Abstract
Many questions remain regarding the formation of ultrathin hydroxapatite (HAP) crystals within the confines of collagen fibrils of bones. These structures form through the interplay of the collagen matrix and non-collagenous proteins, and in vitro mineralization studies employing poly(aspartic acid) (PAsp) as a mimic of the non-collagenous proteins have generated mineralized fibrils with structures comparable to their biogenic counterparts. In this article, we employ the nanoscale cylindrical pores perforating track-etch filtration membranes to investigate the role of PAsp in controlling the infiltration and crystallization of calcium phosphate (CaP) within confined volumes. Oriented polycrystalline HAP and non-oriented octacalcium phosphate (OCP) rods precipitated within the membrane pores via an amorphous calcium phosphate (ACP) precursor, where PAsp increased the proportion of OCP rods. Further, ACP crystallized faster within the membranes than in bulk solution when PAsp was present, suggesting that PAsp inhibits crystallization in solution, but promotes it when bound to a substrate. Finally, in contrast to the collagen system, PAsp reduced the yield of intra-membrane mineral and failed to enhance infiltration. This suggests that a specific interaction between the collagen matrix and ACP/PAsp precursor particles drives effective infiltration. Thus, while orientation of HAP crystals can be achieved by confinement alone, the chemistry of the collagen matrix is necessary for efficient mineralisation with CaP.
Introduction
Bone is a remarkable material, where hierarchical organisation of organic molecules and inorganic calcium phosphate crystals over multiple length scales results in a material with mechanical properties optimised for its function 1–3. The mechanism by which mineralisation occurs – which begins with the growth of an oriented array of ultrathin nonstoichiometric carbonated hydroxyapatite (HAP) crystallites within collagen fibrils – is therefore a subject which attracts considerable interest. One of the most remarkable features of the in vivo mineralisation of collagen is the ability of calcium phosphate to infiltrate so effectively into the nanoscale channels present within the fibrils. Indeed, simple diffusion of ions into the fibril would be anticipated to be very slow at this size regime.4 Crystallization then occurs within the channels in the fibrils such that ultrathin platelets of HAP 30–50 nm wide, 60–100 nm long and just 2–6 nm thick form throughout the fibril,5–7 where these are preferentially oriented with their c-axes parallel to the long axis.8, 9
Integral to the effective mineralisation of collagen in bone are the non-collagenous proteins, which are characteristically highly acidic and constitute less than 10 % of the total organic matrix.7 A range of in vivo and in vitro experiments have been carried out to elucidate their roles, and have shown that the collagen matrix and non-collagenous proteins act in combination to achieve effective mineralization.10, 11 While collagen fibrils cannot be mineralized in vitro in the absence of acidic non-collagenous proteins or suitable analogues, knock-out experiments of a number of non-collagenous proteins have revealed changes in bones and dentin, but none have completely prevented collagen mineralization.12–14 In turn, precipitation of calcium phosphate in the presence of acidic non-collagenous proteins has demonstrated that these can either inhibit or promote nucleation and growth according to experimental conditions, and that they can stabilize metastable mineral phases and modulate crystal sizes and shapes.10, 15–20
Significantly, a number of experiments have been performed which have attempted to mimic the mineralization of collagen fibrils in vitro using poly(aspartic acid) (PAsp) as an analogue of the acidic non-collagenous proteins.9, 11, 21–25 While some approaches simply led to the precipitation of calcium phosphate crystals on the surfaces of the collagen fibres,21, 22 experiments by Olszta et al24 and Deshpande and Beniash23 led to intra-fibrillar mineralization such that HAP platelets formed with their crystalline c-axes oriented along the axis of the fibril. Detailed investigation into the mechanism was subsequently obtained using combined cryo-electron tomography and molecular modelling, which suggested that PAsp acts together with the collagen to control mineralization and that clusters of charged amino acids in gap and overlap regions then act as nucleation sites.11
The mechanism by which the acidic non-collagenous proteins influence the mineralization of collagen has been the subject of considerable speculation. Their binding to the gap region has been suggested to promote nucleation within the fibrils,26–28 while an alternative “size-exclusion” model has proposed that the large noncollagenous proteins inhibit mineralisation outside the fibrils. This then promotes mineralization in the channels, which the protein is too large to access.29, 30 The plausibility of this latter mechanism was demonstrated by experiments using the protein fetuin, which despite being too large to enter the collagen fibrils, promotes intra-fibrillar mineralization.11, 29 It has also been suggested that mineral-protein complexes form outside the fibrils, before diffusing into the collagen structure.28, 31 The experiments carried out with PAsp also provide useful insight into the possible validity of these models. While PAsp, in common with fetuin, inhibits mineralization, it is clearly small enough that it has the potential to enter the fibrils. In this case it has been suggested that mineral-PAsp complexes11 or a so-called “polymer-induced liquid phase” (PILP)24, 25 can diffuse inside the fibrils, and/or that PAsp preferentially binds to specific regions of the collagen fibrils, thereby inducing mineralization.23 In all cases, an amorphous calcium phosphate (ACP) phase formed within the collagen fibres as a precursor to HAP, as has also been observed in a number of in vivo systems.32–34 Recent work has also described a similar result in the absence of mimics of noncollagenous proteins, using instead reaction solutions containing very high concentrations of calcium and phosphate.35
In this article, we build on these observations to explore the possible role of PAsp in controlling the infiltration and crystallization of calcium phosphate within small volumes. A key feature of the biomineralization strategies which lead to the formation of bone is that CaP is generated within confined volumes, which necessarily affects the nucleation and growth phases of the mineral.4, 36–38 However, when investigating mineralization directly within collagen, it is impossible to separate the effects of physical constraint from specific chemical interactions. To overcome this problem, we here precipitate CaP in the presence of PAsp within the cylindrical pores perforating track-etch (TE) filtration membranes, where these provide access to nanometer-scale confinement,39, 40 while offering no structural relationship with the CaP phase. Indeed, we have recently used this system to demonstrate that confinement in pores as large as ≈ 100 nm in diameter can cause a marked orientation of the HAP crystallites and a stabilisation of ACP.41
Materials and methods
Calcium phosphate (CaP) was precipitated within the confines of the pores of polycarbonate track-etch membranes in the presence and absence of poly(aspartic acid) (PAsp), and the influence of the pore diameter and the reaction conditions on the resulting particles was investigated.
