Abstract
Regulatory T cells (Tregs) are crucial for peripheral tolerance and are intimately involved in immunological diseases and cancer. Recent studies have highlighted a key role for Tregs in metabolic disorders, for instance as they accumulate in the adipose tissue to protect against obesity-related inflammation and insulin resistance. Conversely, the generation and immunosuppressive functions of Tregs are influenced by both systemic and cellular metabolism. The nutritional status as well as metabolic cues such as those provided by leptin impinge upon the proliferation of Tregs. In addition, the mTOR-dependent lipid metabolism has a crucial role in programming the activity of Tregs under steady-state conditions as well as upon activation. This review discusses the intricate interaction between Tregs and metabolism, focusing on the roles of Tregs in systemic and local metabolic circuitries as well as on the regulation of Treg abundance and function by metabolic signals.
Keywords: Treg, metabolism, mTOR, FOXP3, lipid metabolism
Introduction
The rapidly evolving field of immunometabolism deals with the interaction between metabolism and immunity.1 Given the central role of Forkhead box P3 (FOXP3)+ regulatory T cells (Tregs) in the regulation of immune responses,2 it is of pivotal importance to understand the mechanisms that control their homeostasis and function. In this review, we discuss recent advances that reveal an intricate relationship between metabolism and Tregs. First, we summarize studies describing how Tregs affect systemic and local metabolism, with a particular focus on metabolic disorders. Second, we discuss how metabolic cues and immunological signals, especially those conveyed by mechanistic target of rapamycin (MTOR, best known as mTOR, for mammalian target of rapamycin), regulate Treg differentiation, expansion, and activity. Finally, we present a metabolic perspective on the biology of Tregs by integrating the knowledge originating from these recent studies.
How do Tregs Regulate Systemic and Local Metabolism?
Historically, metabolism and immunology were considered to be two separate disciplines with little overlap. Berk et al. first observed increased levels of C-reactive protein in the course of coronary artery disease.3 Shortly after, Hostamisligil et al. demonstrated that tumor necrosis factor α (TNFα) is overexpressed in the epididymal fat pad of obese mice and that TNFα neutralization improves obesity-associated insulin resistance.4 Since then, macrophages became the first immune cells to be linked to obesity-related inflammatory responses,5,6 and macrophage-mediated inflammation has been established as a central player in the development of several pathologies.7 The dichotomy between “M1-like” or “classically activated” macrophages that promote inflammation and “M2-like” or “alternatively activated” macrophages that dampen inflammatory reactions has been a tenet in such a macrophage-centric view of metabolic diseases including insulin resistance, type 2 diabetes, coronary artery disease, and fatty liver disease.7 Mechanistically, saturated free fatty acids (FAAs) and various other danger signals, which are elevated in obese individuals, stimulate inflammation through Toll-like receptor (TLR) signaling8-11 and the NLR family pyrin domain containing 3 (NLRP3) inflammasome.12-14 Conversely, anti-inflammatory omega-3 fatty acids exert a beneficial effect in metabolic diseases by inhibiting the NLRP3 inflammasome.15 Recent studies have linked other cells of the innate and adaptive immune system to metabolic disorders. Some of them promote inflammation, including neutrophils,16,17 mast cells,18 CD8+ T cells,19 CD4+ interferon γ-producing TH1 cells,20,21 and B cells.22 Others ameliorate inflammation, including γδ T cells,23 eosinophils,24 group 2 innate lymphoid cells25 and Tregs.20,26-28
Tregs Play a Key Protective Role in Metabolic Disorders
Feuerer et al. described that Tregs preferentially accumulate in the visceral adipose tissue (VAT) of normal mice rather than in peripheral lymphoid organs, but their abundance significantly drops in the abdominal fat tissue of various obese mouse models.20 These VAT-associated Tregs are not derived from local conventional T cells (Tconvs), as determined by their distinct TCR repertoire. These cells also have a distinct phenotype as compared with their lymph node counterparts, such as a partial overlap of TCR repertoires and a markedly elevated expression of the anti-inflammatory cytokine interleukin (IL)-10. Moreover, the sequences of the T-cell receptors (TCRs) expressed by VAT-associated Tregs indicate that they