Abstract
The metabolic syndrome and neuropathy are common conditions, especially in the elderly, that are associated with significant morbidity. Furthermore, the metabolic syndrome is reaching epidemic proportions across the world. Current evidence supports the association of the metabolic syndrome and its individual components with neuropathy. Several clinical trials have demonstrated that treating hyperglycemia, one component of the metabolic syndrome, has a significant effect on reducing the incidence of neuropathy in those with type 1 diabetes. However, glucose control only has a marginal effect on preventing neuropathy in those with type 2 diabetes, suggesting that other factors may be driving nerve injury in these patients. Emerging evidence supports the metabolic syndrome as these risk factors for neuropathy. Interventions exist for treatment of all of the metabolic syndrome components, but only glucose control has strong evidence to support its use and is widely employed. Our understanding of the biology of metabolic nerve injury has rapidly expanded over the last several years. Mechanisms of injury include fatty deposition in nerves, extracellular protein glycation, mitochondrial dysfunction, and oxidative stress. Additionally, the activation of counter-regulatory signaling pathways leads to chronic metabolic inflammation. Medications that target these signaling pathways are being used for a variety of diseases and are intriguing therapeutics for future neuropathy clinical trials. As we move forward, we need to expand our understanding of the association between the metabolic syndrome and neuropathy by addressing limitations of previous studies. Just as importantly, we must continue to investigate the pathophysiology of metabolically induced nerve injury.
Global Importance
Obesity is a world-wide epidemic with a 100% increase in all-cause mortality.1 Between 1980 and 2008, the prevalence of obese individuals doubled, reaching greater than half a billion world-wide.2 Obesity is the central element underlying the metabolic syndrome (MetS), a clustering of five risk factors including obesity, insulin resistance, hypertension, hypertriglyceridemia and dyslipidemia. The MetS is principally responsible for the alarming increase in chronic diseases, chiefly diabetes, cardiovascular disease, neurodegenerative disease and cancer.3 According to National Nutrition and Health Survey (NHANES) data from 1988–1994, 22% of the adult United States population met criteria for MetS, with more than 40% of the elderly affected.4 Using the 1999–2002 NHANES data, the prevalence of MetS had climbed to a staggering 34.5%5 and the current prevalence is approaching 50% (www.cdc.org). Like obesity, MetS is not just an American problem. India, Iran, Mexico, Ireland, Scotland, and Turkey are just some of the countries with more than 20% of their population affected by MetS.6 China, with the world’s largest population, has a rapidly increasing MetS prevalence of approximately 30%.7, 8
Peripheral neuropathy is a chronic and common disease, affecting 2–7% of the population, according to estimates from population-based studies in India and Italy.9, 10 As with MetS, the prevalence rises in the elderly, with 15% affected according to a study that focused on a United States population over the age of 40.11 Not only is neuropathy a widespread condition, but it is also quite disabling. Neuropathic pain affects approximately half of patients with diabetic neuropathy.12–14 Moreover, sensory deficits lead to balance difficulties and frequent falls with resulting musculoskeletal injuries, including fractures.15 Neuropathy is also a risk factor for foot ulcerations and lower extremity amputations, particularly in those with diabetes.16 All of these manifestations of neuropathy have a profound effect on an individual’s quality of life.17 Both neuropathy and MetS are frequently encountered conditions that disproportionately affect the elderly, with significant morbidity and mortality.18
When considering the discrete components of the MetS, diabetes and pre-diabetes have the strongest evidence supporting a pathogenic link with neuropathy, but each of the other components also have evidence supporting their association with neuropathy in diabetic populations.17, 19–26 Specifically, obesity has been shown by multiple investigators to be associated with neuropathy in diabetic patients.17, 20, 21 Isomaa and colleagues, Costa and colleagues, and the Metascreen investigators have independently shown that an individual with diabetes is more likely to have neuropathy if other components of the MetS are present.27–29 In a study of 427 diabetic patients with mild to moderate diabetic neuropathy, elevated triglycerides correlated with loss of sural nerve myelinated fiber density, a direct anatomical measurement of neuropathy.30 In contrast, there was no association with glycemic control and neuropathy in this cohort.30 The most telling data are from several large clinical trials, all of which report that glycemic control alone is not enough to prevent type 2 diabetic patients from developing neuropathy.22 Furthermore, patients with normoglycemia and neuropathy have the same prevalence of MetS components as those with IGT and neuropathy, and an even higher prevalence of MetS components than those with diabetes and no neuropathy.31 These results indicate that MetS and its components are likely to be important in non-diabetic populations as well. Given the clustering of MetS components, hypertension, hypertriglyceridemia, dyslipidemia, and particularly obesity are prime candidates to be the essential factors underlying the neuropathy present in patients with type 2 diabetes.
