravenous saline for rehydration | 828 (79) | 152 (59) | 2.58 (1.91, 3.48)* | 1.90 (0.88, 4.11) |
| Use of antimicrobials at home | 454 (43) | 126 (49) | 0.78 (0.59, 1.03) | 1.35 (0.75, 2.45) |
| Red blood cell (1 to >50) | 456 (44) | 167 (66) | 0.39 (0.29, 0.53)* | 0.19 (0.09, 0.39)* |
| Faecal leukocyte (11 to >50) | 508 (49) | 198 (79) | 0.26 (0.18, 0.36)* | 0.35 (0.17, 0.73)* |
| Macrophage (1 to 10) | 292 (28) | 33 (13) | 2.58 (1.72, 3.88)* | 11.75 (4.87, 28.31)* |
*P < 0.05.
aOR: adjusted odds ratio.
4. Discussion
To our knowledge, few studies have assessed differences in sociodemographic and clinical features of multidrug resistant versus susceptible infections due to Shigella spp. within a single nation or to compare these features for multidrug resistant infections due to Shigella spp. and Vibrio cholerae O1 at an urban versus a rural treatment center [19]. Although multidrug resistant infections are a problem globally, the ease of availability of antibiotics to the public at large in nations such as Bangladesh may expedite the rate at which resistance develops [20]. In the present study, the proportion of multidrug resistant Shigella and Vibrio cholerae O1 isolates recovered from patients in urban Dhaka was similar to that in rural Matlab. Of concern, in both hospital settings, there was evidence of resistance to mecillinam and ciprofloxacin, drugs commonly used for the treatment of shigellosis and, in the case of ciprofloxacin, cholera. In general, the emergence of resistance poses a number of challenges by leading, in some instances, to an increase in morbidity and mortality and longer hospital stays, as a result of inadequate initial therapy or increased virulence [15]. Moreover, antibiotic resistance reduces choices for therapy and can cause health care costs to rise due to the need to use antimicrobial agents that are more expensive than those in current use.
While antibiotic resistance in both Shigella spp. and Vibrio cholerae O1 has serious ramifications, for Shigella spp. the phenomenon carries added weight given that susceptibility rarely returns after resistant strains have become endemic in a region [12, 21]. Although S. dysenteriae 1 frequently develops resistance to new antibiotics initially, resistance is often acquired subsequently in the other Shigella species. In contrast, Vibrio cholerae O1 strains often revert to antibiotic susceptibility [12]. This phenomenon was observed during the study period when, abruptly in late 2004, Vibrio cholerae O1 isolates at both Matlab and Dhaka demonstrated tetracycline resistance; however, two years later, in 2006, tetracycline susceptibility reappeared in large part [22].
We observed evidence that multidrug resistant infections due to Shigella spp. were associated with more severe illness compared to non-MDR strains. For example, patients in Dhaka from whom multiresistant Shigella spp. were recovered significantly were more likely to experience diarrhea for >24 hours compared to patients from whom susceptible isolates were recovered. Similarly, in Matlab, patients from whom multidrug resistant Shigella spp. was recovered are more likely than those with susceptible infections to stay in hospital >24 hours and to report having used antimicrobials at home prior to coming to hospital. We also found evidence of differing degrees of severity of multidrug resistant infections due to Shigella spp. and Vibrio cholerae O1 infections depending on whether patients resided in urban or rural areas of the country. For example, for infections with MDR Shigella spp., patients in Dhaka were more likely than those in Matlab to experience dehydration, a frequency of stools >10/day, and duration of diarrhea >24 hours. Similarly, patients with MDR Vibrio cholerae O1 infections in Dhaka were more likely than those in Matlab to experience dehydration, frequency of stools of >10/day, and the presence of macrophages in stools. Further studies are needed to corroborate whether patients with multidrug resistant infections due to Shigella spp. and Vibrio cholerae O1 who reside in urban areas are, in fact, at elevated risk of experiencing more severe illness than patients in rural areas, and, if the findings are borne out, what factors contribute to this phenomenon. In the meantime, we hypothesize that differences in sociodemographic, nutritional, and economic characteristics of urban versus rural populations may, in part, explain our observations, as may differences in sources of drinking water and water-sanitation practices.
The present study has several limitations. Hospital-based data of the type incorporated in the Diarrheal Disease Surveillance System may not adequately represent the ill population at large. For example, a segment of the population in Bangladesh, particularly in rural regions, is known to use medicinal plants and traditional healers as a first-line of health care to cure gastrointestinal disorders [23]. Consequently, it is possible that sociodemographic and clinical features of patients with shigellosis and cholera described here may not be completely representative of all infections that occurred in the study area and study period. In addition, the results of antibiotic resistance testing we used were largely qualitative—presence, absence—as opposed to a quantitative nature as provided by minimum inhibitory concentrations. Thus, we were unable to ascertain pathogen-antibiotic relationships, wherein resistance levels may have approached limits known to confer resistance. Finally, our results did not incorporate the role of virulence factors or genetic typing that could have added valuable insight into outcomes of interest.
Notwithstanding these limitations, we believe that our results justify further research to determine, first, whether multidrug resistant strains of Shigella and Vibrio cholerae O1 are associated with more severe infections than antibiotic-susceptible strains, and, if so, what factors increase the severity of infections, and second, whether multidrug resistant infections caused by these two pathogens produce more severe infections in urban versus rural regions. Answers to these questions may help in efforts to prevent infections due to Shigella spp. and Vibrio cholerae O1, both of which are often leading bacterial causes of diarrhea in developing nations.