are selected by certain unidentified local antigens. However, the robust expression of chemokine receptors and ligands by these cells suggests that they may be migrants from other tissues. The developmental details of VAT-associated Tregs remain to be delineated. Nonetheless, loss- and gain-of-function studies have established a protective role of VAT-associated Tregs in obesity-associated inflammation and metabolic abnormalities.20 This unique function of VAT-associated Tregs was later found to be dependent on their unusually high expression of peroxisome proliferator-activated receptor γ (PPARγ),29 a transcription factor crucial for adipogenesis.30 The Treg-specific deletion of Pparg leads to a reduction of Tregs specifically in the VAT, but not in other tissues. PPARγ-deficient VAT-associated Tregs exhibit reduced levels of GATA-binding protein 3 (GATA3), a transcription factor that is essential for the expression of FOXP3 and the immunosuppressive activity of Tregs.31,32 Strikingly, the insulin-sensitizing effect of the widely-used drug pioglitazone, a PPARγ agonist, appears to be largely dependent on PPARγ expression by VAT-associated Tregs. Mechanistically, pioglitazone appears to enhance lipid uptake by VAT-associated Tregs as it stimulates the expression of the fatty acid transporter CD36, thus potentially activating fatty acid oxidation.29 These studies highlight an unexpectedly dominant role of VAT-associated Tregs in the regulation of systemic metabolism. Thus, adipose tissue-infiltrating Tregs, presumably by inhibiting pro-inflammatory immune cells or by stimulating the development or activity of M2 macrophages,33 suppress obesity-related inflammation and improve various metabolic parameters.
Tregs Control Immune Responses by Regulating Amino Acid Catabolism
In addition to shaping organismal metabolism, Tregs also influence amino acid metabolism in the immune microenvironment. Tregs employ diverse strategies to enforce immune tolerance.34 One of such strategies is to stimulate antigen-presenting cells (APCs) especially dendritic cells (DCs), to express enzymes that catabolize essential amino acids (EAAs). Indoleamine 2,3-dioxygenase (IDO), an enzyme that consumes tryptophan, inhibits T-cell activation, maintains immune tolerance, and prevents fetal rejection.35 IDO is induced in DCs upon the interaction between cytotoxic T lymphocyte-associated protein 4 (CTLA4) on Tregs and CD80/CD86 on DCs.36 Recently, Cobbold et al. have demonstrated that Tregs enforce DCs and skin grafts to express enzymes that catabolize at least 5 different EAAs, including tryptophan. Reduction of one or more of these EAAs prevented T cells activation and induced FOXP3 expression by Tconvs, hence activating “infectious tolerance,” the process whereby Tregs convert Tconvs into novel Tregs.37 Further investigation is required to elucidate whether such mechanism contributes to the beneficial effects of Tregs on metabolic disorders.
How Does Metabolism Affect Tregs?
The leptin link
How do Tregs preferentially accumulate within the VAT of normal mice but decline as obesity progresses? Studies from the group led by Giuseppe Matarese potentially explain this observation.38 These authors found that leptin, an adipocyte-derived hormone that controls food intake and systemic metabolism, reduces the proliferative potential of Tregs upon TCR stimulation. Notably, in vitro anergy, or the lack of proliferative responses to TCR stimulation, is one of the hallmarks of Tregs.39 The same group also demonstrated that Tregs produce leptin and express high amounts of the leptin receptor (LEPR, also known as OBR). The administration of an anti-leptin antibody reversed the anergic status of Tregs in vitro and enabled them to proliferate in response to anti-CD3 and anti-CD28 stimulation.38 Furthermore, OBR-deficient Tregs exhibited increased proliferative responses,38,40 and leptin-deficient mice harbored greater numbers of Tregs than their wild-type counterparts.20,38 These observations partially explain the reduction of VAT-associated Tregs observed in obese mice, as these animals contain elevated levels of leptin in the fat tissue. However, the mechanism that underlies the increased frequency of Tregs in the normal adipose tissue as compared with lymphoid tissues remains to be defined. A recent study demonstrates that hypothalamic agouti-related peptide-expressing (AgRP) neurons, which are essential for feeding and survival, regulate the development and function of Tregs in a leptin-independent manner.41 Therefore, systemic metabolism influences Treg homeostasis via leptin-dependent and -independent mechanisms.