Modern Understanding of Biology
Up until this last decade, it was generally believed that the underlying cause of neuropathy was hyperglycemia, irrespective of the type of diabetes (1 or 2). The more plausible and current hypothesis is that the MetS underlies the onset and progression of neuropathy and that obesity and its consequences are the driving factors leading to nerve injury.
The fundamental property of obesity is energy imbalance, with low energy expenditure compared to high caloric consumption. Excess nutrients are initially stored in “professional” metabolic tissues, such as fat, skeletal muscle and liver. When the storage capacity of these tissues is exceeded, bystander tissues such as the nervous system are subjected to excess nutrients with little ability to handle super-physiologic substrates, resulting in extrinsic and intrinsic cellular dysfunction.32 Extrinsic forces include fatty deposition in the nerve and extracellular protein glycation and oxidation.33–36 The hallmark of intrinsic dysfunction is metabolic imbalance with lipid and glucose dysregulation leading to mitochondrial dysfunction and subsequent oxidative and endoplasmic reticulum stress.18, 33, 34, 37, 38 Neurons also express receptors for low density lipoproteins (LDLs), and elevated levels of oxidized LDLs, a hallmark of obesity and the MetS, activate receptors for oxidized LDLs, such as lectin-like oxidized LDL receptor-1 (LOX-1), to promote additional mitochondrial injury.
With ongoing energy imbalance, there is a vicious feed-forward cycle, activating counter-regulatory signaling pathways which converge to inhibit insulin signaling and promote chronic metabolic inflammation.39–41 These counter-regulatory pathways include extracellular regulated kinases (ERKs), Jun N-terminal kinases (JNK), inhibitor of nuclear factor κB (IκB) kinase β (IKK β), mammalian target of rapamycin (mTOR) and endoplasmic reticulum-to-nucleus signaling 1 (IRE-1), each a potential target for mechanism-based intervention.32, 39 Continued inflammation fosters neuronal insulin resistance and loss of insulin neurotropism while engorged neural adipocytes secrete inflammatory chemokines capable of recruiting pro-inflammatory M1 macrophages to the already stressed nerve, intensifying neural injury.42–45 Systemic inflammation promotes hypertension resulting in nerve ischemia further promoting oxidative and nitrosative stress, aberrant neuronal and axonal mitochondrial function, energy deprivation and nerve injury. Figure 1 depicts the intersection of MetS components with neuronal injury and the central role of inflammation.
Figure 1.
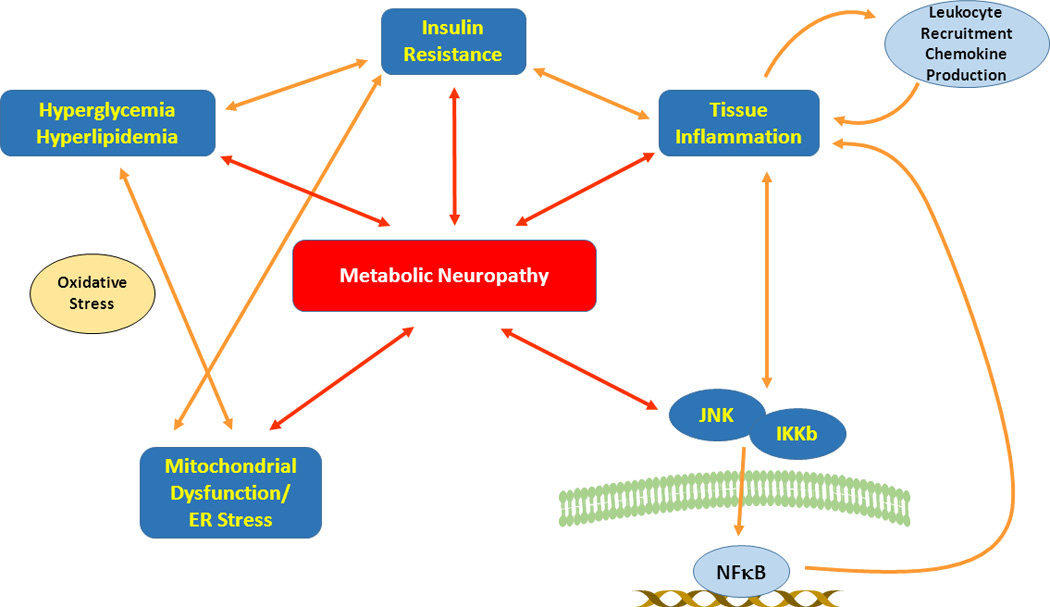
Signaling pathways underlying nutrient excess and metabolic neuropathy. Hyperglycemia and hyperlipidemia incite a feed-forward cycle of cellular damage with production of reactive oxygen species leading to cellular oxidative stress, mitochondrial dysfunction and parallel endoplasmic reticulum (ER) stress. These changes not only lead to direct neuronal injury but also promote nutrient excess-mediated insulin resistance, initiating tissue inflammation, which in turn exacerbates insulin resistance and mediates injury cascades. There is leukocyte recruitment with the production of tissue damaging inflammatory chemokines and activation of Jun N-terminal kinases (JNK) and inhibitor of nuclear factor Kb (IKb) kinase B (IKKb) triggering further insulin resistance, inflammatory responses and tissue damage. JNK and IKKb also mediate NFκB activation leading to production of inflammatory and tissue damaging signals. Collectively these diverse but interlinked pathways reinforce a destructive cycle of cellular impairment and damage linking nutrient excess to metabolic neuropathy.
