Abstract
Delayed reward discounting is a behavioral economic index of impulsivity, referring to how much an individual devalues a reward based on its delay in time. As a behavioral process that varies considerably across individuals, delay discounting has been studied extensively as a model for self-control, both in the general population and in clinical samples. There is growing interest in genetic influences on discounting and, in particular, the prospect of discounting as an endophenotype for addictive disorders (i.e., a heritable mechanism partially responsible for conferring genetic risk). This review assembles and critiques the evidence supporting this hypothesis. Via numerous cross-sectional studies and a small number of longitudinal studies, there is considerable evidence that impulsive discounting is associated with addictive behavior and appears to play an etiological role. Moreover, there is increasing evidence from diverse methodologies that impulsive delay discounting is temporally stable, heritable, and that elevated levels are present in nonaffected family members. These findings suggest that impulsive discounting meets the criteria for being considered an endophenotype. In addition, recent findings suggest that genetic variation related to dopamine neurotransmission is significantly associated with variability in discounting preferences. A significant caveat, however, is that the literature is modest in some domains and, in others, not all the findings have been supportive or consistent. In addition, important methodological considerations are necessary in future studies. Taken together, although not definitive, there is accumulating support for the hypothesis of impulsive discounting as an endophenotype for addictive behavior and a need for further systematic investigation.
Keywords: Delay Discounting, Impulsivity, Behavioral Economics, Genetics, Addiction, Substance Dependence, Review
Situated at the intersection of psychology and economics, behavioral economics is a hybrid discipline that examines the transactions people make with the world around them. The approach has made major contributions to a number of fields, most obviously to its parent disciplines, but also to psychiatry, public health, and cognitive neuroscience. One area that has been particularly fertile has been the study of delayed reward discounting, a behavioral economic index of impulsivity that reflects how much a reward loses values based on its distance in the future (i.e., how much a reward is discounted based on its delay in time). Emerging from the study of reinforcement learning (Ainslie, 1975; Rachlin & Green, 1972) and developmental perspectives in social psychology (Mischel, 1958, 1961), this form of impulsivity has become a robust model for understanding self-control, and the lack thereof, both in the study of general psychological functioning and as a putative factor in a number of psychiatric conditions. This is especially the case for addictive behavior (for a review, see MacKillop, Amlung, Few, Ray, Sweet, & Munafò, 2011). In addition, delay discounting has emerged as a key paradigm in the incipient field of neuroeconomics (e.g., Bickel, Pitcock, Yi, & Angtuaco, 2009; Kable & Glimcher, 2007; McClure, Laibson, Loewenstein, & Cohen, 2004), which further integrates behavioral economics and cognitive neuroscience.
There is increasing evidence that delay discounting may also be relevant to psychiatric genetics and, in particular, the genetic basis for addictive behavior. The goal of the current review is to bring together the diverse sources of evidence supporting this hypothesis and in order to clarify the current knowledge and identify future directions. The first section will review the nature of delay discounting as a phenotype (i.e., any observable characteristic of an organism), both in general and in terms of its association with addictive behavior. The second section will review the endophenotype concept in psychiatric genetics, both at a conceptual level and in terms of the specific criteria that are used to evaluate whether a given phenotype should be designated as an endophenotype. The third section is the intersection of the first and second, the empirical evidence that suggests delay discounting constitutes an endophenotype for addictive disorders. Finally, as this is a relatively new area of interest, the fourth section highlights a number of important methodological considerations for future studies.
Delayed Reward Discounting as a Phenotype and its Association with Addictive Behavior
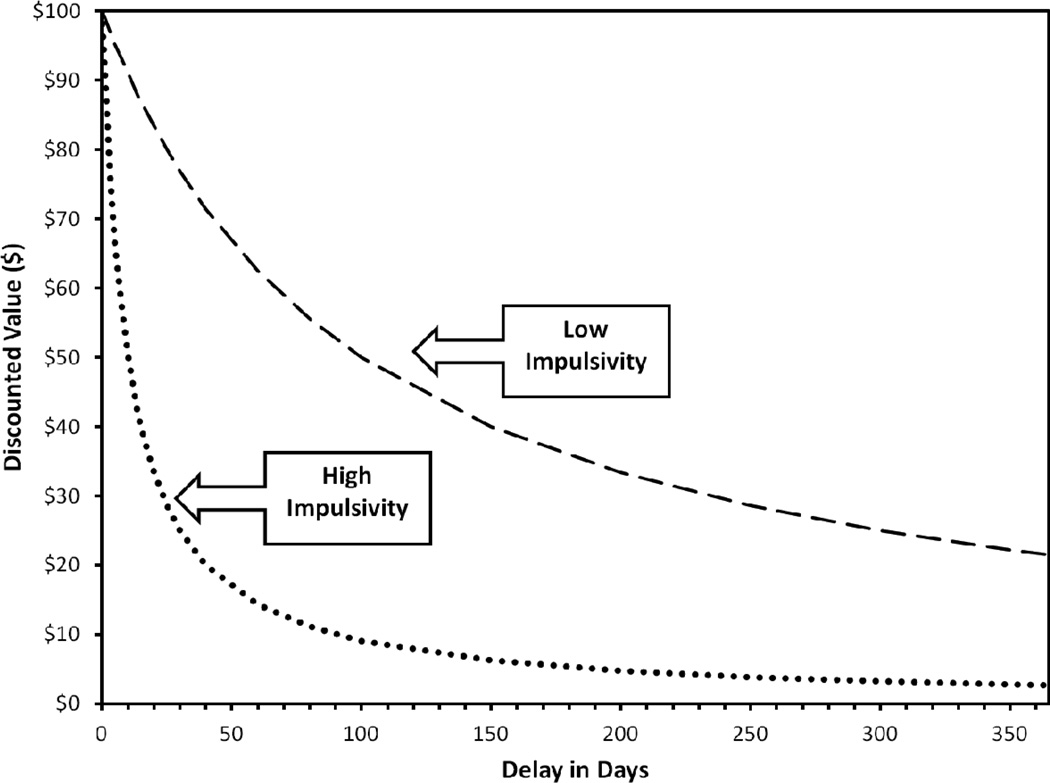
Considering delay discounting as a phenotype simply refers to its phenomenology and measurement. As a behavioral characteristic, delay discounting refers to an individual’s profile of intertemporal reward preferences, or their preferences with regard to the tension between smaller rewards available in the short-term, typically the present, compared versus larger rewards available at a future time point. This profile can be operationalized in a number of ways and for an array of commodities. In children, preferences for smaller immediate rewards at the cost of larger delayed rewards can be assessed using the famous ‘marshmallow test’ (e.g., Mischel, Ebbesen, & Zeiss, 1972), in which participants are offered one marshmallow (or another candy) immediately or two when the experimenter comes back at some later point. In adolescents and adults, discounting is typically assessed using tasks comprising dichotomous items that pit smaller immediate rewards against larger delayed rewards, both in the common currency of money. These tasks use permutations of rewards and delays that systematically vary the options and quantify the effects of delay on the value of the larger reward. By plotting the subjective value of the delayed reward across various delay periods, a discounting curve that reflects the magnitude of devaluation can be generated; two examples are provided in Figure 1. The more precipitously the reward loses value based on delay, the more impulsive the individual is considered. More importantly, the expressed preferences across the choices can be aggregated into a quantitative profile of how rapidly a delayed reward loses value to the individual, the discounting phenotype.
Figure 1.
Prototypic hyperbolic delayed reward discounting curves reflecting the discounted subjective value of $100 delayed from 1 day to 1 year (curves reflect the immediate discounted value). At 100 days, $100 has lost ~50% of its nominal value for the less impulsive profile and ~90% of its nominal value for the high impulsivity profile.
