Abstract
Type 2 inflammatory cytokines including interleukin-4 (IL-4), -5, -9, and -13 drive the characteristic features of immunity against parasitic worms and allergens. Whether IL-9 serves an essential role in the initiation of host-protective responses is controversial and the importance of IL-9 vs. IL-4 producing CD4+ effector T cells in Type 2 immunity is incompletely defined. Herein, we generated IL-9 deficient and IL-9 fluorescent reporter mice that demonstrated an essential role for this cytokine in the early Type 2 immunity against Nippostrongylus brasiliensis. Whereas Th9 cells and Type 2 Innate Lymphoid Cells (ILC2) were major sources of infection-induced IL-9 production, the adoptive transfer of Th9 cells, but not Th2 cells caused rapid worm expulsion, marked basophilia and increased mast cell numbers in Rag2-deficient hosts. Taken together, our data show a critical and non-redundant role for Th9 cells and IL-9 in host protective Type 2 immunity against parasitic worm infection.
Introduction
Naïve T cell differentiation into the CD4+ T helper 2 (Th2) cell subset is considered a central effector mechanism driving adaptive host-protection against parasitic helminths and the pathophysiological sequelae of most allergic diseases (Palm et al., 2012). However, the understanding of Type 2 inflammation has recently increased in complexity to also involve interleukin-9 (IL-9) producing Th cell subsets (Th9), Type 2 Innate Lymphoid Cells (ILC2), and epithelial cell-derived cytokines (Thymic Stromal Lymphopoietin: TSLP, IL-25, and IL-33) (Allen and Maizels, 2011; Lloyd and Hessel, 2010; Paul and Zhu, 2010). While Type 2 inflammatory mechanisms function in both humans and mice to promote elevated IL-4, IL-5, IL-9 and IL-13 amounts, eosinophilia, mastocytosis, basophilia, goblet cell metaplasia, and immunoglobulin E (IgE) production, the pathways responsible for initiating these responses remain controversial (Pulendran and Artis, 2012). Moreover, the functional redundancy among Type 2 cytokines and effector mechanisms has generated considerable debate over which pathway, if any, serves an essential and non-redundant function.
IL-9 is a prototypical Type 2 cytokine implicated in human and murine asthma, anaphylaxis, resistance to nematode infection, antiviral immunity and tumorigenesis (Dodd et al., 2009; Fallon et al., 2000; Faulkner et al., 1997; Faulkner et al., 1998; Forbes et al., 2008; Gessner et al., 1993; Knoops et al., 2005; Lu et al., 2012; Osterfeld et al., 2010; Shimbara et al., 2000). IL-9 has been reported to be mainly produced by T cells but can also be produced by mast cells, eosinophils and ILC2 (Gounni et al., 2000; Hultner et al., 2000; Schmitt et al., 1989; Uyttenhove et al., 1988; Wilhelm et al., 2011). IL-9 stimulates the growth, proliferation and survival of T cells, enhances the production of IgE from B cells, promotes the proliferation and differentiation of mast cells and hematopoietic progenitors and induces secretion of mucus and chemoattractant molecules by mucosal epithelial cells (Dong et al., 1999; Gounni et al., 2004; Petit-Frere et al., 1993; Uyttenhove et al., 1988; Van Snick et al., 1989). Recent identification of Th9 cells as a specialized T helper subset secreting IL-9 in vitro, has caused a reevaluation of the natural sources of this cytokine in vivo. Although previously considered as a Th2 cell derived cytokine, Th9 cells (differentiated via TGF-β and IL-4), Th17 cells (differentiated with TGF-β and IL-6) and Foxp3+ T regulatory cells (differentiated with TGF-β alone) produce IL-9 as an effector cytokine (Dardalhon et al., 2008; Elyaman et al., 2009; Lu et al., 2006; Veldhoen et al., 2008). As such, whether Th9 cells serve a distinct biological role that is not shared by other T cell lineages is currently unknown.