Currently Available Therapies
The only component of MetS with an established treatment for the prevention of neuropathy is diabetes. Enhanced glucose control has been shown to decrease the incidence of neuropathy in patients with type 1 diabetes, with little effect in those with type 2 diabetes (Table 1).46–49 In type 1 diabetes, enhanced glucose control can be achieved through diet and exercise and insulin. Similar diet and exercise regimens with the addition of metformin, sulfonylureas, and other less common drugs, provides improved glycemic control but little protection against neuropathy in type 2 diabetes. Diet and exercise in those with pre-diabetes and neuropathy has been shown to increase nerve fiber density, but no controlled clinical trial has been performed to confirm this finding.26 Furthermore, diet, exercise, and metformin reduce the incidence of diabetes in those with pre-diabetes, but the effect on the prevention of neuropathy is unclear.50 While effective pharmaceutical treatments exist for hypertension, hypertriglyceridemia, and dyslipidemia, no studies have investigated the effect of these interventions on the prevention or improvement of neuropathy. Similarly, while diet and exercise programs and medications can be effective in the treatment of obesity, no current data exist on the effect of these interventions on peripheral neuropathy in this population. Importantly, diet and exercise regimens have the potential to treat MetS as a whole; however, compliance and long term maintenance on these regimens are notoriously difficult. The good news is that there are many currently available treatments of MetS components. The bad news is that our only currently established therapy is glucose control in patients with diabetes and this has little effect on neuropathy in patients with type 2 diabetes and MetS neuropathy.
Table 1.
| Investigator | Trial Size |
Length of study |
Clinical outcome |
Other outcomes |
Enhanced glycemic control superior? |
|---|---|---|---|---|---|
| Type 1 diabetes | |||||
| Holman 1983 | 74 | 2 years | No | QST | Yes |
| Lauritzen 1985 | 30 | 2 years | No | QST | No |
| Dahl-Jorgensen 1986 | 45 | 2 years | No | NCS | Yes |
| Jakobsen 1988 | 24 | 2 years | No | QST | Yes |
| DCCT 1993 | 1,441 | 5 years | Yes | NCS | Yes |
| Reichard 1993 | 102 | 7.5 years | No | NCS, QST | Yes |
| Linn 1996 | 49 | 5 years | Yes | None | Yes |
| Type 2 diabetes | |||||
| Kawamori 1991 | 50 | 4 years | No | NCS | Yes |
| UKPDS 1998 | 3,867 | 10 years | No | QST | Yes |
| Tovi 1998 | 38 | 1 year | Yes | None | No |
| Azad 1999 | 153 | 2 years | Yes | None | No |
| Shichiri 2000 | 110 | 8 years | No | NCS, QST | Yes |
| Gaede 2003 | 160 | 8 years | No | QST | No |
| Duckworth 2009 | 1,791 | 5.6 years | Yes | None | No |
| ACCORD 2010 | 10,251 | 3.7 years | Yes | None | No |
Reprinted from Lancet Neurology, Vol. number 11 (6), Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL, Diabetic Neuropathy: clinical manifestations and current treatments, Pages No. 521-34, Copyright (2012), with permission from Elsevier.