Importantly, however, individual discounting decision-making profiles can be characterized using a number of different methods: modeling the temporal discounting function via nonlinear regression, area-under-the-curve analysis, or simply the proportion of impulsive choices (Green & Myerson, 2004; Mazur, 1987; Mitchell, Fields, D'Esposito, & Boettiger, 2005; Myerson, Green, & Warusawitharana, 2001). In terms of mathematical models, the most widely used approach is a hyperbolic single parameter model (Mazur, 1987), but hyperbola-like (hyperboloid) two-parameter models can also be applied (Laibson, 1997; Myerson & Green, 1995) and an additive-utility model has recently been proposed (Killeen, 2009). These models seek to quantitatively capture the typical form of the discounting curve, namely, that the value of a delayed reward rapidly loses its value during initial delays and then decays at a more moderate rate across subsequent delays. For example, in the hyperbolic discounting curves in Figure 1, in comparing the slope during the first month of delay and the slope from 11 to 12 months of delay, it is clear that although the absolute delay durations are identical, the devaluation applied in the short run is considerably steeper. Abbreviated choice tasks also have been developed to more efficiently characterize discounting (e.g., Kirby, Petry, & Bickel, 1999; Madden, Petry, & Johnson, 2009) and are based on the hyperbolic model but can be also be analyzed using impulsive choice ratios (e.g., Murphy & MacKillop, 2012). Indeed, even very small numbers of items or a single item can be used to infer discounting rates (Anokhin, Golosheykin, Grant, & Heath, 2011; Bradford, 2010; Reimers, Maylor, Stewart, & Chater, 2009; Wulfert, Block, Santa Ana, Rodriguez, & Colsman, 2002). At this point, while there is no single methodology that is uniformly accepted as the most valid index of discounting, it appears that measures with greater precision are more sensitive (MacKillop et al., 2011).
In the context of addictive behavior, delay discounting is highly relevant for a number of reasons. At a clinical level, impulsive discounting is a prototypic pattern in substance use disorders and pathological gambling. In other words, alcoholism, heroin addiction, and other forms of addictive behavior all commonly reflect behavioral patterns of dramatically and persistently overvaluing immediate drug rewards at the very high cost of future outcomes for the individual. The second connection between delay discounting and addictive behavior is at a mathematical level, where impulsive discounting may explain self-control failures in individuals with addictive disorders. In behavioral economics, self-control failures are referred to as preference reversals (i.e., person changing their mind about their preference for one option over another), which are very common in the context of addictive disorders. For example, large proportions of individuals with substance use disorders report being motivated to change their behavior and do indeed seek treatment (e.g., Etter, Perneger, & Ronchi, 1997; Hogue, Dauber, & Morgenstern, 2010), but later voluntarily drop out of treatment to resume drug use or relapse despite successfully completing treatment (e.g., McKay, Franklin, Patapis, & Lynch, 2006). This may be explained by delay discounting because, as noted above, hyperbolic/hyperboloid discounting means that changes in value are not constant over time (Ainslie, 2001; Frederick, Loewenstein, & O'Donoghue, 2003). More specifically, rewards appear to both disproportionately lose and gain value based on temporal proximity (either as the time to receipt approaches or as initial delays are implemented), which quantitatively predicts a change in overall preference from a larger delayed reward to a smaller immediate reward as it becomes temporally near at hand (i.e., a preference reversal). Moreover, the more steeply hyperbolic the individual’s discounting curve, the more likely this is to happen. Thus, hyperbolic discounting provides a mathematical model for preference reversals from outcomes that have larger long-term benefits to those that are smaller, but immediately available (for more comprehensive reviews, see Ainslie, 2001 and Odum, 2011a, b).
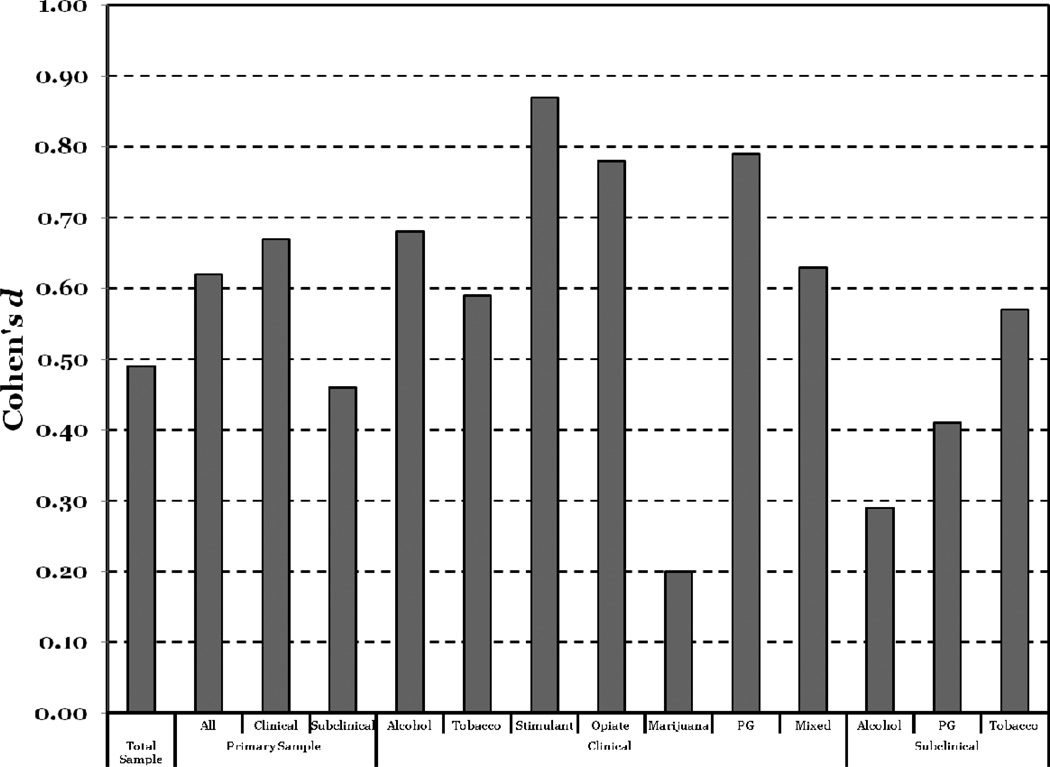
There is also a very robust empirical connection between discounting and addictive behavior. Many cross-sectional categorical studies have been completed, comparing delay discounting in a criterion group of individuals exhibiting addictive behavior to a control group. More impulsive discounting has been found in criterion groups based on levels of alcohol misuse and dependence (e.g., Petry, 2001; Vuchinich & Simpson, 1998), nicotine dependence (e.g., Bickel, Odum, & Madden, 1999), stimulant dependence (e.g., Coffey, Gudleski, Saladin, & Brady, 2003), opiate dependence (e.g., Madden, Petry, Badger, & Bickel, 1997), and pathological gambling (e.g., MacKillop, Anderson, Castelda, Mattson, & Donovick, 2006). Moreover, a recent meta-analysis of 46 cross-sectional studies found consistent and substantial evidence of differences between groups in these categorical studies (MacKillop et al., 2011). Specifically, as illustrated in Figure 2, the meta-analysis revealed a statistically significant medium effect size difference between groups across studies, and also revealed statistically significant and medium effect size differences within addictive behavior categories, suggesting this was common across addictive drugs (albeit with the exception of marijuana use disorders). In addition, the meta-analysis revealed a significantly larger effect size in studies using clinical samples, suggesting impulsive discounting is more robustly associated with clinically-relevant addictive behavior, not simply participation in these behaviors. In addition to categorical studies, continuous analyses have also reported significant positive associations between level of impulsive discounting and the level of addictive behavior (e.g., Alessi & Petry, 2003; Bobova, Finn, Rickert, & Lucas, 2009; MacKillop et al., 2010; Sweitzer, Donny, Dierker, Flory, & Manuck, 2008). Thus, there is very robust evidence of the association between impulsive delay discounting and addictive behavior in cross-sectional studies.
Figure 2.
Aggregated differences between criterion groups comprising individuals exhibiting various forms of addictive behavior and control participants in units of effect size (Cohen’s d); data from MacKillop et al. (2011).
Of course, cross-sectional associations cannot alone demonstrate causality, but a number of lines of evidence suggest that impulsive discounting at least partially predates addictive behavior and plays an etiological role. For example, in a large sample of adolescents, Audrain-McGovern et al. (2009) examined discounting and smoking behavior over a six year period and found that discounting predicted smoking initiation, not the other way around. These findings are similar to a previous finding that discounting assessed in pre-schoolers was associated with adult drug use 20 years later (Ayduk, Mendoza-Denton, Mischel, Downey, Peake, & Rodriguez, 2000). In addition, two retrospective studies have found high discounting in adolescence has been found to be associated with an earlier onset of symptoms of alcohol use disorders (Dom, D'Haene, Hulstijn, & Sabbe, 2006; Kollins, 2003). Finally, studies using animal models of discounting have found that impulsive discounting predicts acquisition and escalation of drug self-administration in drug-naïve animals (Anker, Perry, Gliddon, & Carroll, 2009; Marusich & Bardo, 2009; Perry, Larson, German, Madden, & Carroll, 2005; Perry, Nelson, Anderson, Morgan, & Carroll, 2007), convincingly suggesting that impulsive delay discounting serves as a predisposition to addictive disorders. In addition to contributing to the development of addictive disorders, several prospective studies have found that delay discounting predicts negative outcomes in smoking cessation treatment (Krishnan-Sarin et al., 2007; MacKillop & Kahler, 2009; Sheffer et al., 2012; Yoon et al., 2007). Similarly, impulsive discounting has been found to be significantly associated with poorer opiate treatment response (Passetti, Clark, Mehta, Joyce, King, 2008). Most recently, Stanger et al. (2012) found that discounting predicted treatment outcome in adolescents with marijuana use disorders. Thus, these studies suggest that, in addition to predating addictive behavior, impulsive discounting also plays an important role in maintaining addictive disorders, serving as a risk factor for treatment failure.