Conflict of Interests
The authors declared no potential conflict of interests. All authors confirmed that there is no professional affiliation, financial agreement, or other involvement with any company whose product figures prominently in the submitted paper.
Acknowledgments
Hospital surveillance was funded by icddr,b and the Government of the People's Republic of Bangladesh through IHP-HNPRP. icddr,b acknowledges with gratitude the commitment of the Government of the People's Republic of Bangladesh for their research efforts. icddr,b also gratefully acknowledges the following donors who provide unrestricted support to the Centre's research efforts: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID).
References
- 1.Das SK, Ahmed S, Ferdous F. Changing emergence of Shigella sero-groups in Bangladesh: observation from four different diarrheal disease hospitals. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062029.e62029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhury F, Khan AI, Faruque ASG, Ryan ET. Severe, acute watery diarrhea in an adult. PLoS Neglected Tropical Diseases. 2010;4(11, article e898) doi: 10.1371/journal.pntd.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- 4.Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges & management issues. Indian Journal of Medical Research. 2004;120(5):454–462. [PubMed] [Google Scholar]
- 5.Pazhani GP, Niyogi SK, Singh AK, et al. Molecular characterization of multidrug-resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. Journal of Medical Microbiology. 2008;57(7):856–863. doi: 10.1099/jmm.0.2008/000521-0. [DOI] [PubMed] [Google Scholar]
- 6.Dick MH, Guillerm M, Moussy F, Chaignat CL. Review of two decades of cholera diagnostics–how far have we really come? PLOS Neglected Tropical Diseases. 2012;6(10) doi: 10.1371/journal.pntd.0001845.e1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. American Journal of Medicine. 2006;119(supplement 1):S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Thong K-L, Hoe CH, Koh YT, Yasin RM. Prevalence of multidrug-resistant Shigella isolated in Malaysia. Journal of Health Population and Nutrition. 2002;20(4):356–358. [PubMed] [Google Scholar]
- 9.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. British Medical Journal. 1982;285(6349):1185–1188. doi: 10.1136/bmj.285.6349.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das SK, Ahmed S, Ferdous FD, et al. Etiologic studies of patients visiting different diarrhoeal disease facilities in Bangladesh. Journal of Infection in Developing Country. In press. [Google Scholar]
- 11.Ashkenazi S, May-Zahav M, Sulkes J, Zilberberg R, Samra Z. Increasing antimicrobial resistance of Shigella isolates in Israel during the period 1984 to 1992. Antimicrobial Agents and Chemotherapy. 1995;39(4):819–823. doi: 10.1128/aac.39.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sack DA, Lyke C, McLaughlin C, Voravit S. Geneva, Switzerland: World Health Organization; 2001. Antimicrobial resistance in shigellosis, cholera and campylobacteriosis. [Google Scholar]
- 13.Lima AAM, Lima NL, Pinho MCN, et al. High frequency of strains multiply resistant to ampicillin, trimethoprim- sulfamethoxazole, streptomycin, chloramphenicol, and tetracycline isolated from patients with shigellosis in northeastern Brazil during the period 1988 to 1993. Antimicrobial Agents and Chemotherapy. 1995;39(1):256–259. doi: 10.1128/aac.39.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta PG, Mandal S, Sen D, Das P, Deb BC, Pal SC. Multidrug resistant epidemic shigellosis in a village in west Bengal, 1984. Indian Journal of Public Health. 1990;34(1):15–19. [PubMed] [Google Scholar]
- 15.Spicknall IH, Foxman B, Marrs CF, Eisenberg JN. A modeling framework for the evolution and spread of antibiotic resistance: literature review and model categorization. American Journal of Epidemiology. 2013;178:508–520. doi: 10.1093/aje/kwt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manual For Laboratory Investigation of Acute Enteric Infections. Geneva, Switzerland: World Health Organization; 1987. Programme for control of diarrheal disease. [Google Scholar]
- 17.Qadri F, Khan AI, Faruque ASG, et al. Enterotoxigenic Escherichia coli and Vibrio cholerae diarrhea, Bangladesh, 2004. Emerging Infectious Diseases. 2005;11(7):1104–1107. doi: 10.3201/eid1107.041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standard Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement (June 2010, Update) CLSI document M100-S20-U, CLSI, Clinical and Laboratory Standard Institute, Fort Wayne, Ind, USA, 2010.
- 19.Beatty ME, Ochieng JB, Chege W, et al. Sporadic paediatric diarrhoeal illness in urban and rural sites in Nyanza Province, Kenya. East African Medical Journal. 2009;86(8):387–398. doi: 10.4314/eamj.v86i8.54159. [DOI] [PubMed] [Google Scholar]
- 20.Karmakar P, Sattar MM. Antibiotic prescribing pattern in Bangladesh. Banglaesh Journal of Progressive Science & Technology. 2012;10(1):13–16. [Google Scholar]
- 21.von Seidlein L, Deok RK, Ali M, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Medicine. 2006;3(9, article e353) doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faruque ASG, Alam K, Malek MA, et al. Emergence of multidrug-resistant strain of Vibrio cholerae OI in Bangladesh and reversal of their susceptibility to tetracycline after two years. Journal of Health, Population and Nutrition. 2007;25(2):241–243. [PMC free article] [PubMed] [Google Scholar]
- 23.Kadir MF, Bin Sayeed MS, Mia MM. Ethnopharmacological survey of medicinal plants used by traditional healers in Bangladesh for gastrointestinal disorders. Journal of Ethnopharmacology. 2013;147(1):148–156. doi: 10.1016/j.jep.2013.02.023. [DOI] [PubMed] [Google Scholar]