mTOR signaling negatively controls Treg cellularity
mTOR signaling orchestrates an evolutionarily conserved pathway that couples cell growth and homeostasis to nutrient availability and metabolic cues.42 mTOR is the catalytic (kinase) subunit of two distinct signaling complexes, mTORC1 and mTORC2, that differ from each other by the scaffold proteins, regulatory associated protein of MTOR, complex 1 (RPTOR, best known as RAPTOR) and RPTOR-independent companion of MTOR, complex 2 (RICTOR), respectively. mTORC1 activates anabolic metabolism, in particular protein and lipid synthesis, and inhibits autophagy, while mTORC2 regulates cytoskeletal organization.42 The immunosuppressive drug rapamycin preferentially inhibits mTORC1, but also interferes with the activity of mTORC2 under specific conditions.43 The molecular composition, signaling characteristics and immunological functions of mTORC1 and mTORC2 have been extensively reviewed42-45. Here, we focus on the interplay between mTOR signaling and Treg cellularity and function.
The activation of mTOR by the phophosphonositide-3-kinase (PI3K)-AKT axis inhibits the expression of FOXP3 and other Treg signature molecules, while the inhibition of mTOR with rapamycin or in response to limited EAA availability stimulates FOXP3 expression and the differentiation of induced Tregs (iTregs).37,46-49 Delgoffe et al. provided the first genetic evidence in support of the ability of mTOR signaling to negatively regulate the development of iTregs.50 These authors showed that mTOR-deficient Tconvs failed to differentiate into TH1, TH2 or TH17 effector T cells, but spontaneously converted into FOXP3+ iTregs upon TCR stimulation, even in the absence of exogenous Treg-polarizing cytokines. Both mTORC1 and mTORC2 appear to contribute to this negative regulation, as the deletion of either Ras homolog enriched in brain (RHEB, an important activator of mTORC1) or RICTOR alone fails to spontaneously induce the differentiation of iTregs.50,51 These observations demonstrate that mTOR signaling is a negative regulator of de novo FOXP3 induction.
In order to fully realize the therapeutic potential of Tregs, it is necessary to expand them in vitro. Battaglia et al. found that the administration of rapamycin allows the selective expansion of Tregs in vitro while preserving their immunosuppressive activity.52 Subsequent studies confirmed that the concomitant administration of anti-CD3/CD28 antibodies, IL-2 and rapamycin drives the preferential expansion of Tregs while inhibiting that of Tconvs.53-55 Such an in vitro expansion protocol may translate into promising Treg-based immunotherapeutic strategies for clinical applications.56
Of note, leptin inhibits Treg proliferation by activating mTOR signaling.40 The in vitro anergy of Tregs correlates with increased mTORC1 activity at baseline. The transient inhibition of mTOR with rapamycin has been shown to break such an anergic state, recapitulating the effect of anti-leptin antibodies. Furthermore, acute starvation, which reduces circulating leptin levels, results in decreased mTOR activity and thus promotes the proliferation of Tregs upon TCR stimulation. In line with these observations, the administration of leptin to starved mice activates mTOR, hence restoring the anergic state of Tregs.40 Thus, leptin-mediated mTOR activation dampens Treg proliferation.