Cytokines released from distinct T helper cell lineages dramatically influence disease outcome, particularly in the context of parasitic helminth infections, which affect more than one billion people worldwide (Brooker, 2010). Previous studies directed to elucidate the role of IL-9 in hookworm infection, suggested a dispensable role for IL-9 in promoting worm clearance (Townsend et al., 2000). However, other reports supported a protective role for IL-9 during Trichinella spiralis and Trichuris muris infections (Faulkner et al., 1997; Faulkner et al., 1998; Richard et al., 2000; Veldhoen et al., 2008). Thus contradictory evidence has made it difficult to conclude the real contribution of IL-9 in the control of Type 2 immune responses. In addition, definitive evidence identifying the cellular sources of IL-9 in infection models in vivo is still missing.
In this report, we investigated whether IL-9 was necessary and/or sufficient for host protective Type 2 immunity in vivo. Nippostrongylus brasiliensis infection induced IL-9 expression in both mucosal tissues and secondary lymphoid organs, which preceded IL-4, IL-5, and IL-13 expression at these sites. IL-9 deficiency abrogated canonical Type 2 cytokine production, basophilia, eosinophilia, mast cell amplification and worm expulsion. Furthermore, we produced a strain of IL-9 fluorescent reporter mice and demonstrated that both CD4+ T cells and ILC2 cells were major sources of IL-9 secretion upon infection. Th9 cells were markedly more efficient than Th2 cells at driving basophilia, increased mast cell numbers and rapid worm expulsion when adoptively transfer into Rag2 deficient hosts. Thus, our data show that IL-9 serves a critical role in the early stages of Type 2 immunity and its production from effector CD4+ T cells is alone sufficient for host protection against worm infection.
Results
IL-9 expression precedes IL-4, IL-5 and IL-13 in vivo
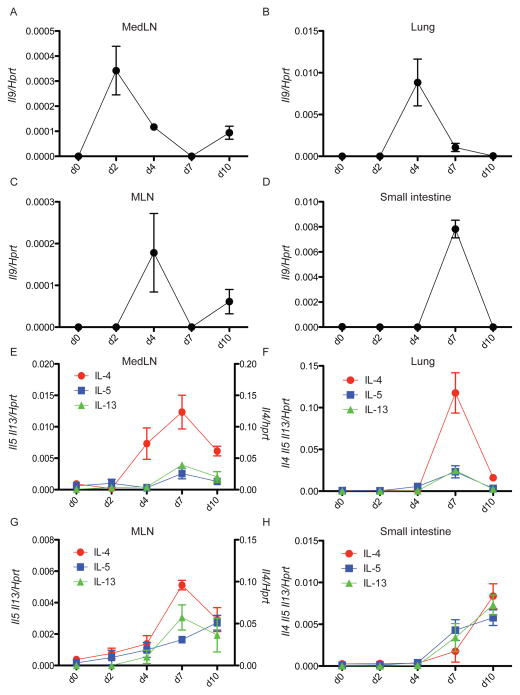
Rapid induction of Type 2 cytokines is critical for host immunity against most parasitic helminthes, but whether distinct cytokines are responsible for orchestrating host protection remain unclear. To gain a better understanding of Type 2 cytokine regulation in vivo, we investigated the spatiotemporal expression patterns of IL-4, 5, 9, and 13 during N. brasiliensis infection. We found a strong induction of all Type 2 cytokines upon infection, which are otherwise low or undetectable at steady state. As expected, the induction of these cytokines correlated with the presence of the parasite in the different target organs and associated lymphoid tissues, peaking first in lung and later in small intestine (Figure 1). Comparison of mRNA expression in wild-type (C57BL/6) infected mice showed that IL-9 was the first among these cytokines induced by N. brasiliensis infection. IL-9 expression in mediastinal lymph nodes (medLN) was detected as early as day 2 post infection (p.i) (Figure 1A) and peaked later around day 4 in lung (Figure 1B). In the mesenteric lymph nodes (MLN) we observed the highest expression at day 4 p.i, (Figure 1C) followed by a strong increase by day 7 in the small intestine (Figure 1D). IL-9 expression was strictly transient, suggesting a very tight control in the expression of this cytokine in vivo, and was detected earlier than IL-4, IL-5 and IL-13 in all tissues examined (Figures 1E–1H). In the spleen IL-9 expression also preceded that of IL-4, IL-5 and IL-13 (Figure S1). Thus, IL-9 is expressed early upon helminth infection and could potentially act upstream of other Type 2 cytokines in vivo.
Figure 1. IL-9 expression precedes IL-4, IL-5 and IL-13 during N. brasiliensis infection.