Precipitation of Calcium Phosphate in Track-Etch (TE) Membrane Pores
A tris-saline buffer was prepared by dissolving 8.77g of NaCl, 6.61g of Tris-HCl and 0.96g of Tris-base in 1L of UltraPure H2O, to give a solution of pH 7.59 at 25°C.24 By the use of this buffer stock solution, separate solutions of 9 mM CaCl2.2H2O and 4.2 mM K2HPO4.3H2O were prepared, where the pH of both solutions was adjusted to 7.4 at 37° C using NaOH and HCl. Isopore GTTP polycarbonate track-etch membranes with pore sizes of 50 nm and 200 nm (Millipore) were used for the experiments, where these are supplied coated with polyvinyl pyrrolidone (PVP) to increase their hydrophilicity and improve filtration performance in aqueous media. The membranes were pre-treated in a plasma cleaner for 1 min under an oxygen plasma to increase their hydrophilic character and were then transferred into vials containing the buffered CaCl2.2H2O solution (4.5 mM). A solution of poly(aspartic acid) sodium salt (PAsp, Mw 2000 – 11000 Da) was added to half of the vials to give the desired concentration of 1 – 100 µg/ml and the solutions were then degassed under vacuum and left overnight to ensure filling of the membrane pores with the solution was complete.
CaP particles were precipitated inside the membrane pores using two methods: an immersion and a double diffusion (DD) method. In the immersion method, a membrane which had been previously soaked in the buffered CaCl2.2H2O solution was placed vertically in a vial containing fresh 9 mM CaCl2.2H2O solution. PAsp was added to the vial to give a concentration between 1 and 100 µg/ml, as required. In the final step K2HPO4 was added to give final concentrations of 4.5 mM CaCl2.2H2O and 2.1 mM K2HPO4.3H2O. In the double diffusion method, a membrane was sealed between a pair of U-tube arms, and buffered 9 mM CaCl2.2H2O and 4.2 mM K2HPO4.3H2O solutions, each containing the desired amount of PAsp (at concentrations between 1 and 100 µg/ml), were added to the two U-tube arms, which were finally sealed with Parafilm to prevent evaporation of the solution. Final concentrations of CaCl2.2H2O and K2HPO4.3H2O were in this way respectively 4.5 mM and 2.1 mM. Both of these experimental set-ups were then incubated in an oven at 37 °C, to mimic body temperature, for times between 3 h and 1 month.
When the reaction was finished, the membranes were removed from the reaction solution, rinsed with ethanol and their surfaces were wiped with the edge of glass cover slip to remove the crystals located on the surface of the membrane. The membranes were then sonicated in a vial of ethanol for ten minutes, rinsed in ethanol and dried with air. To isolate the CaP particles formed inside the membrane pores, the TE membranes were dissolved by putting them inside dichloromethane (DCM), sonicated for two minutes and then centrifuged at 13.2 rpm to separate the inorganic precipitate from the solution. The DCM was then subsequently changed, and the sonication, centrifugation and DCM exchange protocol was then repeated for four more times to completely ensure the removal of the dissolved polymer from the CaP particles. Finally, the remaining DCM was removed from the centrifuged sample and was exchanged for methanol. The sample was then again sonicated for two minutes and centrifuged for another four minutes, before exchanging for ethanol. This process was finally repeated two more times with ethanol.
Control experiments were also carried out under identical reaction conditions, but in bulk solution rather than with a membrane present. Glass slides were placed at the base of the reaction vials, in order to sample the precipitated crystals, and these were removed from the solution after 1 h to 6 days, before being washed with ethanol and dried with nitrogen. Carbon-coated Ni TEM (Transmission Electron Microscope) grids were also placed in the solution on the bottom of the vial, such that the precipitates formed in bulk solution could be examined by TEM, after which they were washed in ethanol and left to dry. XRD and Raman microscopy analysis of the particles precipitated in bulk solution was carried out by isolating the particles by filtration, washing with ethanol twice and the air-drying. The possibility that adsorption of PAsp onto the membrane surface might affect CaP nucleation and growth was also investigated by comparing CaP precipitation from a PAsp-free solution on the surfaces of a native TE membrane and a membrane which had been prior-soaked in a solution of 50 µg/ml PAsp using SEM. CaP was only observed on the surfaces of the TE membrane which had been soaked in PAsp after 6 days.
Characterization of the CaP Particles
The isolated CaP crystals were characterized using Scanning Electron Microscopy (SEM), TEM, X-ray Diffraction (XRD) and Raman microscopy to determine their mineral phase, dimensions and morphologies. Samples for SEM were prepared by placing a droplet of an ethanol suspension of CaP particles onto a glass slide, allowing it to dry, mounting the slide on an SEM stub using adhesive carbon pads, and then sputter coating with 10 nm Pt/Pd. In an alternative method, the ethanol suspension was filtered through a 50 nm track-etch membrane to trap the particles formed in the dissolved membranes. After drying, the filter membrane was mounted on an SEM stub using adhesive carbon pads, before sputter coating as before. SEM was then performed using a LEO 1530 Gemini Field-Emission Gun SEM (FEG-SEM) system operating at 2.00 kV. TEM was performed by placing a droplet of the ethanol suspension containing the CaP particles on a carbon-covered Cu TEM grid, and then examining the dried sample in a Tecnai TF20 TEM operating at 200 kV. Identification of the polymorphs present was achieved using selected area electron diffraction techniques (SAED), carried out in the TEM, and micro-Raman spectroscopy. Raman microscopy was performed using a Renishaw inVia-Raman microscope system, operating with a 785 nm diode laser as excitation source. To determine the different polymorphs, powder XRD (PXRD) was performed using a Bruker D8 Advanced diffractometer equipped with an X-ray source emitting Cu Ka1 radiation. The dry sample was placed on a piece of corundum wafer, and XRD data were collected in an angular range between 5° and 60° with intervals of 0.02°, and a scan rate of 1° min−1.