Taken together, delay discounting as a phenotype reflects a decision-making orientation toward smaller immediate rewards compared to larger delayed rewards. This orientation can be thought of as an aspect of personality (e.g., Odum, 2011b) or simply as a decision-making profile. In either case, however, this orientation toward intertemporal rewards can be objectively and precisely characterized using decision-making tasks. Across methodologies and in both cross-sectional and longitudinal designs, there is converging and convincing evidence that impulsive discounting contributes to the development and maintenance of addictive disorders.
The endophenotype concept in psychiatric genetics
The substantial role of genetics in addictive disorders is now well established (for a review, see Goldman, Oroszi, & Ducci, 2005). Additive genetic factors are estimated to have a heritability of 50–60% (Goldman et al., 2005), meaning that approximately half of the statistical probability of developing an addictive disorder is attributable to genetic influences. Moreover, the recent revolutionary advances in human molecular genetics and high-throughput bioinformatics have offered an unprecedented opportunity to potentially identify the specific genetic polymorphisms responsible for conferring genetic risk. Paradoxically, however, from early candidate gene association studies examining one variant in a single gene to more recent genome-wide association studies using 1M or more polymorphisms across genome1, the results have been disappointing because the genetic associations observed in early studies have often not been replicated and, where present, the effect sizes have been very small (Goldman et al, 2005; Treutlein & Rietschel, 2011). In other words, in spite of the established high levels of heritability, the specific genetic variants that confer risk for (or protection against) addictive disorders remain largely obscure. This disconnect between moderate heritability and very small individual genetic associations applies to most psychiatric disorders (Turkheimer, 2011).
One reason why this may be the case is increasing consensus that diagnosis of a clinical syndrome constitutes a low quality phenotype. This is because a diagnostic phenotype by its nature is inherently heterogeneous. Two individuals could receive an identical diagnosis with many different permutations of symptoms that entirely do not overlap. Severity introduces further variability, with some individuals barely meeting criteria and others exhibiting the most severe manifestation of the disorder. Moreover, subtypes within disorders have been empirically identified (e.g., Hesselbrock & Hesselbrock, 2006; Johnson, van den Bree, & Pickens, 1996; Moss, Chen, & Yi, 2007), reflecting clusters of characteristics that meaningfully aggregate and may be diversely genetically influenced. Furthermore, the course of substance use disorders is highly variable; large portions of individuals recover with or without formal treatment, while others exhibit stable patterns over long periods of time and still others exhibit a progressive course (e.g., Guttmannova et al., 2011). This means that individuals may be classed together as reflecting the phenotype of interest at one point but actually reflect very different profiles when considered longitudinally. Taken together, the common problem is that dichotomous clinical diagnosis has manifold instances in which ‘apples and oranges’ may be combined and this creates a very heterogeneous and imprecise phenotype.
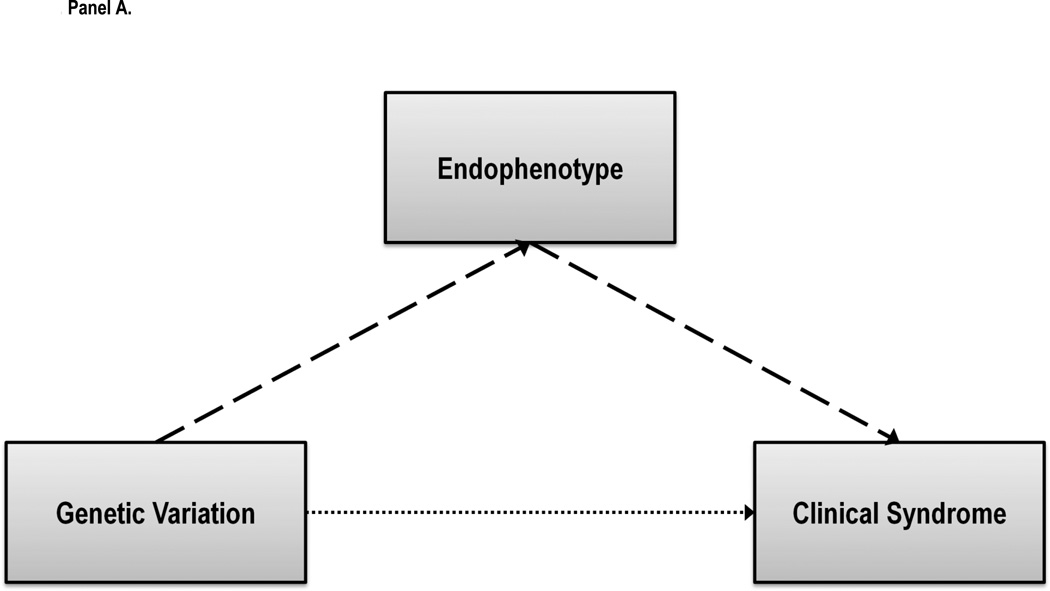
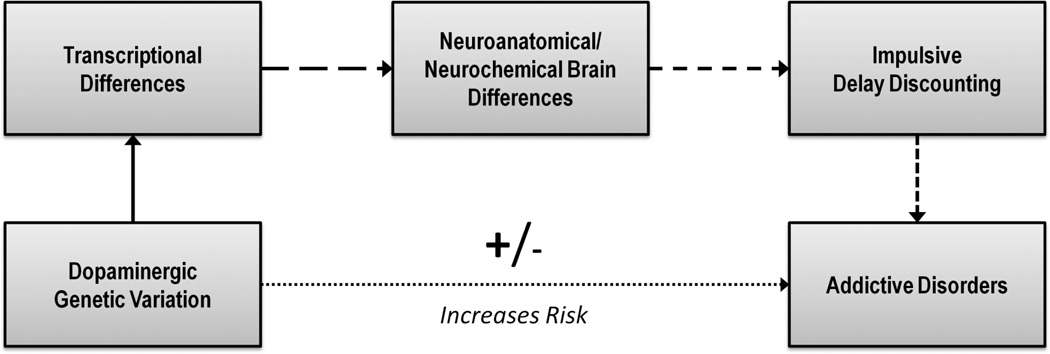
Toward clarifying the genetic basis for psychiatric disorders, an endophenotype approach focuses on characterizing narrower mechanistic processes that are more proximally related to genetic variation and in turn confer risk for a given clinical condition (Gottesman & Gould, 2003; Gottesman & Shields, 1973). Endophenotypes are defined as genetically-influenced characteristics that putatively connect the dots from specific sources of genetic variation to increases or decreases in the probability of having a disorder, as illustrated in Figure 3. Genetic variation is theorized to directly result in transcriptional differences, which then result in systems-level differences, which, in turn, result in the observed endophenotypic variation, and thereby proximally influences risk for the disorder. More simply, endophenotypes are the intervening psychobiological mediators between specific forms of genetic variation and disorder liability. Thus, a key element for the endophenotype approach is the importance of refining the complex pathways by which risk is communicated from the genome to an individual’s clinical status. By being more closely associated with genetic variation, endophenotypes are theorized to be more reliable and reflect larger magnitude relationships, making them easier to detect and replicate in smaller samples. Furthermore, by clarifying the pathophysiological and motivational processes that contribute to the clinical condition, endophenotypes have the potential to provide the foundation for implementing genetically-informed treatment or prevention.
Figure 3.