(A–D) C57BL/6 mice were subcutaneously infected with 625 L3 N. brasiliensis larvae. MedLN (A), lung (B), MLN (C) and small intestine (D) were collected and homogenized at different days p.i for assessment of il9 mRNA expression by real time RT-PCR. The experiment was performed two times with similar results with 2–3 mice per day p.i. Statistically significant p values were determined by one-way ANOVA when comparing basal expression (d0) with at least one other time point for each gene.
(E–H) Same samples as above analyzed for Il4, Il5 and Il13 mRNA expression. Data represent the mean +/− SEM ratio of cytokine gene to Hprt expression as determined by the relative quantification method (ΔΔCt). The experiment was performed two times with similar results with 2–3 mice per day p.i. Statistically significant p values were determined by one-way ANOVA when comparing basal expression (d0) with at least one other time point for each gene.
IL-9 is necessary for IL-5 and IL-13 induction, eosinophilia, basophilia and worm clearance in vivo
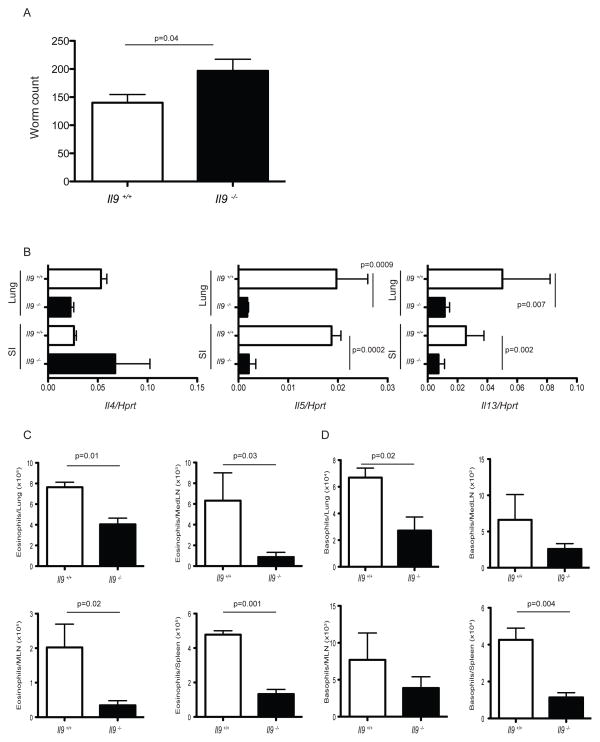
To investigate the role of IL-9 during the spontaneous development of Type 2 immunity against N. brasiliensis, IL-9 deficient mice were generated on a BALB/c background through targeted deletion of exons 1–4. The targeting vector contained a neo resistance cassette flanked by homology regions upstream of exon 1 and downstream of exon 4 of the IL-9 gene (Figure S2A). Genotyping of wild-type (+/+), heterozygous (+/−) and homozygous null (−/−) mice by PCR is shown in Figure S2B. IL-9 deficient mice were viable and fertile and did not showed any gross phenotypic abnormalities. Analysis of the IL-9 deficient mice confirmed that Il9 mRNA and protein expression was undetectable in supernatants of sorted naive CD4+ T cells polarized under Th9 cell culture conditions in vitro (Figure S2C and data not shown). During N. brasiliensis infection, IL-9 deficiency had a negative impact on worm clearance shown by a significant increase in worm burden in Il9−/− mice compared to wild-type mice at day 7 p.i (Figure 2A). As we found that IL-9 was expressed in the early phase of N. brasiliensis infection, we sought to determine whether in its absence, other Type 2 cytokines were affected. To this end we analyzed Type 2 cytokine induction in this infectious setting. We confirmed the absence of Il9 mRNA in IL-9 deficient mice in vivo (Figure S2D). Compared to wildtype mice, Il5 and Il13 mRNA expression was severely impaired in lung and small intestine of IL-9 deficient mice at day 7 p.i (Figure 2B). Expression of other type 2 related genes such as Gata3, Il10 and Il9r were not affected by IL-9 deletion (data not shown). Infection of IL-9 deficient mice with N. brasiliensis also resulted in a significant reduction in the cellularity of spleen and medLN at day 7 p.i. In contrast, total lymphoid infiltrates in MLN and lung were not affected (Figure S2E). A detailed phenotypic analysis of the infiltrate present in the target organs of Il9−/− mice and wildtype mice infected with N. brasiliensis, showed a significant decrease in the absolute numbers of eosinophils (CD11b+, Siglec F+) in all organs analyzed (Figure 2C). Unexpectedly, we also found a significant decrease in basophilia (CD49b+, FcεR+, c-Kit−) in spleen and lung of Il9−/− mice (Figure 2D). Analysis of other cell subsets including neutrophils (CD11b+ Ly6G+) and ILC2 cells (lin− CD45+ Thy1.2+ Sca+ c-Kit+ CD44+) showed similar numbers in affected tissues (Figure S2F, S2G and data not shown). Consistent with previous findings reporting a role for IL-9 in mast cell degranulation and goblet cell hyperplasia (Townsend et al., 2000), mast cell metalloproteinase 1 (Mcpt1) secretion in serum and mucin5b mRNA expression in small intestine was reduced or absent in Il9−/− infected mice (Figure S2H and S2I). Mast cell numbers were also significantly decreased in lung, MLN and spleen of Il9−/− mice (Figure S2J). Altogether our data supports an important role for IL-9 in the potentiation and orchestration of anti-helminthic responses in vivo.