Results and discussion
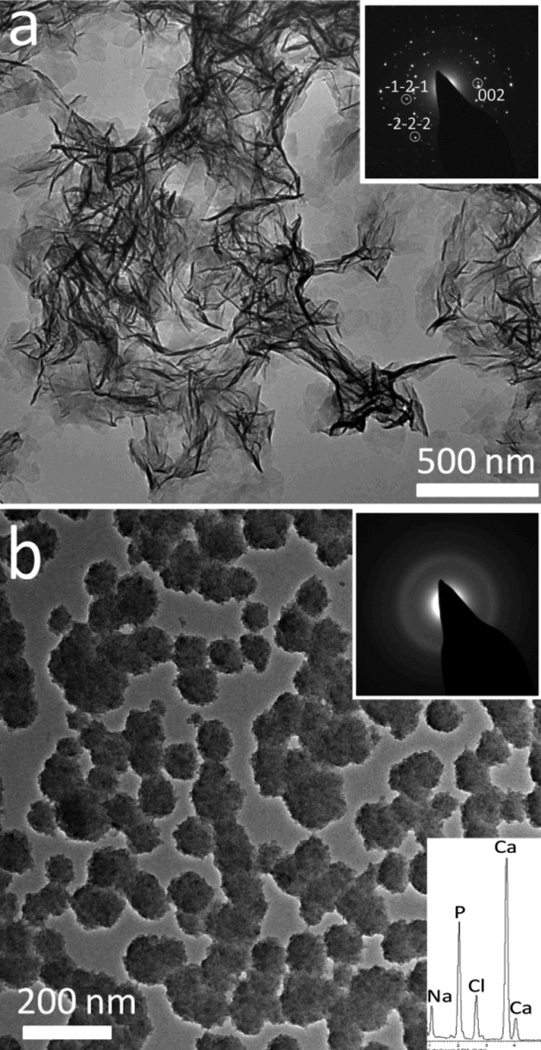
A comparison of the results obtained under the different reaction conditions is provided in Table 1. In order to evaluate the effects of PAsp on the precipitation of calcium phosphate (CaP) in confinement, control experiments were conducted where CaP was precipitated from bulk solution, both in the presence and absence of poly(aspartic acid) (PAsp), under the standard reaction conditions employed. In the absence of PAsp, the material isolated after 1 h comprised amorphous CaP particles with average sizes of 10 nm, as shown by TEM and EDX (Figure S1a). Growth of the amorphous particles to 20 nm in size (Figure S1b) and some formation of 20 nm octacalcium phosphate (OCP) platelets (Figure S1c) was apparent after 2 h, while complete transformation into hydroxyapatite (HAP) occurred after 3 h (Figure 1a). This was confirmed by Raman microscopy (Figure 2a), where the Raman spectrum shows characteristic peaks at 961 cm−1 (symmetric stretching mode ν1), 610 cm−1 and 589 cm−1 (bending modes ν4), and at 430 cm−1 (degenerate bending mode ν2).42 XRD was also used to confirm this result with the peak at 31.78° (121) (Figure 2b) confirming the HAP polymorph.42 TEM examination of these HAP crystals showed that they were plateshaped and approximately 150 nm wide and 200 nm in length (Figure 1a).
Table 1.
Comparison of CaP particles precipitated in bulk solution and within the membrane pores in the presence and absence of PAsp. Both the double diffusion and immersion methods gave similar results, with the only difference that no OCP rods were observed with the immersion method in the absence of polymer.
| Bulk Solution |
Intra-Membrane Particles |
|||
|---|---|---|---|---|
| No PAsp | 50 µg/ml PAsp | No PAsp | 50 µg/ml PAsp | |
| 1 h | Amorphous | Amorphous | - | - |
| 2 h | Amorphous & OCP |
Amorphous | - | - |
| 3 h | HAP | Amorphous | Amorphous precipitates |
- |
| 6 h | HAP | Amorphous | Amorphous & HAP rods |
- |
| 1 day | HAP | Amorphous | HAP rods & few OCP rods |
Amorphous rods |
| 3 days | HAP | Amorphous | HAP rods & few OCP rods |
Amorphous rods |
| 6 days | HAP | Amorphous | HAP rods & few OCP rods |
HAP & 10% OCP rods |
|
3 wks (immersion method) |
HAP | HAP | HAP rods | HAP rods |
Figure 1.
TEM images and corresponding electron diffraction patterns of CaP particles precipitated from buffered solutions. (a) HAP crystals precipitated in the absence of PAsp after 3h. (b) Amorphous CaP particles precipitated in the presence of 50 µg/ml PAsp after 6 days, where EDX shows that the particles have a Ca/P ratio of 1.52.
Figure 2.
(a) Raman and (b) XRD spectra of hydroxyapatite particles precipitated in bulk solution in the absence of PAsp after 3 h.
Addition of PAsp to the system at concentrations of 1 µg/ml and 100 µg/ml had a marked effect on the precipitation in bulk solution, where the polymer retarded crystallization, thereby stabilizing an amorphous calcium phosphate (ACP) phase. Indeed, CaP particles isolated from solutions containing 50 µg PAsp after 6 days were spherical in shape and ≈ 80 nm in diameter, and electron diffraction demonstrated that they were still fully amorphous (Figure 1b). After longer times of between 1 day with 1 µg/ml, 1 week with 10 µg/ml and 3 weeks with 50 µg/ml, the amorphous CaP particles transformed into HAP, as confirmed by TEM and SAED. The HAP particles exhibited average diameters of 40 nm and were therefore significantly smaller than the particles precipitated in the absence of additives (Figure S2).