The endophenotype approach in psychiatric genetics and delay discounting as an endophenotype. Panel A presents the conceptual premise, that for heritable polygenic conditions such as addictive disorders, alternative mechanistic phenotypes are necessary to map the relationship from genetic factors to risk for the condition. These phenotypes are defined as stable and heritable features of the individual that predate the onset of the condition, are independent of the condition itself, but are probabilistically related to the development of the condition. Panel B provides the elaborated endophenotype model that includes the additional relevant levels of analysis, including the direct effects of genetic variation on protein transcription, those effects on larger biological systems, the proximal endophenotype, and, ultimately, disorder liability. For both panels, the number of spaces in the arrows reflecting relationships reflects the putative strength of the relationship, with fewer spaces reflecting stronger relationship and vice versa.
These manifold benefits, however, are predicated on the successful identification of endophenotypes and a number of criteria have been proposed to clarify whether a characteristic is appropriately thought of as an endophenotype. Since the initial introduction of the concept almost forty years ago (Gottesman & Shields, 1973), the term was intended to refer to a characteristic or process that was not readily observable, but was significantly associated with the disorder of interest, could be objectively and reliably assessed over time, and was heritable. In addition, further criteria for an endophenotype are evidence that it is state-independent (i.e., present even when the disorder is not) and present in non-affected family members at a higher prevalence than in the population in general, reflecting transmission patterns of familial association and cosegregation (Flint & Munafò, 2007; Gottesman & Gould, 2003). Additional criteria that have been proposed are that an endophenotype be continuous in nature, part of a biologically plausible etiological process, and closer to the genetic causative factors than to the disorder (Flint & Munafò, 2007). Importantly, it has been noted that an endophenotype may be developmentally-defined (e.g., evident only after a certain age or only pertaining to a specific developmental period) and may only be present in response to a laboratory challenge (Hasler, Drevets, Gould, Gottesman, & Manji, 2006).
Evidence supporting delayed reward discounting as an endophenotype
Although the notion of delay discounting as an addiction endophenotype is relatively recent, there is nonetheless accumulating support from a number of domains. As discussed previously, there is converging evidence that discounting is robustly associated with addictive behavior. In addition, there is evidence that it meets the core endophenotype criteria. To start with, a number of studies have found discounting preferences to be relatively stable over time. Robust reliability has been demonstrated in adolescents and adults over numerous time intervals, including one week (Baker, Johnson, & Bickel, 2003; Clare, Helps, & Sonuga-Barke, 2010; Simpson & Vuchinich, 2000), six weeks (Beck & Triplett, 2009), two months (Takahashi, Furukawa, Miyakawa, Maesato, & Higuchi, 2007), three months (Takahashi et al., 2007; Weatherly, Derenne, & Terrell, 2011), one year (Kirby, 2009), and even multiple years (Anokhin et al., 2011; Audrain-McGovern et al., 2009).
More importantly, there is also evidence from studies with both animals and human twins that delay discounting is heritable. In the former case, a number of studies have investigated differences in discounting between inbred rodent strains. As these strains are isogenetic within each strain (Beck et al., 2000) and receive identical rearing environments, systematic differences in discounting across strains can be attributed to genetic differences. The first study revealing strain differences did so in the context of investigating clomipramine as a pharmacotherapy for impulsivity in Lewis and Fischer 344 rats (Anderson & Woolverton, 2005), finding no effect of the medication, but significantly higher delay discounting in the Lewis rats. This finding has subsequently been replicated twice using a steady-state paradigm to minimize within-session carryover (Madden, Smith, Brewer, Pinkston, & Johnson, 2008; Stein, Pinkston, Brewer, Francisco, & Madden, 2012). Using six strains, Wilhelm and Mitchell (2009) found significantly greater discounting in Fischer rats compared to Copenhagen and Noble rats, but not Lewis rats, although a recent study suggests that this failure to replicate may be a function of procedure rather than strain (Stein et al., 2012). Studies using mice have revealed similar results. In an initial investigation of four strains, significant between-strain differences were present as was covariation between discounting behavior and locomotor activity (Isles, Humby, Walters, & Wilkinson, 2004). Most recently, no differences in discounting were found between dopamine D4 receptor knock-out mice and wild-type controls (Helms, Gubner, Wilhelm, Mitchell, & Grandy, 2008), suggesting that the dopamine D4 receptor is not playing a primary role.
Of particular interest, a number of studies have further linked strain-based differences in discounting with propensity for addictive behavior. For example, rats with a high preference for saccharin have been show to have a propensity for cocaine consumption using a number of indices (e.g., Carroll, Anderson, & Morgan, 2007; Carroll, Morgan, Lynch, Campbell, & Dess, 2002; Perry, Morgan, Anker, Dess, & Carroll, 2006), and also exhibit significantly more impulsive discounting than low-preferring rats (Perry et al., 2007). Similarly, rats and mice from alcohol-preferring strains exhibit significantly greater delay discounting compared to low alcohol preference strains (Oberlin & Grahame, 2009; Wilhelm & Mitchell, 2008). However, this is not the case for all alcohol-preferring strains, as mice in another alcohol-preferring strain, STDRHI2, have not been found to differ compared to STDRL02 mice, a low alcohol preference strain (Wilhelm, Reeves, Phillips, & Mitchell, 2007). In addition, surprisingly, greater impulsivity has been found in DBA/2J mice compared to C57BL/6J mice (Helms, Reeves, & Mitchell, 2006), which is the opposite of what would be predicted by strain-based drug preferences. Although not conclusive at this point, these studies nonetheless reveal the interplay of genetic factors, impulsive discounting, and drug motivation without the typical confound of drug exposure in human studies.
These studies using animal models reveal both aggregate genetic influences on impulsive choice and, in several cases, the connection between these influences and drug motivation. However, there are limits to generalizability, such as whether the same genetic variants are associated with greater discounting across strains or whether variation in discounting in humans is similarly genetically-influenced and, if so, attributable to homologous genes. Toward addressing the question of heritability in humans, the gold standard is the twin methodology, in which a given phenotype is examined in both monozygotic and dizygotic twins and statistical modeling is used to infer the amount of variation attributable to genetic factors based on known differences in the amount of shared genetic variation (i.e., effectively 100% in monozygotic twins versus 50% on average in dizygotic twins). More specifically, this approach permits parsing the relative overlap in the characteristic or condition to additive genetic factors (the sum of individual genetic effects); non-additive genetic factors, such as gene×gene interactions; shared environmental experiences (events or experience that pertain to both twins), and non-shared environmental factors (events or experiences that are specific to an individual). These designs generate heritability estimates of the proportion of variation attributable to these different domains, such as the estimate of 50–60% heritability of addictive disorders (Goldman et al., 2005).
While many twin studies have examined disorder status as a phenotype, only one twin study has assessed discounting. Anokhin et al. (2011) examined the heritability of delay discounting in early adolescent twins who were assessed using a single item discounting measure at age 12 and 14 and found evidence for additive genetic influences at both ages (age 12, a2 = .30; age 14, a2 = .51). Interestingly, however, the best fitting model indicated significant genetic influences at age 12 predicting discounting at both age 12 and 14, but also with significant non-shared environmental influences at both ages 12 and 14. In other words, these results suggest that discounting is indeed a heritable phenotype but both genetic and environmental influences appear to be operative.
Another common methodology for investigating endophenotypes is examining whether the phenotype of interest aggregates in individuals who do not themselves have the disorder, but have a family history (FH) of the condition. This permits assessing the presence of the endophenotype in unaffected but nonetheless at-risk relatives. For heritable disorders, like addictive disorders, the presence of a FH increases risk for the condition and, if FH status is associated with a specific characteristic, it implicates the characteristic as a potential mechanism. To date, only a small number of studies have been conducted using this approach with discounting and the findings have been mixed. Petry, Kirby, and Kranzler (2002) examined paternal alcohol dependence family history status in 122 adults and found significantly more impulsive discounting in FH+ women, but not men. Crean, Richards, and de Wit (2002) also examined paternal alcoholism in the context of a tryptophan depletion laboratory challenge, but found no differences between the two groups and no effect of the manipulation on discounting. In a relatively small sample (N = 33), Herting, Schwartz, Mitchell, and Nagel (2010) detected greater discounting in FH+ adolescents compared to FH− at the level of a statistical trend, although a significant difference was present in terms of choice reaction times.