Figure 2. Impaired worm expulsion in the absence of IL-9.
(A) Infected wildtype Il-9+/+ (white bars) and null Il-9−/− (black bars) mice were sacrificed at day 7 p.i to obtain intestinal worm counts. Mean +/− SEM of 6 mice per group.
(B) At day 7 p.i, lung and small intestine (SI) of Il-9+/+ (white bars) or Il-9−/− (black bars) infected mice, were harvested and homogenized for assessment of Il4, Il5 and Il13 mRNA expression. Data represent the mean +/− SEM expression of the cytokine gene to Hprt using the ΔΔCt method. Similar results were obtained in two independent experiments with 6 mice per group.
(C) Total numbers of eosinophils (CD11b+ Ly6G+) as determined by flow cytometry in lung, medLN, MLN and spleen of Il9+/+ (white bars) and Il9 −/− (black bars) mice at day 7 p.i. Mean +/− SEM of experiments with 6 mice per group.
(D) Total numbers of basophils (CD49b+ FceR+ c-Kit−) as determined by flow cytometry in lung, medLN, MLN and spleen of Il9+/+ (white bars) and Il9−/− (black bars) mice at day 7 p.i. Mean +/− SEM of experiments with 6 mice per group. The p values determined by unpaired two-tailed Student’s t test between groups are shown.
Tracking of IL-9 expressing cells in vivo with an IL-9 fluorescent reporter mice
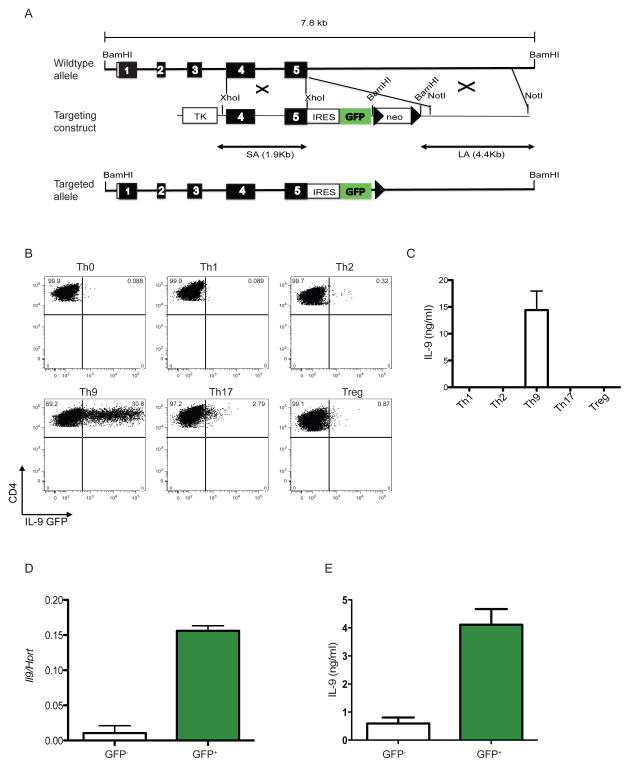
To identify the cellular sources of IL-9 in vivo, we generated an IL-9 reporter knock-in mice designated INFER (Interleukin Nine Fluorescent Reporter). Unlike previous reports using a fate reporter transgenic mouse (Wilhelm et al., 2011), our design allows tracking of IL-9 expressing cells in real time. The targeting design consisted of the insertion of an internal ribosomal entry site sequence (IRES) followed by the coding sequence for green fluorescent protein GFP downstream of the stop codon of Il9 (Figure 3A), thus allowing for reporter expression induced by the endogenous IL-9 promoter. The resulting mice were viable and fertile, showing no gross phenotypic defects.