The CaP particles isolated from the membrane pores, in contrast, were entirely distinct in size, morphology and structure from the particles produced in bulk solution, demonstrating the effect of confinement on the precipitation process. In the absence of PAsp, precipitation of CaP in the membranes using a double diffusion method gave a high yield of rods with average lengths of around 1 µm in the 200 nm pores and about 2 µm in the 50 nm pores, indicating that the CaP only partially infiltrates into the 10 µm long pore. It should also be noted that these membranes are sold for use in filtration, such that the pore diameter quoted is the pore size at the membrane surface, rather than the internal diameter. The rods precipitated in the “200 nm” pores therefore have thicknesses of 200 – 250 nm, giving overall aspect ratios of 5, while the rods formed in the “50 nm” pores have widths of 50 – 100 nm, corresponding to aspect ratios of about 40 (Figure 3). As seen in the SEM images, the majority of rods isolated after 1 day were solid, (Figure 3a), although some hollow rods were also identified, particularly in the 200 nm pores (Figure 3a arrowed). TEM examination of these rods revealed their internal structure and demonstrated that most were composed of small, needle-like particles of around 100 nm in length for rods precipitated in the 200 nm pores (Figure 3a inset) and 30 nm in length for rods precipitated in the 50 nm pores (Figure 3b inset). These rods were identified as HAP using electron diffraction (Figure 3c), as confirmed from the measured d-spacings of 3.41 Å (002), 2.77 Å (112) and 3.1 Å (102), and the 22° angle between the (002) and (102) planes (Figure 3c inset). As noted previously,41 selected area electron diffraction (SAED) patterns of these revealed that many rods exhibited a marked degree of orientation where the [001] axis of HAP is preferentially aligned with respect to the long axis of the rod, especially for the 50 nm pores (Figure 3c).
Figure 3.
CaP rods formed using the double diffusion method in the absence of PAsp. (a) SEM image of rods formed in 200 nm pores after 6 days, where a hollow rod is arrowed and the inset provides a TEM image showing the internal structure of a rod. (b) Rods formed in 50 nm pores after 6 hours, comprising small, needle-like particles of around 30 nm in size (inset). (c) An HAP rod isolated after 1 day from 50 nm pores, where the corresponding electron diffraction pattern demonstrates that the long axis of the rod is coincident with the [001] direction.
In addition to the polycrystalline HAP rods, some single crystal octacalcium phosphate (OCP) rods were also produced under these reaction conditions, amounting to ~0.1% of the total rod population (Figure 4a). TEM images often revealed a low-contrast layer coating the rod, which is likely to correspond to residual polymer resulting from dissolution of the membranes. These OCP rods were of low abundance, were comparable in size to the polycrystalline HAP rods and showed no preferential orientation with respect to the membrane pores. The structural evolution of the intra-membrane particles was also investigated by isolating the rods at different reaction times. After reaction times of 3 hours, only aggregates of spherical, amorphous particles approximately 45 nm in size were present (Figure S3), while after 6 hours both crystalline and amorphous rods were identified in addition to amorphous particles (Figure 4b). These amorphous rods were often hollow and the quantity of the amorphous rods and particles continued to decrease with time such that all material isolated after 1 day was crystalline.
Figure 4.
TEM images of intra-membrane particles produced using the double diffusion method in the absence of PAsp in 50 nm pores. (a) An OCP single crystal rod isolated after 6 days, where the corresponding electron diffraction pattern shows that its long axis corresponds to the [001] direction. (b) A hollow, amorphous CaP particle isolated after 6 h with corresponding electron diffraction pattern and EDX showing a Ca/P ratio of 1.36.
In the presence of 20–100 µg/ml PAsp, rod-like particles were isolated after 6 days, with an ~10 times lower yield being obtained than for comparable reactions performed in the absence of PAsp. This can be attributed to the inhibitory effect of PAsp on the precipitation and crystallisation of CaP.23 These rods were either amorphous or crystalline, where the amorphous rods were typically short, and appeared to comprise poorly-packed spherical particles (Figure 5a). That crystalline rods were also observed is striking given that no crystalline material was formed in control experiments performed in bulk solution in the presence of PAsp after the same reaction time. The crystalline rods formed in the pores were either HAP or OCP and were identical in size, structure and morphology to those precipitated in the membrane pores in the absence of PAsp, with the HAP rods being polycrystalline, and the OCP rods single crystal (Figures 5b and 5c). The HAP rods showed similar degrees of orientation in the 200 nm and 50 nm pores as observed in the absence of PAsp. Notably, the OCP rods were present as a significantly higher proportion (~1% of total rod population) than in the absence of PAsp, and were present in higher numbers in the 50 nm pores as compared with the 200 nm. As observed in the absence of PAsp, the OCP rods were not preferentially aligned with respect to the pore axis. The configuration of the double diffusion method, where precipitation occurs on combination of the calcium and phosphate ions within the membrane pores, leads to effective particle formation even in the absence of PAsp. In order to effectively assess the role of PAsp in promoting infiltration of the mineral into the pores, an “immersion method” was also explored where the membrane was simply immersed in the reaction solution. In the absence of PAsp, a significantly lower yield of rods (by a factor of approximately 100) was obtained as compared with the double diffusion method. These were exclusively polycrystalline HAP in both the 50 nm and 200 nm pores, and were very similar to those produced under the same conditions using the double diffusion method (Figure 6a and inset). In common with the double diffusion method, addition of PAsp to the system resulted in a reduction in the yield of the rods, and a marked increase in the proportion of single crystal OCP rods (Figure 6b). Their crystallographic [001] directions were oriented along the long axis of the rod, and high resolution TEM imaging confirmed their single crystal structure (Figure 6b).
Figure 5.