Therapeutic Pipeline in 2013
The central role of inflammation in the MetS and associated chronic clinical disorders has led to the recent development of mechanism-based therapies which include small molecule kinase, chemokine and cytokine inhibitors and genetically engineered recombinant proteins that target specific inflammatory receptors or ligands, as well as the use of older, more broadly based anti-inflammatory drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) like salsalate. Although these therapies have not yet been used to abrogate the acquired inflammatory microenvironment in the peripheral nervous system, these approaches are in current experimental use in other chronic MetS diseases, including central nervous system neurodegenerative disorders.
For example, small molecule kinase inhibitors targeting JNK, mTOR, and IRE-1 can attenuate inflammation and macrophage activation to provide neuroprotection in neurodegenerative conditions, including traumatic brain and spinal cord injury, Parkinson’s disease, multiple sclerosis, and Alzheimer’s disease.51, 52 JNK inhibitors are currently in Phase 2 clinical trials for inflammatory endometriosis and idiopathic pulmonary fibrosis, demonstrating the translational potential of this therapeutic strategy for metabolic neuropathy.52 Inhibitors of IRE1 are effective in the treatment of endoplasmic reticulum stress-associated diseases, including multiple myeloma.53, 54 mTOR inhibitors are another popular therapeutic strategy, and the role of mTOR in cellular metabolism, autophagy, and survival has supported applications of mTOR inhibitors in breast cancer and spinal cord injury and as an anti-aging treatment.55–57 Interestingly, the type 2 diabetes drug metformin functions by activating AMPK which in turn negatively regulates mTOR signaling, and indirect mTOR regulatory mechanisms of metformin have also been recently uncovered.56, 58, 59
Attention to chemokines as a therapeutic target is also increasing, with evidence of critical implications of CC ligand 2 (Ccl2) and its receptor CCR2 in neuronal injury and multiple sclerosis.60, 61 Small molecule inhibitors of Ccl2 and Ccl5 are currently in Phase 2 trials for the treatment of diabetic nephropathy (www.clinicaltrials.gov; NCT01712061). Salsalate is reported to have significant glucose-lowering effects by blocking low grade inflammation via inhibiting nuclear factor-kappaB (NFκB) and consequently improving insulin sensitivity in multiple small trials and case reports.62 Goldfine and colleagues recently completed a large multicenter randomized trial, the Targeting Inflammation with Salsalate in Type 2 Diabetes (TINSAL-T2D) trial, evaluating the effects of salsalate on serum inflammatory markers, insulin levels and glucose control. They report that salsalate lowers hemoglobin A1C levels and improves glycemic control in patients with type 2 diabetes.63 Together, these approaches provide optimism that a novel and as yet untested therapeutic pipeline exists for neuropathy.
Unmet Needs
While multiple studies have demonstrated associations between MetS and neuropathy, studies to date have largely focused on patients with diabetes, have utilized cross-sectional study designs, and have used inconsistent definitions of neuropathy. Furthermore, the proportion of patients with neuropathy that are attributed to MetS is also unclear. The high prevalence of MetS makes this syndrome a potentially large contributor to the development and progression of neuropathy in those with and without diabetes, but the degree of impact of MetS on neuropathy remains to be defined. Past studies investigating the association between specific MetS components and neuropathy have also yielded inconsistent results. For example, De Block et al did not find an association between obesity and neuropathy, whereas three other investigators found a significant association.17, 19, 21 Identifying the particular components that drive neuropathy is essential in informing future clinical trials. We also have no information on the interactions between the different MetS components and neuropathy. It is possible that a specific combination of MetS components is needed to cause neuropathy or that the effects of the individual components are not additive but synergistic. Much also remains to be learned about the underlying causes and potential treatments of metabolic neuropathy and we contend that targeting inflammation offers a novel and likely effective treatment strategy.