Figure 3. Generation of INFER mouse and expression analysis in vitro.
(A) A 7.8 kb genomic fragment of the Il9 locus was used to create the targeting construct (middle), in which an IRES-GFP cassette followed by a loxp-flanked neomycin resistance gene was inserted downstream of the stop codon. Deletion of neo by Cre recombinase resulted in the final targeted allele (bottom). Filled boxes represent exons 1–5. Loxp sites are shown as arrow heads. Restriction sites are depicted. TK: thymidine kinase.
(B) Sorted naïve CD4+CD62L+CD25−CD44− T cells from INFER mice were cultured for 5 days in Th0, Th1, Th2, Th9, Th17 or Treg polarizing conditions in vitro. Recovered cells were stained for surface CD4 and GFP expression was analyzed by flow cytometry. Numbers indicate the frequencies of CD4+GFP+ cells. The experiment was performed five times with similar results.
(C) Supernatants from cells isolated from cultures in (B) at day 5, stimulated in vitro with anti-CD3 for 24 hrs and analyzed by ELISA for IL-9 quantification.
(D) Sorted GFP− or GFP+ cells from day 5 Th9 cultures with INFER naïve CD4+ T cells were examined for IL-9 expression by real time RT-PCR. Mean +/− SEM normalized to Hprt is shown.
(E) GFP− or GFP+ cells as described in (D) were stimulated with anti-CD3 for 24 hrs. Supernatants where collected and analyzed by ELISA for IL-9 quantification.
Initial characterization of the INFER mice demonstrated that insertion of the IRES-GFP cassette into the IL-9 locus did not disrupt the endogenous expression of the gene or the protein compared to wild-type C57BL/6 mice. Kinetic analysis of Il9 mRNA expression in vitro demonstrated that IL-9 peaked at day 3 and decrease by day 5 in sorted naïve CD4+ T cells from INFER or wildtype mice differentiated in Th9 cell cultures. Measurement of IL-9 in the supernatants of these cultures by ELISA, also demonstrated equivalent protein expression by day 5 (Figure S3A and S3B). We consistently observed a 24 hr delay between the peak of Il9 mRNA expression and IL-9 protein secretion measured in supernatants of Th9 cultures in vitro. To validate IL-9 production and GFP expression, we sorted naïve CD4+ T cells isolated from INFER mice and cultured them in vitro for 5 days in polarizing conditions suited to generate different effector Th cell subpopulations. Notably, GFP expression controlled by the endogenous IL-9 promoter, occurred mostly under Th9 cell polarizing conditions and only a low frequency of cells in Th2 and Th17 cell conditions (Figure 3B). We confirmed by ELISA the specific induction of IL-9 in Th9 cell polarizing conditions and the respective cytokines in the different cultures to validate the corresponding induction of IFN-γ, IL-4 and IL-17A in Th1, Th2 and Th17, respectively (Figure 3C and data not shown). To further demonstrate that GFP expression in Th9 cell cultures of INFER naïve CD4+ T cells corresponded to IL-9 producing cells, we sorted GFP+ and GFP− cells from day 5 cultures and analyze Il9 mRNA and cytokine expression. Il9 mRNA and protein levels where significantly increased in GFP+ compared to GFP− cells (Figure 3D and 3E). Moreover, intracellular staining performed in Th9 cell cultures with INFER naïve T cells in vitro, demonstrated that 80% of the cells that expressed IL-9 also expressed GFP (Figure S3C). Thus, we generated a novel tool that faithfully recapitulates active IL-9 expression and could be efficiently used to determine the source and function of this cytokine in vivo.