TEM images and corresponding electron diffraction patterns of particles obtained after 6 days, using the double diffusion method in the presence of PAsp. (a) Amorphous rods precipitated in 200 nm pores in the presence of 20 µg/ml PAsp, where the rods have a Ca/P ratio of 1.44. (b) A polycrystalline HAP rod isolated from 200 nm pores in the presence of 20 µg/ml PAsp. (c) A single crystal OCP rod precipitated in a 50 nm pore in the presence of 100 µg/ml PAsp, where the long axis of the rod corresponds to the [100] direction.
Figure 6.
CaP rods precipitated in 50 nm pores and isolated after 6 days. (a) TEM and SEM (inset) images of HAP rods formed by the immersion method in the absence of PAsp. The corresponding electron diffraction pattern demonstrates that the long axis of the rod is coincident with the [001] direction. (b) TEM image and corresponding electron diffraction pattern of a single crystal OCP rod precipitated using the immersion method, in the presence of 50 µg/ml PAsp. The rod is oriented with its [001] direction coincident with its long axis. The inset shows a high resolution TEM image of the same rod with the (031) lattice plane (d spacing of 2.77 Å) highlighted.
Finally, the influence of longer incubation times on the structures of the intra-membrane CaP particles was investigated (Figure 7). CaP particles, precipitated in the presence of 100 µg/ml PAsp using the “immersion method”, were maintained in the membrane in the reaction solution for one month. Subsequent analysis using TEM showed that all rods were now crystalline. Just as with shorter reaction times, a mixture of polycrystalline and single crystal rods were observed, where the majority were polycrystalline HAP. Analysis of the single crystal rods showed though that in contrast to the OCP rods present after a reaction time of 6 days, these were single crystals of HAP, which were oriented with their [001] direction coincident with the long axis of the rod (Figure 7).
Figure 7.
TEM image and corresponding electron diffraction pattern of a single crystal HAP rod obtained using the immersion method in the presence of 100 µg/ml pAsp, in a 50 nm pore. The rod was isolated after 1 month incubation, and its long axis corresponds to the [001] direction.
With their well-defined structures, track-etch membranes provide a highly attractive system for investigating the effects of confinement on the precipitation of CaP, and for determining how polyelectrolytes such as poly(aspartic acid) (PAsp) – which are suggested to promote the formation of a polymer-induced liquid precursor (PILP) phase24, 43 – influence the infiltration of the mineral into small volumes. Considering first CaP precipitation in the absence of PAsp, comparison of the precipitates formed in bulk solution with those produced in the membrane pores demonstrates that confinement on the length scales used here (50–200 nm) has a number of significant effects on the precipitation of CaP, influencing the size, morphology, orientation, polymorph and the rate of crystallization. While bundles of nanoplatelets of HAP were generated in bulk solution, morphologically distinct polycrystalline rods of HAP with aspect ratios of up to 20–40 times were generated in the membrane pores. Inspection of these by SEM and TEM revealed an internal structure comprising HAP platelets, although the ability of the rods to remain completely intact during isolation from the membrane suggests significant intergrowth. Importantly, although polycrystalline, the rods also show a preferred crystallographic orientation, especially in the case of the smaller pore sizes, where the [001] axis of the HAP crystallites is preferentially aligned with respect to their long axes.
We have previously attributed this effect to the competitive growth of the individual crystallites within the pore,44 and a similar orientation effect has been observed on precipitating HAP within uniaxially deformed gelatin films,45 and within bundles of nanofibres generated through supramolecular assembly,46, 47 or a filamentous phage.48, 49 Such competitive growth has also been observed to give rise to orientational effects and elongation of OCP and HAP crystals in a range of gel and membrane systems.50, 51
Our results also demonstrate that the pores in track-etch membranes can support the formation of large single crystal rods of both OCP and HAP. These were entirely distinct from any particles observed in bulk in control reactions. A number of previous studies have described the formation of single crystal OCP rods, although the vast majority were formed under hydrothermal conditions, in the presence of organic additives.52, 53 Large single crystal rods of HAP have also been formed under similar conditions,54–58 and in some cases have been shown to form via similarly-sized OCP rods as an intermediary phase.52, 53, 59, 60 Study of the transformation of OCP to HAP has suggested that this can occur by two mechanisms; the OCP dissolves and HAP then precipitates, or a direct solid-state transformation can occur, where hydrolysis of an OCP unit cell leads to a two unit cell thick layer of HAP.16 In the latter case, the product HAP crystallites are pseudomorphs of the parent OCP, where the [001] axis of the parent OCP crystal is coincident with the [001] axis of the HAP product.61, 62 Sequential transformation of ACP to octacalcium phosphate (OCP) to HAP has also been observed within the confines of cross-linked gelatine nanoparticles.63
Addition of PAsp to the system had some influence on the intra-membrane precipitation process, although this was far less dramatic than had been anticipated based on the ability of CaCO3 PILP phases to infiltrate into small pores.43 Particle sizes remained unchanged at lengths of about 1 µm, and fewer rods were obtained, a result which can be attributed to the inhibitory effect of PAsp on CaP precipitation.23 We have previously studied the precipitation of CaCO3 in the pores of track-etch membranes and have observed that the presence of a PILP phase, as generated in the presence of PAsp or poly(acrylic acid) (PAA), significantly enhances the ability of the mineral to infiltrate into the membrane pores.38 This results in particles precipitating in a much higher fraction of the membrane pores and significantly increased efficiencies of filling small pores. Further, particles are generated such that they fill the entire membrane pores, thereby reaching 10 µm lengths equivalent to the thickness of the membrane. Indeed, very few short rods were obtained; when mineral precursor is drawn into the pore, it fills the entire volume. These effects were attributed to the liquid-like properties of the PILP phase, where an ability to wet the membrane surface leads to its ability to be drawn into the pore via capillary action.38