The findings from these studies modestly implicate discounting as a mechanism conferring the risk from family history, but significant methodological issues make this conclusion far from definitive. For example, two of the three preceding studies had very small samples that were underpowered for effects below relatively large magnitude group differences. Moreover, these studies focused specifically on family history of alcohol misuse, but have not necessarily ruled out the presence of other drug misuse or other forms of addictive behavior in the FH− samples. This is a problem because, as noted above, impulsive discounting is associated with an array of addictive disorders (MacKillop et al., 2011) and the ostensible ‘control group’ may have been contaminated with individuals who also had a family history of addictive behavior, substantially undermining the experimental comparison. Most recently, in the largest study to date (N = 298), some of these considerations were addressed via careful characterization of family history status for alcohol and other drugs, and also quantification of the density of the family history (Acheson, Vincent, Sorocco, & Lovallo, 2011). In this study, FH+ status was dichotomously associated with more impulsive discounting and significantly positively related to density of family history of addictive disorders. Thus, although the data are somewhat mixed with regard to family history status and discounting, it appears that when sample sizes are sufficiently large and the status of both FH+ and FH− are thoroughly characterized, there is support for the aggregation of impulsive delay discounting with family history status.
The preceding studies provide oblique inferences about genetic influences on delay discounting, but a small number of molecular genetic association studies have directly investigated the role of specific polymorphisms on discounting2. For example, in a moderately-sized nonclinical sample of young adults (N = 166), discounting was examined in relation to two genetic variants, the DRD2/ANKK13 Taq IA single nucleotide polymorphism (SNP; rs1800497) and the variable number of tandem repeats polymorphism in exon 3 of the dopamine D4 receptor gene (DRD4 VNTR) (Eisenberg et al., 2007). In this case, possession of at least one DRD2/ANKK1 A1 allele was associated with significantly greater discounting and possession of both the A1 allele and the long form of DRD4 VNTR was interactively associated with substantially more impulsive discounting. In other words, there was no main effect of DRD4 VNTR genotype, but the A1 allele carriers who also had at least one long version of DRD4 VNTR exhibited disproportionately high levels of impulsive discounting compared to the other genotype combinations. In a separate study of a locus related to the dopamine D2 gene, nonclinical young adults who were C allele carriers of the DRD2 C957T (rs6277) SNP exhibited more rapid responding during discounting, although not more impulsive discounting (White, Lawford, Morris, & Young, 2009).
Two studies have examined the relationship between COMT val158met SNP (rs4680) and discounting. In the first, a study of alcoholics and healthy controls (N = 19), individuals who were homozygous for the val variant exhibited significantly more impulsive discounting (Boettiger et al., 2007). In the second study, individuals with attention deficit/hyperactivity disorder (ADHD) were compared to control participants (N = 68) and discounting differed by COMT val158met genotype, but met-met homozygotes exhibited significantly greater discounting independent of ADHD diagnosis (Paloyelis, Asherson, Mehta, Faraone, & Kuntsi, 2010), a result that differs from Boettiger et al. (2007), where val-val homozygotes exhibited the greatest discounting. Paloyelis et al. also examined discounting according to genetic variation in the DAT1 (the dopamine transporter gene) 10/6 haplotype4 and DRD4 VNTR. Interestingly, diagnosis interacted with genotype, such that control participants with two copies of the DAT1 10/6 haplotype exhibited significantly more impulsive discounting than those with fewer than two copies and exhibited a profile of preferences that was equivalent to the ADHD participants. This is important because the presence of the 10/6 haplotype is robustly associated with ADHD (Asherson et al., 2007) and it suggests that 10/6 haplotype may be responsible for differences in discounting, but that this effect may be obscured in individuals with the clinical condition who exhibit a generally high level of discounting. In other words, because clinical populations are comprised of individuals who have a convergence of risk factors and by their nature exhibit non-normative levels of performance, it may be that nonclinical populations will be especially important for successfully detecting genetic effects. Notably, no DRD4 VNTR genotype main effect was present in the Paloyelis et al. study, converging with Eisenberg et al. (2007) and also a recent study by Garcia et al. (2010). It also is worth noting that these associations were present only for a decision-making discounting task, not an experiential discounting task using actual delays, a point discussed in greater detail below.
Clearly only a small number of molecular genetic studies have been conducted on humans and those that have include small to moderate sample sizes, often comprising both clinical and nonclinical populations. As such, it is challenging to make definitive conclusions, but one theme that is emerging is the implication of genetic variation that results in changes in dopamine neurotransmission and, in particular, reduced levels of dopamine neurotransmission. For example, in the case of the association between DRD2/ANKK1 Taq IA and interaction of DRD2/ANKK1 and DRD4 VNTR (Eisenberg et al., 2007), the alleles associated with greater impulsivity have both been associated with dopaminergic hypofunction (Jonsson et al., 1996; Jonsson et al., 1999; McGeary, 2009). Similarly, in the association between impulsive discounting and COMT val-val genotype, the val allele is associated with greater enzymatic metabolism of dopamine (i.e., more rapid degradation of dopamine, terminating the activity faster) (Savitz, Solms, & Ramesar, 2006), again implicating dopamine hypofunction. Finally, in the case of the DRD2 C957T polymorphism, the C allele is associated with reduced striatal D2 binding (Hirvonen et al., 2004), further supporting a role for reduced dopamine activity as a neurobiological mechanism for genetic influences on delay discounting.
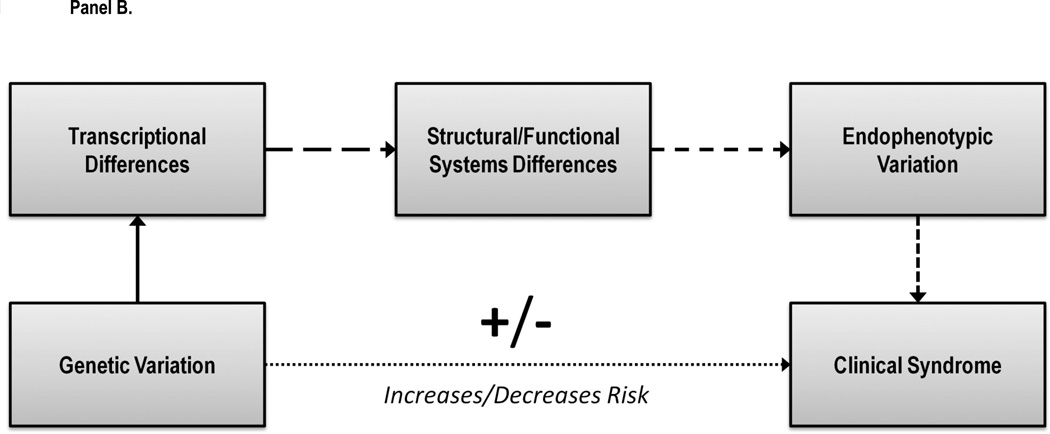
This hypothesis is also consistent with studies using animal models and fMRI that have implicated the ventral striatum in discounting decision-making, a key region in the corticomesolimbic dopamine system (for reviews, see Cardinal, 2006; Carter, Meyer, & Huettel, 2010). For example, lesioning the ventral striatum in an animal model of discounting has been shown to induce significantly more impulsive delay and probability discounting (Cardinal & Howes, 2005; Cardinal, Pennicott, Sugathapala, Robbins, & Everitt, 2001). Similarly, compared to Fischer rats, Lewis rats have fewer dopamine D2 and D3 receptors in the striatum and also fewer dopamine transporters in these regions (Flores, Wood, Barbeau, Quirion, & Srivastava, 1998), paralleling the behavioral findings. Moreover, a recent meta-analysis of brain activity during fMRI discounting paradigms revealed the ventral striatum was consistently recruited (Carter et al., 2010). However, it is important to note that dopaminergic hypofunction as an underlying mechanism is not consistent with all the published findings (e.g., Koffarnus, Newman, Grundt, Rice, & Woods, 2011; Pine, Shiner, Seymour, & Dolan, 2010). Moreover, other neurotransmitter systems have also been implicated in delay discounting (Bevilacqua et al., 2010; Mobini, Chiang, Al-Ruwaitea, Ho, Bradshaw, & Szabadi, 2000; Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000), as have important interactions between neurotransmitter systems (Winstanley, Dalley, Theobald, & Robbins, 2003; Winstanley, Theobald, Dalley, & Robbins, 2005). Thus, although diverse neurotransmitters are likely involved, an emerging theme is that genetic variation that contributes to reduced corticomesolimbic dopamine tone is associated with more impulsive discounting. This hypothesized model is presented in Figure 4.
Figure 4.