mTOR-mediated lipid synthesis promotes Treg activity
Despite these studies pointing to a negative effect of mTOR signaling on FOXP3 induction and Treg expansion, very little is known about how mTOR signaling affects the immunosuppressive activity of Tregs. Notably, although rapamycin does not affect Tregs functions, this chemical only inhibits mTORC1 to partial extents.57 Moreover, it is unclear whether mTOR signaling inhibits the development of Tregs in a physiological setting, even though previous studies have observed a negative association between mTOR activity (e.g., as forced by AKT activation) and the abundance of thymus-derived Tregs (tTregs).47,58 Interestingly, although the transient inhibition of mTOR promotes the cellularity of Tregs, chronic rapamycin treatment impairs Treg proliferation in vivo.40 Moreover, the deletion of Sirt1 (coding for sirtuin 1) in AgRP neurons decreases the abundance of tTregs while impairing their immunosuppressive activity. This phenotype correlates with decreased mTOR activity in Tregs, suggesting a positive role for mTOR in tTreg homeostasis and function.41
To obtain new insights into the role of mTOR in Treg homeostasis and function, we deleted Rptor and/or Rictor specifically in Tregs.59 Surprisingly, the Treg-specific deletion of Rptor led to severe autoimmune diseases and early lethality, while deletion of RICTOR did not affect immune homeostasis. RAPTOR-deficient Tregs had intrinsic proliferative defects, expressed reduced levels of Treg effector molecules such as CTLA-4 and inducible T cell co-stimulator (ICOS), and exhibited highly impaired immunosuppressive functions in vivo. Such a functional impairment was associated with reduced lipid biosynthesis, in particular with defects in the mevalonate pathway. The treatment of Tregs with statins, pharmacological inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR, the rate-limiting enzyme of the mevalonate pathway), impaired their immunosuppressive activity, a functional defect that could be resolved upon the addition of mevalonate, the immediate downstream product of HMGCR. In this setting, mTORC1 signaling was potently activated upon TCR and IL-2 stimulation, pointing to a link between immune signals and lipid biosynthesis that underlies the functional competence of Tregs. Although the deletion of Rictor in Tregs did not lead to obvious immune defects, mutant mice exhibited modestly reduced cellularity of Tregs, suggesting that mTORC2 positively regulates Treg homeostasis. Importantly, mTORC1 appeared to promote Treg function at least in part by inhibiting mTORC2, because deletion of both Rptor and Rictor in Tregs partially ameliorated the autoimmune disease of mice bearing a Treg-specific deletion of Rptor only. Hence, in line with the association between PPARγ-dependent lipid metabolism and the homeostasis of VAT-associated Tregs,29 Tregs require mTORC1 signaling, in particular mTORC1-mediated lipid biosynthesis, to maintain their homeostasis and function.59
A Metabolic Perspective on Tregs
The discoveries that the leptin-mTOR axis controls Treg cellularity and that mTORC1 promotes Treg function allow us to tackle some of the perplexing observations about Tregs. Tregs are anergic and hyporesponsive in vitro, but highly proliferative in vivo,60 and express high levels of effector molecules. A combination of rapamycin and high-dose IL-2 can expand Tregs in vitro while maintaining their immunosuppressive activity.52,53 However, these two agents have opposite effects on mTOR: rapamycin inhibits mTOR while IL-2 activates the PI3K-AKT-mTOR signal transduction cascade. It has been challenging to reconcile these seemingly contradictory facts,61 but recent advances have provided fresh insights into these observations.
T cells experience two metabolic switches when they are activated by cognate antigen-MHC complexes. First, when naïve T cells are activated and become effector T cells, they switch from a catabolic to an anabolic metabolism. Second, as effector T cells differentiate into memory T cells, they switch back from an anabolic to a catabolic metabolism.43 Different T-cell subsets also exhibit distinct metabolic features, which correlate with mTOR activity. Naïve T cells and Tregs primarily utilize fatty acid oxidation and exhibit low mTOR activity, while effector T cells heavily rely on aerobic glycolysis along with high glucose uptake, also known as the Warburg effect, and display high mTOR activity.62-64 The differentiation of effector T cells and iTregs can be reciprocally modulated by metabolic means. For instance, the pharmacological inhibition of glycolysis or mTOR promotes the accumulation of iTregs, but inhibits TH17 differentiation.62,65 However, the glycolytic rate of Tregs is higher than that of naïve T cells.64 Compared with naïve T cells, Tregs also exhibit higher levels of CD71 (the transferrin receptor) and CD98 (a subunit of the l-amino acid transporter), two nutrient receptors whose expression is dependent on mTOR activity. Indeed Tregs are characterized by pronounced mTORC1 activity in steady-state conditions as compared with naïve T cells.40,59 Therefore, Tregs exhibit an intermediate metabolic activity between naïve and effector T cells.