Possible New Directions for Research
An evolving literature indicates that type 1 and type 2 diabetes are substantially different diseases with disparate mechanisms.64, 65 The MetS and its individual components are potential explanations for this observation, with a much greater prevalence in those with type 2 diabetes. Future, investigations are needed to define the underlying pathophysiologic differences between the two different types of diabetes, with a focus on MetS components and inflammation. This information would have significant implications for the development of new therapeutics in this area. There is also a need for epidemiologic studies that address some of the shortcomings of existing trials, such as studying patients with MetS with and without diabetes, utilizing longitudinal study designs, and employing rigorous definitions of neuropathy. This information has the potential to give further evidence that there is a causal relationship between MetS and neuropathy. We also must define the impact of MetS on neuropathy, the role of its individual components, and the interactions between them. Enhancing our knowledge of the underlying scientific mechanisms and epidemiology of metabolic neuropathy has the potential to rapidly lead to clinical trials, since all MetS components have currently available treatments. Hopefully, this new knowledge will also help us develop novel therapeutics with the potential to prevent, halt, or reverse this common, disabling disease.
Acknowledgements
Dr. Stacey Sakowski Jacoby contributed to critical review of the manuscript.
Study Funding: Dr. Callaghan and Dr. Feldman are supported by the Taubman Medical Institute, the Katherine Rayner Program, and the Program for Neurology Research & Discovery. Dr. Callaghan is also supported by an American Diabetes Association Junior Faculty Award and a NIH K23 award. Dr. Feldman is also supported by NIH RO1 NS077982, NIH 1DP3DK094292, NIH/NIA 2P01 AG020591-06A1, and NIH/NIDDK 1 R24 082841.
Footnotes
Author Disclosures:
Dr. Callaghan and Dr. Feldman report no disclosures.
Author contributions:
Dr. Callaghan and Dr. Feldman participated in the literature review and writing of the manuscript.
References
- 1.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. The New England journal of medicine. 2006 Aug 24;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011 Feb 12;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013 Jan 11;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005 Nov;28(11):2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 6.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004 Jun;33(2):351–375. doi: 10.1016/j.ecl.2004.03.005. table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Xu WH, Ruan XN, Fu XJ, et al. Prevalence of the metabolic syndrome in Pudong New Area of Shanghai using three proposed definitions among Chinese adults. BMC Public Health. 2010;10:246. doi: 10.1186/1471-2458-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo H, Shi Z, Hu X, Wu M, Guo Z, Hussain A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism. 2009 Aug;58(8):1102–1108. doi: 10.1016/j.metabol.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology. 1991 Aug;41(8):1315–1317. doi: 10.1212/wnl.41.8.1315. [DOI] [PubMed] [Google Scholar]
- 10.Savettieri G, Rocca WA, Salemi G, et al. Prevalence of diabetic neuropathy with somatic symptoms: a door-to-door survey in two Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Neurology. 1993 Jun;43(6):1115–1120. doi: 10.1212/wnl.43.6.1115. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004 Jul;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 12.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011 Oct;34(10):2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004 Sep;21(9):976–982. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 14.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000 Feb;47(2):123–128. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010 Dec;31(9):1445–1450. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- 16.Margolis DJ, Malay DS, Hoffstad OJ, et al. Incidence of Diabetic Foot Ulcer and Lower Extremity Amputation Among Medicare Beneficiaries 2006 to 2008: Data Points #2. 2011 [PubMed] [Google Scholar]
- 17.Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009 Jun;35(3):206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013 Jan;4(1):4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Block CE, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care. 2005 Jul;28(7):1649–1655. doi: 10.2337/diacare.28.7.1649. [DOI] [PubMed] [Google Scholar]
- 20.Straub RH, Elbracht R, Kramer BK, Roth M, Palitzsch KD, Scholmerich J. Influence of digoxin-like immunoreactive factor on late complications in patients with diabetes mellitus. Eur J Clin Invest. 1994 Jul;24(7):482–487. doi: 10.1111/j.1365-2362.1994.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 21.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005 Jan 27;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 22.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol. 1990 Apr;131(4):633–643. doi: 10.1093/oxfordjournals.aje.a115547. [DOI] [PubMed] [Google Scholar]
- 24.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001 Sep;24(9):1229–1231. doi: 10.1002/mus.1137. [DOI] [PubMed] [Google Scholar]