Delay discounting as an endophenotype for addictive disorders. Genetic variation related to dopamine neurotransmission is hypothesized to affect the neuroanatomical and neurochemical substrates responsible for variation intertemporal choice. Variants associated with more impulsive discounting as a trait are hypothesized to constitute a risk factor for addictive disorders. The number of spaces in the arrows reflecting relationships reflects the putative strength of the relationship, with fewer spaces reflecting stronger relationship and vice versa.
Taken together, acknowledging the relatively small number of studies and ambiguities to date, the assembled findings nonetheless ‘tick-the-boxes’ for delay discounting as an endophenotype. There is evidence that delay discounting decision-making is a stable and heritable phenotype; that elevations in impulsive discounting are present in otherwise unaffected individuals who are FH+ for addictive disorders; and that specific forms of genetic variation are associated with significant differences in discounting preferences. These findings thus provide the critical second link, that genetic factors are partially responsible for discounting decision-making. Moreover, in the case of strain-based differences in discounting, there is also evidence that differences aggregate with strains that also engage in more addictive behavior. This connection has not been made directly in humans, however, and while the findings to date suggest this is a highly promising prospect, it is by no means established or a foregone conclusion. Future progress will depend on further articulating the links between genetic factors, discounting preferences, and risk for developing an addictive disorder.
Important methodological considerations for future studies
The preceding sections provide the theoretical and empirical basis for pursuing delay discounting as an endophenotype for addictive disorders. This is clearly a promising area of research and success could have a major impact in terms of understanding the genetic basis of addiction, the nature of impulsivity, and even prevention and treatment. Importantly, however, there are a number of considerations and challenges that pertain to delay discounting in general, but especially genetic research. To start, there are issues of measurement with regard to discounting, as a wide array of assessments are used in the field (MacKillop et al., 2011) and the discounting indices vary in terms of how well they correlate with each other, ranging from very high magnitude to negligible correlations (e.g., Epstein et al., 2003; Krishnan-Sarin et al., 2007; MacKillop et al., 2010; Paloyelis et al., 2010). This may seem surprising at first glance, but when the diversity of commodities, delay durations, and size of commodity are considered, these variable relationships make more sense. As such, it will be critical for investigators to use consistent assessments across investigations and, ideally, to use multiple measures to determine whether different magnitudes of rewards are particularly sensitive to genetic influences or create latent variables that reflect discounting across reward magnitudes.
In addition, one critical issue is that of reliability, as the magnitude of genetic influences will be necessarily bounded by the reliability of the discounting assessment over time. For example, the experiential discounting task was intentionally designed to be sensitive to state changes (Reynolds & Schiffbauer, 2004), favoring sensitivity to dynamic changes in preferences as a result of experimental manipulations over general reliability across time. This makes the resulting phenotype more generally fluctuating and in turn less likely to be sensitive to genetic effects. These important differences may clarify variability in findings within studies (e.g., Paloyelis et al., 2010). In general, trait-focused assessments that have established temporal reliabilities would be predicted to be most sensitive as endophenotypic measures.
The issue of consistency applies also to the index of discounting. In the case of curve fitting, nonlinear regression is the most typical procedure but multiple models can be applied and the solutions almost always includes some level of error (i.e., R2 <1.0). For this reason, metrics like assumption-free area under the curve (Myerson et al., 2001; Odum, 2011b) or impulsive choice ratio (Mitchell et al., 2005; Odum, 2011b) may be more appropriate to avoid incorporating that error. Alternatively, differences in the sensitivity of different methods of deriving discounting functions to genetic influences may be informative with regard to the nature of discounting and the optimal derivation methods. For example, if reliable associations with specific loci are consistently of larger magnitude using one model compared to the others, it would provide further support for that analytic strategy. In any case, it will be important for these methodological issues to be thoroughly considered and addressed as findings in this area accumulate.
A second consideration represents diverse domains, but the common theme is the importance of assessing alternative aspects of the individual. The risk here is essentially of ‘third-variable’ confounds, or potential relationships with alternative or unmeasured variables that lead to spurious conclusions. In other words, genetic variables or discounting variables may be associated with each other by virtue of a common association with an alternative characteristic, so it is important to thoroughly examine and incorporate variation on related domains to rule out false positives (or false negative). In particular, this applies to both collateral demographic and cognitive factors. As a decision-making phenotype, the typical delay discounting task using money as a commodity is necessarily contextualized within factors such as the individual’s socioeconomic status; as well as cognitive processing factors, such as working memory and attention. In the first case, income has typically not been found to be substantially related to discounting (e.g., MacKillop & Tidey, 2011), but this is not always the case and the role of income should be considered nonetheless. In contrast, aspects of cognitive function have been consistently associated with discounting (Bickel, Yi, Landes, Hill, & Baxter, 2011; Hinson, Jameson, & Whitney, 2003; Jaroni, Wright, Lerman, & Epstein, 2004; Shamosh et al., 2008). This is not surprising, as the process of discounting decision-making involves simultaneously juxtaposing the appeal of one immediately available reward in the present with another of larger magnitude in the future, requiring abstraction, working memory, inhibition, and other cognitive faculties. Moreover, genetic factors have also been implicated in cognitive functioning (e.g., Dickinson & Elvevag, 2009), making these processes high-risk confounds if not simultaneously considered. Thus, high-quality assessment of the discounting phenotype would optimally also characterize these related factors to incorporate their role and to avoid misattribution of genetic associations.
Similar to these preceding factors, another source of potential confounding is a failure to address comorbidities in sampling. The association between impulsive discounting and addiction is arguably the most well established, but there is also evidence that it is associated with several related conditions, such as ADHD (e.g., Scheres et al., 2006; Hurst, Kepley, McCalla, & Livermore, 2011), antisocial personality disorder (e.g., Acheson et al., 2011; Petry, 2002), and bipolar disorder (e.g., Ahn et al., 2011). As such, case-control studies using clinical cases compared to a matched control group will need to be sure that both groups are fully characterized in terms of present and past psychiatric conditions to avoid potential confounding. More generally, collateral assessment of relevant variables on continua, such as attentional control and antisociality, would be optimal in order to characterize the relationship to the discounting phenotype simultaneously in genetic studies. This issue is essentially the same as the potential demographic and cognitive confounds; a well-designed study should be able to specifically attribute genetic influences to the discounting phenotype and in turn to addictive behavior, but not other conditions. Of course, it is also possible that genetic influences may be determined to be more robust with other comorbid conditions. Alternatively, it is entirely plausible that impulsive discounting will be best understood as a risk factor for a number of conditions, not only addictive disorders, but perhaps disorders characterized by poor self-control or perhaps externalizing disorders in general. As a consequence, genetic variation that contributes to impulsive discounting may be risk loci for a more broad domain of psychopathology. In light of these possibilities, it is critical for these collateral characteristics and conditions to be ascertained to examine these relationships empirically.
A further consideration is that although there is relatively extensive evidence that impulsive discounting predates addictive behavior, as reviewed above, there is also evidence that persistent drug exposure can also result in long-term increases in impulsive delay discounting (Dallery & Locey, 2005; Roesch, Takahashi, Gugsa, Bissonette, & Schoenbaum, 2007; Simon, Mendez, & Setlow, 2007) and probability discounting (Nasrallah, Yang, & Bernstein, 2009). Not all studies have found this to be the case and important methodological considerations apply (for a review, see Stein & Madden, in press), but, nonetheless, the aggregated findings suggest that impulsive delay discounting represents a recursive etiological process. In other words, impulsive discounting may well serve as both a risk factor and a characteristic that can be exacerbated by addictive behavior. Impulsive discounting from this perspective operates as a ‘Matthew Effect’ (i.e., advantage potentiating further advantage, disadvantage potentiating further disadvantage), with individuals with innate propensities to discount the future being at greatest risk of developing addictive disorders and in turn become progressively more impulsive, and vice versa. This recursive perspective further complicates the phenotypic picture, as a reliably observed level of discounting would nonetheless reflect influences from both innate factors and the longstanding effects of chronic addictive behavior. As such, fully characterizing the genetic basis of discounting in nonclinical samples will be critical foundational research. Prospective studies characterizing both genetic factors and addictive behavior across the lifespan will ultimately be essential for validly investigating discounting as an endophenotype for addictive disorders.