The failure of PAsp to enhance filling of the membrane pores with CaP as compared to the additive-free system suggests that CaP/ PAsp behaves quite differently from the CaCO3/ PAsp system. Formation of a PILP phase can be attributed to a microphase separation,44, 64–66 driven in this case by the association of the PAsp polyelectrolyte and Ca2+ ions.44 In the calcium carbonate system, subsequent addition of carbonate ions results in the generation of Ca2+/ CO32−/ PAsp species which gradually convert to amorphous CaCO3 with time, before ultimately crystallising.64 The ability of the system to behave in this way therefore relies upon a subtle balance between the strength of the Ca2+/ PAsp interaction, and the driving force for precipitation of the mineral product. If the cation/polyelectrolyte interaction is very strong, immediate precipitation of these complexes will occur, while a strong cation/ anion interaction will limit formation of the Ca2+/ anion/ PAsp species which characterise the PILP phase. Indeed, it is interesting to note that studies of the nucleation of CaCO3 and CaP in additive-free systems have suggested some important differences between these systems. Mineral clusters form during the early stages of formation of CaCO3, where these comprise an ionic polymer, composed of alternating calcium and carbonate ions, that interchanges between chains, branches and ring structures.67 By comparison, under conditions where ACP forms, discrete calcium triphosphate complexes form, which serve as building blocks of octaclacium phosphate and apatite.68
Given that CaP/PAsp also fails to form thin films – which in addition to effective infiltration and the generation of fibres is a key signature of a PILP phase43, 44, 66 – raises doubt about the formation of a PILP phase in the CaP system. The principal evidence for CaP PILP comes from the effective infiltration of CaP into the gaps in collagen fibres in the presence of PAsp, where it has been suggested that liquid-like droplets of CaP/PAsp were drawn into the collagen fibres by capillarity.24, 25 However, the penetration depth required is only at the nanometre-level and filling of the gaps in the collagen fibres may also be driven by electrostatic interactions between the net negative surface charge of the stabilised PAsp/ACP complex/ nanoparticle and the positively charged regions in the collagen fibril.11 A liquid-like mineral precursor is therefore not necessarily required to drive infiltration into the collagen. We therefore suggest that the PAsp is effective in stabilising ACP particles, where polymer is expected to be adsorbed to the particle surfaces, but that these do not demonstrate the “liquid-like” character which leads to film formation or effective infiltration into pores, and which defines a PILP phase. This may be related to the lower solubility, and thus higher driving force for the precipitation of CaP as compared with CaCO3 (where values for the solubility products of ACP and amorphous calcium carbonate (ACC) are 10−25 and 10−6 respectively.69–71)
Although PAsp did not lead to superior infiltration of the membrane pores, a number of differences in the polymorphs of the crystal products were noted. In addition to polycrystalline HAP rods identical to those formed in the additive-free system, both single crystal OCP and amorphous rods were obtained after 6 days reaction time in the presence of PAsp, where the OCP rods were about 10 times more abundant than in the absence of PAsp. This effect is consistent with the recognised ability of PAsp to retard the transformation of OCP to HAP.72 Prolonged incubation in solution then resulted in the crystallization of all amorphous material, and in the conversion of the OCP rods to single crystal rods of HAP, with preservation of the morphology and orientation (where topotactic transformation of OCP to HAP leads to [100] OCP with respect to [100] HAP).61, 62
PAsp also had a surprising effect on the rate of crystallization of CaP in confinement such that crystallization proceeded faster in the membrane pores than in bulk solution; while no crystalline material was detected in solution after 6 days, crystalline rods were isolated from the membrane pores after the same time. In the absence of PAsp, in contrast, crystallization of CaP proceeded more slowly within the membrane pores such that HAP was produced in bulk solution after 3 hours while only amorphous calcium phosphate (ACP) was isolated from the membrane pores after the same time. This is consistent with previous studies of calcium carbonate precipitation in confinement where amorphous calcium carbonate (ACC) was stabilised in an annular wedge when the walls of the wedge are separated by distances of less than a micron,36 and also within the pores of track-etch membranes.38 Stabilisation was attributed to kinetic factors rather than a thermodynamic stabilisation of ACC with respect to the crystalline polymorphs of CaCO3, where it was suggested that that these may derive from the restricted contact between the ACC precipitates and solution. An increased lifetime of ACC was also observed in droplets,37 vesicles73 and in small ACC particles isolated in a microfluidic device,74 which is consistent with the volume dependence of nucleation rates.
While it is clearly impossible at this stage to offer a definitive explanation for this effect, it is likely to derive from the ability of changes in the barrier to nucleation of crystalline materials within the amorphous phase. Indeed, study of the influence of environmental factors such as additive molecules and ions, pH, ionic strength, and temperature on ACP crystallization has shown that while these can give rise to significant differences in the time taken for initial nucleation of the crystalline phase to occur, crystallization subsequently proceeds at a similar rate for all samples.75 Thus, it is possible that the PAsp acts to promote crystallization when it is located on a solid surface provided by the membrane pores, while it acts as an inhibitor in bulk solution. These apparently opposing behaviours have been observed in a range of systems, where it is suggested that when the soluble additive is bound to a substrate it creates a surface that provides a reduced barrier to heterogeneous nucleation. In free solution, however, the same soluble additive can bind to a developing nucleus, therefore inhibiting its growth.76 Indeed, this scenario has been discussed for non-collagenous proteins adsorbed into collagen.7
Conclusions
In conclusion, the results presented here demonstrate that confinement at length scales of up to ≈ 100 nm, as offered by the track-etch membrane pores, can significantly affect the precipitation of calcium phosphate, generating oriented, high aspect ratio polycrystalline hydroxyapatite (HAP) rods, together with a small number of single crystal octacalcium phosphate (OCP) rods. We then profited from this system to evaluate the effect of the polyelectrolyte poly(aspartic acid) (PAsp) – which has been used as a mimic of the highly acidic non-collagenous proteins – on crystallization of CaP in confinement, where the matrix itself is structurally disordered. While PAsp has been demonstrated to give rise to effective mineralization of collagen fibrils,11, 23, 24 its presence in the current system reduced the yield of rods and failed to increase their lengths. This both challenges the idea that a polymer-induced liquid precursor (PILP) phase forms in the CaP/ PAsp system, and suggests that a specific interaction between the collagen matrix and ACP/PAsp precursor particles11 or PAsp molecules23 drives its efficient mineralization. The most surprising effect, however, was that crystallization of ACP occurred faster in the membrane pores than in bulk solution when PAsp was present. This effect was attributed to an ability of PAsp to promote nucleation when located on a substrate, while behaving as an inhibitor in solution. Overall, these results indicate that while confinement alone can lead to orientation of HAP crystals comparable to that seen in bone, the chemistry of the collagen matrix itself plays an essential role in its effective mineralization with CaP.