IL-9 is expressed in CD4+ T cells and ILC2 cells in vivo
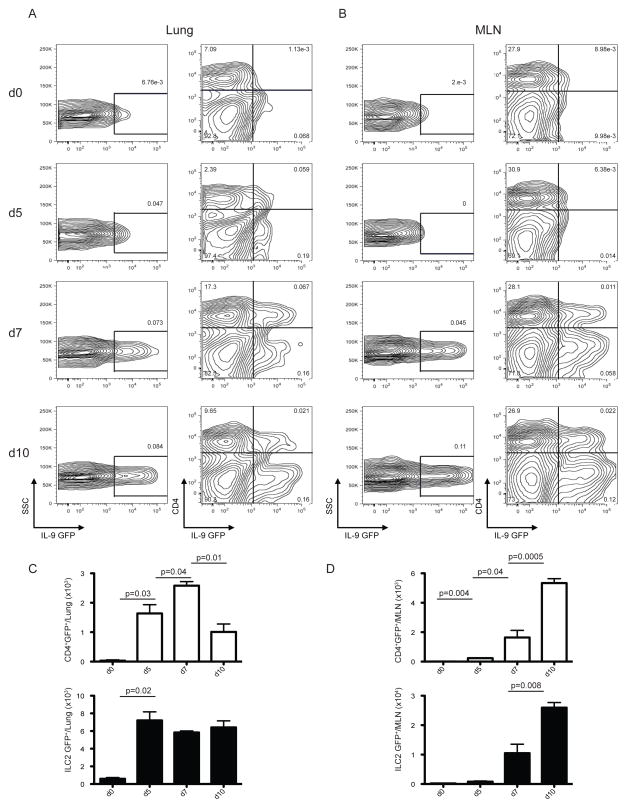
To determine the source of IL-9 during helminth infection, we infected INFER mice with N. brasiliensis and analyzed GFP expression at day 0, 3, 5, 7 and 10 p.i. We did not find detectable expression of IL-9 (measured as GFP) under steady state conditions in spleen, lymph nodes, thymus and bone marrow of INFER mice (data not shown). GFP expression was detected as early as day 3 p.i in medLN, day 5 in lung and later by day 7 in MLN of infected INFER mice (Figure 4A, 4B, S4A and S4B). A detailed analysis, demonstrated that the GFP expression observed, corresponded to CD4+ T cells and ILC2 cells.
Figure 4. IL-9 is expressed in CD4+ T cells and ILC2 cells during N. brasiliensis infection.
INFER mice were subcutaneously infected with 625 L3 N. brasiliensis larvae. Lung (A) and MLN (B) was harvested and homogenized at different days p.i for assessment of IL-9 expression by flow cytometry. Numbers represent the frequencies of total GFP+ cells (left column) or GFP+ among CD4+ T cells (right column).
Total numbers of CD4+ T cells (white bars) and ILC2 cells (black bars) expressing IL-9 (GFP+) in lung (C) or MLN (D) of INFER infected mice as determined by flow cytometry.
Data are represented as mean +/− SEM from one out of three independent experiments with 3–4 mice per day p.i. The p values determined by unpaired two-tailed Student’s t test between days are shown.
Quantification of GFP+ cells in lung, showed that CD4+ GFP+ T cells increased from day 5 to day 7 p.i, however there was a significant reduction by day 10 p.i, indicating transient expression of IL-9 in this cellular compartment. In contrast, ILC2 GFP+ cells in the lung increased by day 5 and were maintained throughout the analysis (Figure 4C). In MLN, we found a significant increase of CD4+ T cells as well as ILC2 cells expressing GFP by day 7 p.i that was further amplified by day 10 (Figure 4D). Importantly, the kinetics of IL-9-GFP expression closely correlated with worm trafficking and expulsion in most immunocompetent mouse strains. IL-9-GFP was not significantly expressed in eosinophils or mast cells during N. brasiliensis infection (data not shown). To definitively prove that GFP+ cells corresponded to IL-9 expressing cells in vivo, we sorted GFP+ and GFP−, CD4+ T cells or ILC2 cells from lung and MLN at day 10 p.i. GFP+ CD4+ and GFP+ lLC2 cells expressed higher levels of IL-9 when compared to GFP− cells (Figure S4C and S4D), hence GFP expression efficiently reported IL-9 expressing cells in vivo. Thus, using INFER mice we demonstrated that during N. brasiliensis infection Th9 cells develop and represent, together with ILC2 cells, the cellular sources for IL-9 expression. The kinetic of IL-9 expression as well as the original source changes over time and depends on the presence of the relevant insult in vivo.