Finally, it is worth noting that the issue at hand is a hypothesis, not a truism. The prospect of impulsive discounting as an endophenotype should be systematically investigated and the results considered critically, with the recognition that it may not be supported. It is notable that because endophenotypes are putatively more proximal to genetic variation, the associations between endophenotypes and specific genotypes are theorized to be larger in magnitudes and more reliable. In a meta-analysis of several of the most widely studied endophenotypes, however, Flint and Munafò (2007) did not find evidence of larger effect sizes. They recommended caution against presuming that endophenotypes necessarily have a simple genetic architecture, and this may well be the case for discounting also. Furthermore, in thinking critically about genetic influences on discounting, there are potentially influences beyond individual genetic factors. For example, impulsive discounting may in fact be a function of one or more gene × environment interactions, meaning that genetic influences may only be evident in individuals with certain environmental exposures, such as early life stress (e.g., Enoch, 2012). Alternatively, if level of impulsive discounting is indeed largely a function of low corticomesolimbic dopamine tone, it is possible that an array of combinations of genetic variants might contribute to this systems-level characteristic, making impulsive discounting nonspecific to individual loci and an emergent property of the system. For example, variants responsible for transporter molecules clearing dopamine from the synapse and variants responsible for differences in the density or binding potential of dopamine receptors subtypes could both lead to the same aggregate profile of dopaminergic activity, high or low. Based on the model in Figure 4, these systems-level effects would be associated with commensurate differences in discounting, but this would happen by way of entirely different loci. Capturing relationships like this may require novel strategies, such as examining aggregate genetic risk scores (e.g., Derringer et al., 2010; Karlson et al., 2010) or examining combinatorial genetic profiles that have known systemic effects (e.g., contrasting individuals who are positive and negative for a panel of risk or hypofunction variants). Fundamentally, although the findings to date are promising, it is important to recognize that the endophenotype approach is not the only possible framework for understanding genetic influences on discounting and the multiplicity of influences and mechanisms are worthy of consideration.
Conclusions
The goal of the current review was to lay out the theoretical and empirical basis for considering impulsive delay discounting as an endophenotype for addictive disorders. Although it is only a recent area of focus, there is considerable evidence supporting this perspective. As a phenotype, impulsive discounting is stable over time, heritable, and robustly associated with addictive behavior. A small number of studies have identified direct associations between genetic variation and variation in discounting, and several preclinical studies have triangulated genetics, discounting, addiction liability, but these links remain considerably less well established. Moreover, caution is warranted in light of a history of highly promising initial findings that are ultimately false starts (or at least much more modest starts) in addiction genetics. This is particularly important because there are methodological issues pertaining to discounting as a phenotype that require careful consideration. Nonetheless, the existing literature provides strong basis for systematically investigating this hypothesis.
Acknowledgments
The review was partially supported by NIH grants (K23 AA016936, P30 DA027827, R01 DA032015) and is based on an invited lecture given at the Association for Behavior Analysis International (ABAI) conference Behavioral Economics: From Demand Curves to Public Policy (Chicago, IL, March 2011). Many thanks to Dr. Tim Hackenberg for organizing the conference, to Maria Malott and her staff for its coordination, and to the ABAI Science Board for its sponsorship. I am also grateful for Dr. Tim Hackenberg and Dr. Greg Madden for editorial feedback on earlier versions of this manuscript.
Footnotes
Candidate gene studies involve one or more specific genes that have been identified as promising targets. Typically, different frequencies of one or more variants within the candidate gene are examined between groups of individuals who do and do not have the disorder of interest (cases vs. controls), with the disproportionate frequency of an allele in either the affected group or control group reflecting risk or protection. These are also referred to as association studies as they examine the association of certain genotypes with the condition. Genome-wide association studies are similar to candidate gene association studies, but are atheoretical high-bandwidth investigations that examine frequency variation in many thousands of alleles across the genome, providing a more comprehensive perspective.
Several definitions may be useful for this section. Genetic variation across individuals can come in diverse forms and a polymorphism refers to any instance in which the same portion of a gene exists in multiple forms. These different versions are termed alleles. DNA is comprised of nucleotide base pairs and a single nucleotide polymorphism (SNP) refers to genetic variation reflecting a single difference at a given base. Alternatively, a single nucleotide may be deleted or inserted into the sequence. In contrast, variable number of tandem repeats (VNTR) polymorphisms refer to DNA sequences that include the repetition of the same series of nucleotides multiple times, with individuals varying on the number of times the series repeats. Larger scale structural differences are also present between individuals, such as copy number variations (CNVs) which are large scale portions (thousands to millions of base-pairs) of the genome that are repeated or deleted. Thus, across the ~3.2B nucleotide base-pairs that comprise the genome, human genetic variation can be present from the level of a single base-pair all the way up to segments of DNA thousands and even millions of base-pairs in length.
This polymorphism was initially erroneously identified as being within the DRD2 gene but is in fact within the nearby ANKK1 gene (Neville, Johnstone, & Walton, 2004).
A haplotype refers to a combination of alleles that are physically close to one another and tend to be inherited together.
I have no conflicts of interest to declare with regard to this review.
References
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the oklahoma family health patterns project. Alcoholism: Clinical and Experimental Research. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, et al. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. Journal of Abnormal Psychology. 2011;120(4):911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82(4):463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Breakdown of Will. Cambridge University Press; 2001. [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64(3):345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacology, Biochemistry, and Behavior. 2005;80(3):387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, Biochemistry, and Behavior. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behavioral Genetics. 2011;41(2):175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP, Buitelaar J, Banaschewski T, Sonuga-Barke E, Eisenberg J, Manor I, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Faraone SV. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. American Journal of Psychiatry. 2007;164:674–677. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. Journal of Personality and Social Psychology. 2000;79(5):776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112(3):382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, et al. Genealogies of mouse inbred strains. Nature Genetics. 2000;24(1):23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Beck RC, Triplett MF. Test-retest reliability of a group-administered paper-pencil measure of delay discounting. Experimental and Clinical Psychopharmacology. 2009;17(5):345–355. doi: 10.1037/a0017078. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. Journal of Neuroscience. 2009;29(27):8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Experimental and Clinical Psychopharmacology. 2009;17(1):51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. Journal of Neuroscience. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford WD. The association between individual time preferences and health maintenance habits. Medical Decision Making. 2010;30(1):99–112. doi: 10.1177/0272989X09342276. [DOI] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19(8):1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Cheung TH. Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat. BMC Neuroscience. 2005;6:9. doi: 10.1186/1471-2202-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neuroscience. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Regulation of intravenous cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacology (Berl) 2007;190(3):331–341. doi: 10.1007/s00213-006-0600-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: A review. Journal of Neuroscience, Psychology, and Economics. 2010;3(1):27–45. [Google Scholar]
- Clare S, Helps S, Sonuga-Barke EJ. The quick delay questionnaire: a measure of delay aversion and discounting in adults. Attention Deficit Hyperactivity Disorder. 2010;2(1):43–48. doi: 10.1007/s12402-010-0020-4. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11(1):18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behavioral Brain Research. 2002;136(2):349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behavioral Pharmacology. 2005;16(1):15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Saccone S, Grucza RA, Agrawal A, Lin P, Almasy L, Edenberg HJ, Foroud T, Nurnberger JI, Jr, Hesselbrock VM, Kramer JR, Kuperman S, Porjesz B, Schuckit MA, Bierut LJ Gene Environment Association Studies (GENEVA) Consortium. Predicting sensation seeking from dopamine genes. A candidate-system approach. Psychological Science. 2010;21:1282–1290. doi: 10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164(1):72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, D'Haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101(1):50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behavior and Brain Functions. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Current Psychiatry Reports. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Experimental and Clinical Psychopharmacology. 2003;11:131–138. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- Etter J-F, Perneger TV, Ronchi A. Distributions of smokers by stage: International comparison and association with smoking prevalence. Preventive Medicine: An International Journal Devoted to Practice and Theory. 1997;26(4):580–585. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Research. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O'Donoghue T. Time discounting and time preference: A critical review. In: Loewenstein G, Read D, Baumeister R, editors. Time and decision: Economic and psychological perspectives on intertemporal choice. New York, NY US: Russell Sage Foundation; 2003. pp. 13–86. [Google Scholar]