Supplementary Material
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC) for funding of a Leadership Fellowship (FCM and BC, EP/H005374/1) and the NIH for financial support via grant NIH R56 DE016703 (EB).
Footnotes
Electronic Supplementary Information (ESI) available: further characterisation of the precipitates.See DOI: 10.1039/b000000x/.
Notes and References
- 1.Fratzl P, Gupta HS, Paschalis EP, Roschger P. J. Mater. Chem. 2004;14:2115–2123. [Google Scholar]
- 2.Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Proc. Natl. Acad. Sci. USA. 2006;103:17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner S, Wagner HD. Ann. Rev. Mater. Sci. 1998;28:271–298. [Google Scholar]
- 4.Wang Y-W, Christenson HK, Meldrum FC. Adv. Funct. Mater. 2013 [Google Scholar]
- 5.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Microsc. Res. Tech. 1996;33:192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Camacho NP, Rinnerthaler S, Paschalis EP, Mendelsohn R, Boskey AL, Fratzl P. Bone. 1999;25:287–293. doi: 10.1016/s8756-3282(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 7.Beniash E. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2011;3:47–69. doi: 10.1002/wnan.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traub W, Arad T, Weiner S. Proc. Natl. Acad. Sci. USA. 1989;86:9822–9826. doi: 10.1073/pnas.86.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner S, Traub W. Faseb J. 1992;6:879–885. [PubMed] [Google Scholar]
- 10.Deshpande AS, Fang PA, Zhang XY, Jayaraman T, Sfeir C, Beniash E. Biomacromolecules. 2011;12:2933–2945. doi: 10.1021/bm2005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk N. Nat. Mater. 2010;9:1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 13.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Calcif. Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 14.Ling YF, Rios HF, Myers ER, Lu YB, Feng JQ, Boskey AL. J. Bone Miner. Res. 2005;20:2169–2177. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Chem. Rev. 2008;108:4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LJ, Nancollas GH. Chem. Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman L, Boskey AL. Calcif. Tissue Int. 2004;75:494–501. doi: 10.1007/s00223-004-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Biochem. J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Bone and Mineral. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 20.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, Boskey AL. Calcif. Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradt J-H, Mertig M, Teresiak A, Pompe W. Chem. Mat. 1999;11:2694–2701. [Google Scholar]
- 22.Zhang W, Liao SS, Cui FZ. Chem. Mat. 2003;15:3221–3226. [Google Scholar]
- 23.Deshpande AS, Beniash E. Cryst. Growth Des. 2008;8:3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB. Mater. Sci. Eng. R. 2007;58:77–116. [Google Scholar]
- 25.Jee SS, Kasinath RK, DiMasi E, Kim YY, Gower L. Crystengcomm. 2011;13:2077–2083. [Google Scholar]
- 26.Veis A. In: Biomineralization. Dove PM, DeYoreo JJ, Weiner S, editors. Chantilly: Mineralogical Soc Amer; 2003. pp. 249–289. [Google Scholar]
- 27.Beniash E, Traub W, Veis A, Weiner S. J. Struct. Biol. 2000;132:212–225. doi: 10.1006/jsbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- 28.George A, Veis A. Chem. Rev. 2008;108:4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price PA, Toroian D, Lim JE. J. Biol. Chem. 2009;284:17092–17101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toroian D, Lim JE, Price PA. J. Biol. Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 31.He G, Gajjeraman S, Schultz D, Cookson D, Qin CL, Butler WT, Hao JJ, George A. Biochem. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li CH, Siegel S, Paris O, Fratzl P, Weiner S, Addadi L. Proc. Natl. Acad. Sci. USA. 2010;107:6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beniash E, Metzler RA, Lam RSK, Gilbert P. J. Struct. Biol. 2009;166:133–143. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahamid J, Sharir A, Addadi L, Weiner S. Proc. Natl. Acad. Sci. USA. 2008;105:12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Azais T, Robin M, Vallee A, Catania C, Legriel P, Pehau-Arnaudet G, Babonneau F, Giraud-Guille MM, Nassif N. Nat. Mater. 2012;11:724–733. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 36.Stephens CJ, Ladden SF, Meldrum FC, Christenson HK. Adv. Funct. Mater. 2010;20:2108–2115. [Google Scholar]
- 37.Stephens CJ, Kim Y-Y, Evans SD, Meldrum FC, Christenson HK. J. Am. Chem. Soc. 2011;133:5210–5213. doi: 10.1021/ja200309m. [DOI] [PubMed] [Google Scholar]