- 25.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001 Aug;24(8):1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 26.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006 Jun;29(6):1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 27.Bonadonna RC, Cucinotta D, Fedele D, Riccardi G, Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care. 2006 Dec;29(12):2701–2707. doi: 10.2337/dc06-0942. [DOI] [PubMed] [Google Scholar]
- 28.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med. 2004 Mar;21(3):252–255. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 29.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001 Sep;44(9):1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 30.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009 Jul;58(7):1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008 Oct 15;273(1–2):25–28. doi: 10.1016/j.jns.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013 Jan 11;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinder LM, Vincent AM, Burant CF, Pennathur S, Feldman EL. Bioenergetics in diabetic neuropathy: what we need to know. J Peripher Nerv Syst. 2012 May;17(Suppl 2):10–14. doi: 10.1111/j.1529-8027.2012.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. The Journal of endocrinology. 2013 Jan;216(1):1–11. doi: 10.1530/JOE-12-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009 Dec;14(4):257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent AM, Perrone L, Sullivan KA, et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007 Feb;148(2):548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 37.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011 Oct;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 38.Lupachyk S, Watcho P, Obrosov AA, Stavniichuk R, Obrosova IG. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Experimental neurology. 2012 Nov 8; doi: 10.1016/j.expneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 40.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature medicine. 2012 Jul 29; doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 41.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012 Mar 2;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng HT, Dauch JR, Hayes JM, Yanik BM, Feldman EL. Nerve growth factor/p38 signaling increases intraepidermal nerve fiber densities in painful neuropathy of type 2 diabetes. Neurobiol Dis. 2012 Jan;45(1):280–287. doi: 10.1016/j.nbd.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009 Jun;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herder C, Lankisch M, Ziegler D, et al. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany) Diabetes Care. 2009 Apr;32(4):680–682. doi: 10.2337/dc08-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. Journal of neurophysiology. 2011 Aug;106(2):905–914. doi: 10.1152/jn.01123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995 Dec;38(6):869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 47.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 48.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009 Jan 8;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 49.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010 Aug 7;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Pan J, Chen SD. Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson's disease. Prog Neurobiol. 2012 Aug;98(2):207–221. doi: 10.1016/j.pneurobio.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Graczyk PP. JNK inhibitors as anti-inflammatory and neuroprotective agents. Future Med Chem. 2013 Apr;5(5):539–551. doi: 10.4155/fmc.13.34. [DOI] [PubMed] [Google Scholar]
- 53.Mimura N, Fulciniti M, Gorgun G, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012 Jun 14;119(24):5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkmann K, Lucas JL, Vuga D, et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. The Journal of biological chemistry. 2011 Apr 8;286(14):12743–12755. doi: 10.1074/jbc.M110.199737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanno H, Ozawa H, Sekiguchi A, et al. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. 2012 Sep 1;11(17):3175–3179. doi: 10.4161/cc.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013 Mar 1;123(3):980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology (Williston Park) 2013 Jan;27(1):38–44. 46, 48, passim. [PubMed] [Google Scholar]
- 58.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer research. 2011 Jul 1;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 59.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010 May 5;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang YS, Cha JJ, Hyun YY, Cha DR. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs. 2011 Jun;20(6):745–756. doi: 10.1517/13543784.2011.575359. [DOI] [PubMed] [Google Scholar]
- 61.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010 Mar;30(3):459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rumore MM, Kim KS. Potential role of salicylates in type 2 diabetes. Ann Pharmacother. 2010 Jul-Aug;44(7–8):1207–1221. doi: 10.1345/aph.1M483. [DOI] [PubMed] [Google Scholar]
- 63.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010 Mar 16;152(6):346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callaghan B, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol. 2012 Oct;25(5):536–541. doi: 10.1097/WCO.0b013e328357a797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012 Jun;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