- Garcia JR, MacKillop J, Aller EL, Merriwether AM, Wilson DS, Lum JK. Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PLoS ONE. 2010;5(11):e14162. doi: 10.1371/journal.pone.0014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews: Genetics. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A Discounting Framework for Choice With Delayed and Probabilistic Rewards. Psychological Bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, et al. Sensitive periods for adolescent alcohol use initiation: predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. Journal of Studies on Alcohol and Drugs. 2011;72(2):221–231. doi: 10.15288/jsad.2011.72.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Helms CM, Gubner NR, Wilhelm CJ, Mitchell SH, Grandy DK. D4 receptor deficiency in mice has limited effects on impulsivity and novelty seeking. Pharmacology, Biochemistry, and Behavior. 2008;90(3):387–393. doi: 10.1016/j.pbb.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188(2):144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2010;34(9):1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock VM, Hesselbrock MN. Are there empirically supported and clinically useful subtypes of alcohol dependence? Addiction. 2006;101(Suppl 1):97–103. doi: 10.1111/j.1360-0443.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Experimental Psychology: Learning, Memory, and Cognition. 2003;29(2):298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hirvonen M, Laakso A, Någren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Molecular Psychiatry. 2004;9:1060–1061. doi: 10.1038/sj.mp.4001561. [DOI] [PubMed] [Google Scholar]
- Hogue A, Dauber S, Morgenstern J. Validation of a contemplation ladder in an adult substance use disorder sample. Psychology of Addictive Behaviors. 2010;24(1):137–144. doi: 10.1037/a0017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RM, Kepley HO, McCalla MK, Livermore MK. Internal consistency and discriminant validity of a delay-discounting task with an adult self-reported ADHD sample. Journal of Attention Disorders. 2011;15:412–422. doi: 10.1177/1087054710365993. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. Journal of Neuroscience. 2004;24(30):6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroni JL, Wright SM, Lerman C, Epstein LH. Relationship between education and delay discounting in smokers. Addictive Behaviors. 2004;29(6):1171–1175. doi: 10.1016/j.addbeh.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Johnson EO, van den Bree MB, Pickens RW. Subtypes of alcohol-dependent men: a typology based on relative genetic and environmental loading. Alcoholism: Clinical and Experimental Research. 1996;20(8):1472–1480. doi: 10.1111/j.1530-0277.1996.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Jonsson E, Sedvall G, Brene S, Gustavsson JP, Geijer T, Terenius L, et al. Dopamine-related genes and their relationships to monoamine metabolites in CSF. Biological Psychiatry. 1996;40(10):1032–1043. doi: 10.1016/0006-3223(95)00581-1. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson EW, Chibnik LB, Peter Kraft P, Jing Cui J, Brendan T, Keenan BT, Ding B, Raychaudhuri S, Klareskog L, Alfredsson L, Plenge RM. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Annals of the Rheumatic Diseases. 2010;69:1077–1085. doi: 10.1136/ard.2009.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR. An additive-utility model of delay discounting. Psychological Review. 2009 Jul;116(3):602–619. doi: 10.1037/a0016414. [DOI] [PubMed] [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychonomic Bulletin and Review. 2009;16(3):457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behavioral Pharmacology. 2011;22(4):300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addictive Behaviors. 2003;28(6):1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson D. Golden Eggs and Hyperbolic Discounting. The Quarterly Journal of Economics. 1997;112(443–477) [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Anderson EJ, Castelda BA, Mattson RE, Donovick PJ. Divergent validity of measures of cognitive distortions, impulsivity, and time perspective in pathological gambling. Journal of Gambling Studies. 2006;22(3):339–354. doi: 10.1007/s10899-006-9021-9. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug and Alcohol Dependence. 2009;104(3):197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda JR, Monti PM, Ray LA, Tidey JW, Rohsenow DJ, et al. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. Journal of Abnormal Psychology. 2010;119:115–125. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl) 2011;216(1):91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Experimental and Clinical Psychopharmacology. 2009;17(5):283–290. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. Journal for the Experimental Analysis of Behavior. 2008;90(3):333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behavioural Pharmacology. 2009;20(5–6):447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The Effect of Delay and of Intervening Event on Reinforcement Value. Quantitative Analyses of Behavior. Vol. 5. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1987. pp. 55–73. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacology, Biochemistry, and Behavior. 2009;93(3):222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clinical Psychology Review. 2006;26(2):109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mischel W. Preference for delayed reinforcement: An experimental study of a cultural observation. Journal of Abnormal and Social Psychology. 1958;56(1):57–61. doi: 10.1037/h0041895. [DOI] [PubMed] [Google Scholar]
- Mischel W. Father-absence and delay of gratification: crosscultural comparisons. Journal of Abnormal and Social Psychology. 1961;63:116–124. doi: 10.1037/h0046877. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss AR. Cognitive and attentional mechanisms in delay of gratification. Journal of Personality and Social Psychology. 1972;21(2):204–218. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29(12):2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2000;149(3):313–318. doi: 10.1007/s002130000385. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152(4):390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug and Alcohol Dependence. 2007;91(2–3):149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, MacKillop J. Living in the here and now: Interrelationships between impulsivity, mindfulness, and alcohol misuse. Psychopharmacology (Berl) 2012;219:527–536. doi: 10.1007/s00213-011-2573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: Models of individual choice. Journal for the Experimental Analysis of Behavior. 1995;64(3):263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, Bernstein IL. Long-term risk preference and suboptimal decision making following adolescent alcohol use. PNAS. 2009;106(41):17600–17604. doi: 10.1073/pnas.0906629106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcoholism: Clinical and Experimental Research. 2009;33(7):1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: trait variable? Behavioural Processes. 2011a;87(1):1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior. 2011b;96:427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35(12):2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug and Alcohol Dependence. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178(2–3):193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006;186(2):235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacology, Biochemistry, and Behavior. 2007;86(4):822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psychopharmacology (Berl) 2002;162(4):425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. Journal of Studies on Alcohol. 2002;63(1):83–90. [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. Journal of Neuroscience. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17(1):15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personality and Individual Differences. 2009;47:973–978. [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behavioural Processes. 2004;67(3):343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. Journal of Neuroscience. 2007;27(1):245–250. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes, Brain, and Behavior. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, et al. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44(11):2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stein JS, Madden GJ. Delay Discounting and Drug Abuse: Empirical, Conceptual, and Methodological Considerations. In: MacKillop J, de Wit H, editors. The Wiley-Blackwell Handbook on Addiction Psychopharmacology. Oxford, UK: Wiley-Blackwell; (in press). [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychological Science. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sheffer C, MacKillop J, McGeary J, Landes R, Carter L, Yi R, Jones B, Christensen D, Stitzer M, Jackson L, Bickel W. Delay Discounting, Locus of Control, and Cognitive Impulsiveness Independently Predict Tobacco Dependence Treatment Outcomes in a Highly Dependent, Lower Socioeconomic Group of Smokers. American Journal on Addictions. 2012;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behavioral Neuroscience. 2007;121(3):543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. The Psychological Record. 2000;50:3–16. [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Experimental and Clinical Psychopharmacology. 2012;20:205–212. doi: 10.1037/a0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Pinkston JW, Brewer AT, Francisco MT, Madden GJ. Delay discounting in Lewis and Fischer 344 rats: Steady-state and rapid-determination adjusting-amount procedures. Journal of the Experimental Analysis of Behavior. 2012;97:305–321. doi: 10.1901/jeab.2012.97-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine and Tobacco Research. 2008;10(10):1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Furukawa A, Miyakawa T, Maesato H, Higuchi S. Two-month stability of hyperbolic discount rates for delayed monetary gains in abstinent inpatient alcoholics. Neurology and Endocrinology Letters. 2007;28(2):131–136. [PubMed] [Google Scholar]
- Treutlein J, Rietschel M. Genome-wide association studies of alcohol dependence and substance use disorders. Current Psychiatry Reports. 2011;13:147–155. doi: 10.1007/s11920-011-0176-4. [DOI] [PubMed] [Google Scholar]
- Turkheimer E. Still missing. Research in Human Development. 2011;8:227–241. [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6(3):292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Weatherly JN, Derenne A, Terrell HK. Testing the reliability of delay discounting of ten commodities using the fill-in-the-blank method. The Psychological Record. 2011;61(1):113–126. [Google Scholar]
- White MJ, Lawford BR, Morris CP, Young RM. Interaction between DRD2 C957T polymorphism and an acute psychosocial stressor on reward-related behavioral impulsivity. Behavioral Genetics. 2009;39(3):285–295. doi: 10.1007/s10519-008-9255-7. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes, Brain, and Behavior. 2008;7(7):705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes, Brain, and Behavior. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcoholism: Clinical and Experimental Research. 2007;31(11):1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170(3):320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30(4):669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. Journal of Personality. 2002;70(4):533–552. doi: 10.1111/1467-6494.05013. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology. 2007;15(2):176–186. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]