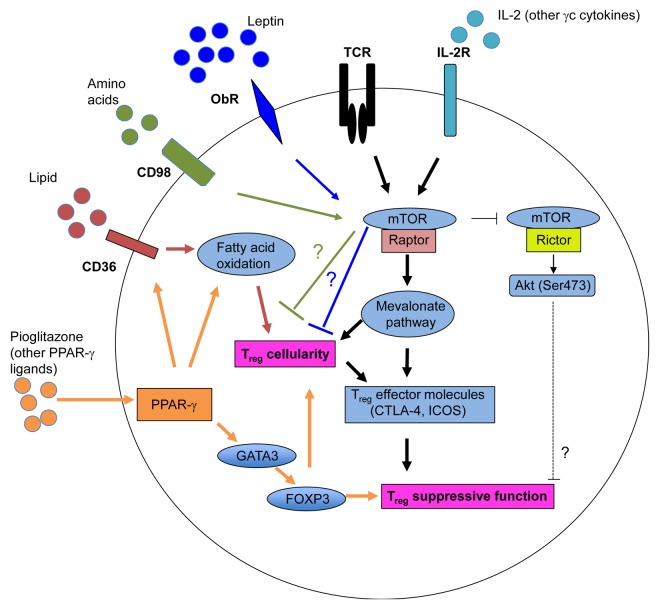
Based on the current literature, we propose that Tregs are constantly under dynamic regulation by both immune signals and metabolic cues (Fig. 1). The conversion of naïve T cells to FOXP3+ Tregs is negatively regulated by mTOR activation and glycolysis. However, the recognition of endogenous or commensal microbial antigens, together with IL-2 and other γc cytokines, activates mTORC1 signaling in tTregs, hence upregulating lipid biosynthesis through the mevalonate pathway. Elevated lipid biosynthesis promotes the expression of effector molecules and the immunosuppressive activity of Tregs, which enable them to control Tconvs to maintain immune homeostasis. Upon foreign antigen stimulation, Tregs further boost mTORC1-dependent metabolism to potentiate their proliferative and immunosuppressive potential. However, such a functional maturation also depends on additional signals, in particular IL-2 released by activated Tconvs, thereby ensuring that this process does not interfere with immune priming but acts in a delayed fashion to prevent immunopathology. These data potentially explains why Tregs exhibit high metabolic and proliferative rate under steady-state conditions in vivo but are less responsive to antigen stimulation than naïve T cells.
Figure 1. Impact of metabolism and mTOR signaling on the abundance and immunosuppressive activity of regulatory T cells. The T-cell receptor (TCR) and interleukin-2 (IL-2) receptor transduce two major immune inputs that activate the mTOR complex 1 (mTORC1), which promotes cholesterol/lipid biosynthesis. In particular, the mevalonate pathway stimulates the proliferation of regulatory T cells (Tregs) and the expression of effector molecules on their surface, hence establishing their functional competence. mTORC1 also negatively regulates the activity of mTORC2 to modulate Treg function. The leptin-dependent activation of mTOR maintains the anergic status of Tregs through an unknown mechanism. Blocking the leptin receptor (OBR) or reducing the levels of leptin enhances Treg proliferation in vitro and in vivo. Amino acids also activate mTOR, which limits the generation of inducible Tregs (iTregs) through an undefined mechanism. Tregs preferentially accumulate in the visceral adipose tissue (VAT), where they exhibit increased peroxisome proliferator-activated receptor γ (PPARγ) expression levels. The activation of PPARγ by either endogenous ligands or exogenous agonists stimulates fatty acid metabolism, hence promoting Treg proliferation as well as the expression of GATA-binding protein 3 (GATA3) and forkhead box P3 (FOXP3), which sustain the immunosuppressive activity of these cells.