- 38.Kim YY, Hetherington NBJ, Noel EH, Kroger R, Charnock JM, Christenson HK, Meldrum FC. Angew. Chem.-Int. Edit. 2011;50:12572–12577. doi: 10.1002/anie.201104407. [DOI] [PubMed] [Google Scholar]
- 39.Loste E, Meldrum FC. Chem. Commun. 2001:901–902. [Google Scholar]
- 40.Loste E, Park RJ, Warren J, Meldrum FC. Adv. Funct. Mater. 2004;14:1211–1220. [Google Scholar]
- 41.Cantaert B, Beniash E, Meldrum FC. Chem.-Eur. J. 2013 doi: 10.1039/C3TB21296C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutsopoulos S. J. Biomed. Mater. Res. 2002;62:600–612. doi: 10.1002/jbm.10280. [DOI] [PubMed] [Google Scholar]
- 43.Gower LB. Chem. Rev. 2008;108:4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantaert B, Kim YY, Ludwig H, Nudelman F, Sommerdijk N, Meldrum FC. Adv. Funct. Mater. 2012;22:907–915. [Google Scholar]
- 45.Falini G, Gazzano M, Ripamonti A. J. Mater. Chem. 2000;10:535–538. [Google Scholar]
- 46.Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. Small. 2012;8:2195–2202. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 48.He T, Abbineni G, Cao BR, Mao CB. Small. 2010;6:2230–2235. doi: 10.1002/smll.201001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang FK, Cao BR, Mao CB. Chem. Mat. 2010;22:3630–3636. doi: 10.1021/cm902727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iijima M, Moriwaki Y. J. Cryst. Growth. 1991;112:571–579. [Google Scholar]
- 51.Iijima M, Moriwaki Y. J. Cryst. Growth. 1998;194:125–132. [Google Scholar]
- 52.Zhan JH, Tseng YH, Chan JCC, Mou CY. Adv. Funct. Mater. 2005;15:2005–2010. [Google Scholar]
- 53.Ito N, Kamitakahara M, Murakami S, Watanabe N, Ioku K. J. Ceram. Soc. Jpn. 2010;118:762–766. [Google Scholar]
- 54.Jevtic M, Mitric M, Skapin S, Jancar B, Ignjatovic N, Uskokovic D. Cryst. Growth Des. 2008;8:2217–2222. [Google Scholar]
- 55.Tao JH, Jiang WG, Pan HH, Xu XR, Tang RK. J. Cryst. Growth. 2007;308:151–158. [Google Scholar]
- 56.Zhang Y, Lu J. Cryst. Growth Des. 2008;8:2101–2107. [Google Scholar]
- 57.Teshima K, Lee S, Sakurai M, Kameno Y, Yubuta K, Suzuki T, Shishido T, Endo M, Oishi S. Cryst. Growth Des. 2009;9:2937–2940. [Google Scholar]
- 58.Ren FZ, Ding YH, Ge X, Lu X, Wang KF, Leng Y. J. Cryst. Growth. 2012;349:75–82. [Google Scholar]
- 59.Lu X, Wang Y-B, Wang J-X, Qu S-X, Weng J, Xin R-L, Leng Y. J. Cryst. Growth. 2006;297:396–402. [Google Scholar]
- 60.Iijima M, Moradian-Oldak J. J. Mater. Chem. 2004;14:2189–2199. [Google Scholar]
- 61.Arellano-Jimenez MJ, Garcia-Garcia R, Reyes-Gasga J. J. Phys. Chem. Solids. 2009;70:390–395. [Google Scholar]
- 62.Fernandez ME, Zorilla-Cangas C, Garcia-Garcia R, Ascencio JA, Reyes-Gasga J. Acta Crystallogr. Sect. B-Struct. Sci. 2003;59:175–181. doi: 10.1107/s0108768103002167. [DOI] [PubMed] [Google Scholar]
- 63.Ethirajan A, Ziener U, Chuvilin A, Kaiser U, Colfen H, Landfester K. Adv. Funct. Mater. 2008;18:2221–2227. [Google Scholar]
- 64.Bewernitz MA, Gebauer D, Long J, Cölfen H, Gower LB. Faraday Disc. 2012;159:291–312. [Google Scholar]
- 65.Jiang Y, Gower L, Volkmer D, Colfen H. Phys. Chem. Chem. Phys. 2012;14:914–919. doi: 10.1039/c1cp21862j. [DOI] [PubMed] [Google Scholar]
- 66.Schenk AS, Zope H, Kim YY, Kros A, Sommerdijk NAJM, Meldrum FC. Faraday Discuss. 2012;159:327–344. [Google Scholar]
- 67.Demichelis R, Raiteri P, Gale JD, Quigley D, Gebauer D. Nat. Commun. 2011;2 doi: 10.1038/ncomms1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habraken W, Tao JH, Brylka LJ, Friedrich H, Bertinetti L, Schenk AS, Verch A, Dmitrovic V, Bomans PHH, Frederik PM, Laven J, van der Schoot P, Aichmayer B, de With G, DeYoreo JJ, Sommerdijk N. Nat. Commun. 2013;4 doi: 10.1038/ncomms2490. [DOI] [PubMed] [Google Scholar]
- 69.Meyer JL, Eanes ED. Calcif. Tiss. Res. 1978;25:59–68. doi: 10.1007/BF02010752. [DOI] [PubMed] [Google Scholar]
- 70.Onuma K, Ito A. Chem. Mat. 1998;10:3346–3351. [Google Scholar]
- 71.Ogino T, Suzuki T, Sawada K. Geochim. Cosmochim. Acta. 1987;51:2757–2767. [Google Scholar]
- 72.Bigi A, Boanini E, Bracci B, Falini G, Rubini K. J. Inorg. Biochem. 2003;95:291–296. doi: 10.1016/s0162-0134(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 73.Tester CC, Brock RE, Wu C-H, Krejci MR, Weigand S, Joester D. CrystEngComm. 2011;13:3975–3978. [Google Scholar]
- 74.Nudelman F, Sonmezler E, Bomans PHH, de With G, Sommerdijk N. Nanoscale. 2010;2:2436–2439. doi: 10.1039/c0nr00432d. [DOI] [PubMed] [Google Scholar]
- 75.Termine JD, Peckausk RA, Posner AS. Arch. Biochem. Biophys. 1970;140:318–325. doi: 10.1016/0003-9861(70)90072-x. [DOI] [PubMed] [Google Scholar]
- 76.Gunthorpe ME, Sikes CS, Wheeler AP. Biol. Bull. 1990;179:191–200. doi: 10.2307/1541769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.