Abstract
Many malignant cells produce increased amounts of lactate, which promotes the development of myeloid-derived suppressor cells (MDSCs). MDSCs, lactate, and a low pH in the tumor microenvironment inhibit the function of natural killer (NK) cells and T lymphocytes, hence allowing for disease progression. Ketogenic diets can deplete tumor-bearing animals from MDSCs and regulatory T cells, thereby improving their immunological profile.
Keywords: glycolysis, ketogenic diet, lactate, MDSCs, NK cells
After being largely disregarded for decades, tumor immunology has recently come back into prominence, at least in part because of the approval by the US Food and Drug Administration (FDA) of a monoclonal antibody targeting cytotoxic T lymphocyte-associated protein 4 (CTLA4) and the development of dendritic cell (DC)-based therapies. There is widespread consensus on the notion that the optimal efficacy of these immunotherapeutic agents depends upon the elimination of immunosuppressive tumor-infiltrating cells. Indeed, malignant transformation is generally accompanied by the secretion of soluble factors that stimulate the production of immunosuppressive cells in the bone marrow and recruit them the tumor microenvironment. Immunosuppressive cells are involved in the regulation of a variety of processes, including angiogenesis, inflammation and immunosuppression. The most prominent bone marrow-derived cell population that exerts broadly immunosuppressive functions is constituted by myeloid-derived suppressor cells (MDSC). These cells potently suppress both innate and adaptive immunity by inhibiting T-cell activation, suppressing natural killer (NK)-cell cytotoxicity, favoring the development of regulatory T cells (Tregs) and preventing the maturation of DCs.1
While Otto Warburg reported his milestone discovery more than 80 years ago,2 only recently has progress been made in understanding the role of metabolism in oncogenesis and tumor progression. Many tumor types are indeed characterized by enhanced glycolytic flux and convert a major fraction of pyruvate into lactate, even under normoxic conditions. The latter effect is mediated by lactate dehydrogenase A (LDHA), a hypoxia-inducible factor 1α (HIF-1α) target. It is clear that the Warburg effect serves to generate biosynthetic precursors, thus facilitating the survival and rapid proliferation of malignant cells.3 Is it possible that a tumor environment enriched in lactate also influences anticancer immune responses?
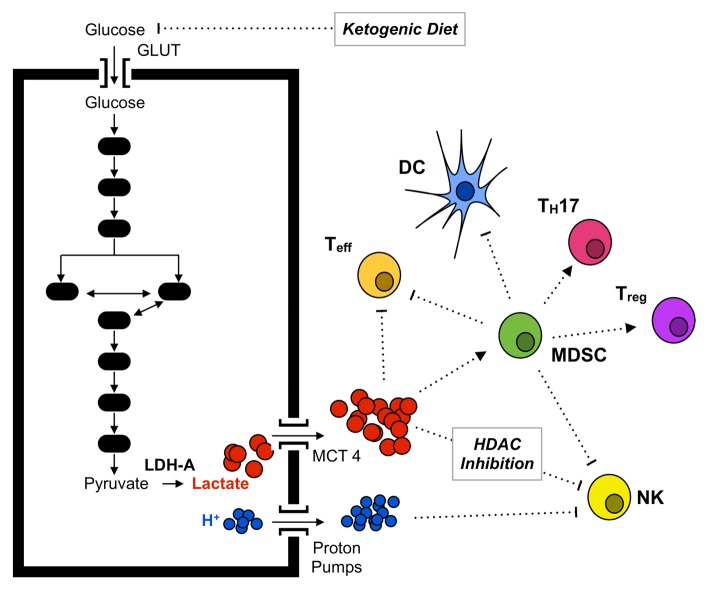
It has recently been suggested that the production of lactate by tumor cells impairs immune effectors by inhibiting the cytolytic functions of T cells and by polarizing immune responses toward a TH17/TH23 pro-inflammatory profile.4,5 In addition, lactate is known to inhibit the maturation of DCs.6 Finally, our recent study adds lactate to the long list of cancer-derived factors that stimulate the generation of MDSCs,7 which already included interleukin IL-1β, IL-6, granulocyte colony-stimulating factor (G-CSF), granulocyte monocyte colony stimulating factor (GM-CSF), prostaglandin E2 (PGE2), tumor necrosis factor α (TNFα), and vascular endothelial growth factor (VEGF). In particular, lactate turned out to exacerbate immunosuppression (by increasing the frequency of MDSCs) and to limit the activity of innate immune effectors (by inhibiting the functions of NK cells), further demonstrating that lactate contributes to the establishment of an immunosuppressive microenvironment for developing tumors (Figure 1).7 Moreover, a recent study suggests that lactate operates as an endogenous inhibitor of histone deacetylases,8 hence regulating (at the transcriptional level) a number of genes that not only are involved in metabolism and transcriptional control but also participate in immune responses, such as natural cytotoxicity triggering receptor 1 (NCR1), which encodes an NK-cell activating receptor. Since it has been reported that effector T cells and Tregs differ from each other with regard to bioenergetic metabolism, high levels of lactate (and/or the consequent decrease in microenvironmental pH) may affect these T-cell subsets in a different fashion.5 We observed an increased frequency of T cells to infiltrate LDHA-deficient tumors as well as LDHA-proficient lesions developing in mice subjected to a ketogenic diet, a suboptimal surrogate for LDHA inhibition. These observations suggest that reduced levels of lactate may be associated with an improved survival or recruitment of effector T cells. Interestingly, a recent report demonstrates that a ketogenic diet lowers the levels of lactate in head and neck cancer lesions.9 It would be interesting to investigate how the kinetic of lactate reduction affects the generation of MDSCs and the overall immunological profile in this setting.
Figure 1. Impact of lactate on tumor-infiltrating immune cells. Cancer cells acquire an altered metabolic state that involves increased glucose uptake and glycolytic metabolism in spite of normal oxygen availability. Cells adapting to this state often exhibit the upregulation of hypoxia-inducible factor 1α (HIF-1α), in turn promoting the synthesis of glucose transporters (GLUTs) and monocarboxylate transporters (MCTs) to allow for glucose intake and lactate secretion, respectively. Increased lactate secretion results in the acidification of the tumor microenvironment and in the recruitment of increased amounts of myeloid-derived suppressor cells (MDSCs). MDSCs inhibit the activity of natural killer (NK) cells and T lymphocytes, skew the immune response toward a TH17 polarization and inhibit the maturation of dendritic cells (DCs). Lactate may also interfere with antitumor immune responses by affecting the transcription of histone deacetylase (HDAC)-regulated genes.
Several reports indicate that the pH of the tumor microenvironment can impact on antitumor immune responses.10 Our study also shows that lowering the local pH from 6.8 to 6.0 results in a significant decrease in the activity of NK cells. Moreover, by utilizing a system for the in vitro generation of MDSCs from mouse bone marrow cells, we demonstrated a decrease in the individual cytotoxic activity of NK cells against target cells at low pH.
In summary, our study highlights a role for tumor-derived lactate in the regulation of NK cells, MDSCs and Tregs, lending further support to the notion that tumor metabolism and immunity are tightly interconnected.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Husain Z, Seth P, Sukhatme V. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. OncoImmunology 2013; 2:e26383; 10.4161/onci.26383
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26383
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O, Posener K, Negelein E. Ueber den stoffwechsel der tumoren. Biochem Z. 1924;152:319–44. [Google Scholar]
- 3.Locasale JW, Cantley LC. Altered metabolism in cancer. BMC Biol. 2010;8:88–90. doi: 10.1186/1741-7007-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–5. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 5.Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–83. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 6.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–21. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 7.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–95. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 8.Latham T, Mackay L, Sproul D, Karim M, Culley J, Harrison DJ, Hayward L, Langridge-Smith P, Gilbert N, Ramsahoye BH. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65:843–9. doi: 10.1080/01635581.2013.804579. [DOI] [PubMed] [Google Scholar]
- 10.Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–56. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]