In the adipose tissue, Tregs require high levels of PPARγ to maintain FOXP3 expression and homeostasis. The activation of PPARγ by endogenous or pharmacological ligands increases fatty acid metabolism and FOXP3 expression in Tregs, possibly through the expression of GATA3.29 The fat-sensing hormone leptin appears to act as a brake to restrain Treg proliferation by activating mTOR. However, how the leptin-induced activation of mTOR inhibits Treg responsiveness awaits future investigation. The nutritional status controls circulating leptin levels. In obese individuals, increased leptin production leads to a reduction in adipose tissue-infiltrating Tregs, hence exacerbating local inflammation. Conversely, in a underfed individuals, decreased leptin levels relieve the brake on Treg proliferation and drive the accumulation of Tregs, perhaps contributing to starvation-induced immunosuppression.40 The seemingly conflicting use of rapamycin and high-dose IL-2 to expand Tregs in vitro likely reflects the need for breaking the anergy of these cells38,40 while preventing the expansion of Tconvs (as achieved with rapamycin),52,54,55 and maintaining Treg function (as achieved with IL-2).
Concluding Remarks
We are just beginning to appreciate the importance of the metabolic regulation of immunological functions. Many important questions remain to be answered regarding the interplay between metabolism and Tregs. What are the endogenous signals that induce the expansion of Tregs in the VAT and activate PPARγ in VAT-associated Tregs? How does the PPARγ-controlled metabolism of fatty acids promote VAT-associated Treg homeostasis? How do the interactions between Tregs and DCs promote the expression of EAA-catabolizing enzymes? What is the mechanism that underlies the leptin-mTOR-dependent anergic Treg phenotype? How does lipid synthesis promote the functional maturation of Tregs? Finally, whether and how metabolic processes other than lipid metabolism affect the activity of Tregs is an interesting open question.
The interaction between metabolism and Tregs and/or other immune cells suggests that metabolic diseases may benefit from immunotherapeutic regimens and conversely, immunological functions might be modulated by targeting metabolic processes. Indeed, Tregs have been shown to protect against insulin resistance in animal models of diet-induced obesity.66 However, caution should be applied when the intricate interplay between metabolism and Tregs is considered for therapeutic manipulation. For example, the anti-inflammatory effects of statins have been recognized for some time but their efficacy is highly variable,67 and our results suggest that this may reflect the deleterious effect of statins on Treg function.59 Further investigation on the interplay between metabolism and Tregs may lead to innovative strategies for the treatment of both metabolic and inflammatory disorders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by grants from NIH AI101407, AI094089 and NS064599 and American Cancer Society (RSG-13-248-01-LIB).
Citation: Zeng H, Chi H. The interplay between regulatory T cells and metabolism in immune regulation. OncoImmunology 2013; 2:e26586; 10.4161/onci.26586
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26586
References
- 1.Rathmell JC. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol Rev. 2012;249:5–13. doi: 10.1111/j.1600-065X.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–72. doi: 10.1016/0002-9149(90)90079-G. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–9. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 12.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–9. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Talukdar S, Oh Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 20.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Wang J, Xia N, Yan XX, Tang TT, Chen H, Zhang HJ, Liu J, Kong W, Sjöberg S, et al. A guanidine-rich regulatory oligodeoxynucleotide improves type-2 diabetes in obese mice by blocking T-cell differentiation. EMBO Mol Med. 2012;4:1112–25. doi: 10.1002/emmm.201201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–59. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–62. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–70. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–48. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. GATA3 controls Foxp3⁺ regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–15. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 36.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 37.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A. 2009;106:12055–60. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–55. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 40.Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–41. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matarese G, Procaccini C, Menale C, Kim JG, Kim JD, Diano S, Diano N, De Rosa V, Dietrich MO, Horvath TL. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc Natl Acad Sci U S A. 2013;110:6193–8. doi: 10.1073/pnas.1210644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013;25:347–55. doi: 10.1016/j.coi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–8. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 47.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–74. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–56. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 53.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 54.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–9. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 56.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–77. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–90. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Procaccini C, Matarese G. Regulatory T cells, mTOR kinase, and metabolic activity. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1058-6. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–76. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass Bak-Jensen K, McGeachy MJ, Do JS, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeiser R, Maas K, Youssef S, Dürr C, Steinman L, Negrin RS. Regulation of different inflammatory diseases by impacting the mevalonate pathway. Immunology. 2009;127:18–25. doi: 10.1111/j.1365-2567.2008.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]