IL-9 derived from CD4+ T cells is sufficient to promote host protection against N. brasiliensis
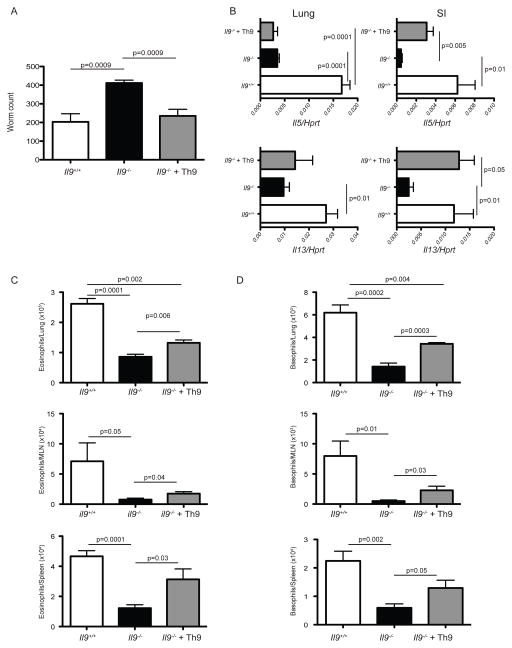
As ILC2 and CD4+ T cells produce IL-9 in vivo, we sought to determine the contribution of IL-9 produced by CD4+ T to host protection against N. brasiliensis. Thus, we transferred Th9 cells generated in vitro into IL-9-deficient hosts in the context of nematode infection. This experimental setting is devoid of IL-9 produced from the innate compartment (i.e. ILC2). IL-9 derived from CD4+ T cells is sufficient to promote a significant decrease in worm loads in IL-9 deficient hosts, equivalent to wildtype levels (Figure 5A). Moreover, IL-13 expression, which is significantly reduced in the absence of IL-9 (Figure 2B), increased to levels comparable to wildtype mice in the small intestine upon reintroduction of Th9 cells (Figure 5B). Likewise, IL-5 expression was partially restored in the small intestine (Figure 5B). Interestingly, the levels of Il13 and Il5 mRNA in lung did not show a significant change when compared to Il9−/− mice that did not receive Th9 cells (Figure 5B). A partial rescue was also observed in the numbers of eosinophils and basophils in lung, MLN and spleen of IL-9 deficient mice transferred with Th9 cells (Figures 5C and 5D). Thus, although ILC2 derived IL-9 could play a critical role in the promotion of optimal immune responses against nematodes; IL-9 derived from CD4+ T cells is sufficient to promote an effective anti-helminth response in vivo.
Figure 5. T cell derived IL-9 is sufficient to promote worm expulsion in IL-9 deficient hosts.
(A) Sorted effector Th9 (5 × 105) cells (GFP+) from day 5 in vitro cultures with naïve CD4+ T cells isolated from INFER mice, were intravenously transferred into IL-9−/− recipient mice (gray bars). 24 hrs later, mice were subcutaneously infected with 625 L3 N. brasiliensis larvae. For comparison Il9+/+ (white bars) and Il9−/− (black bars) mice that did not received Th9 cells were used. Worm counts in small intestine at day 7 p.i are shown.
(B) At day 7 p.i, lung and small intestine (SI) of mice described in (A), were harvested and homogenized for assessment of Il5 and Il13 mRNA expression. Data represent the mean +/− SEM expression of the cytokine gene to Hprt using the ΔΔCt method. Similar results were obtained in three independent experiments with 6 mice per group.
(C) Total eosinophil numbers in lung, MLN and spleen of mice described in (A) as determined by flow cytometry.
(D) Total basophil numbers in lung, MLN and spleen of mice described in (A) as determined by flow cytometry.
Data are represented as mean +/− SEM. The experiment was repeated three times with similar results. n= 5–6 mice per group. Statistics were calculated by unpaired two-tailed Student’s t test. The p values between groups are shown.
Th9 cells promote basophilia and increased mast cell numbers leading to a rapid helminth expulsion in vivo
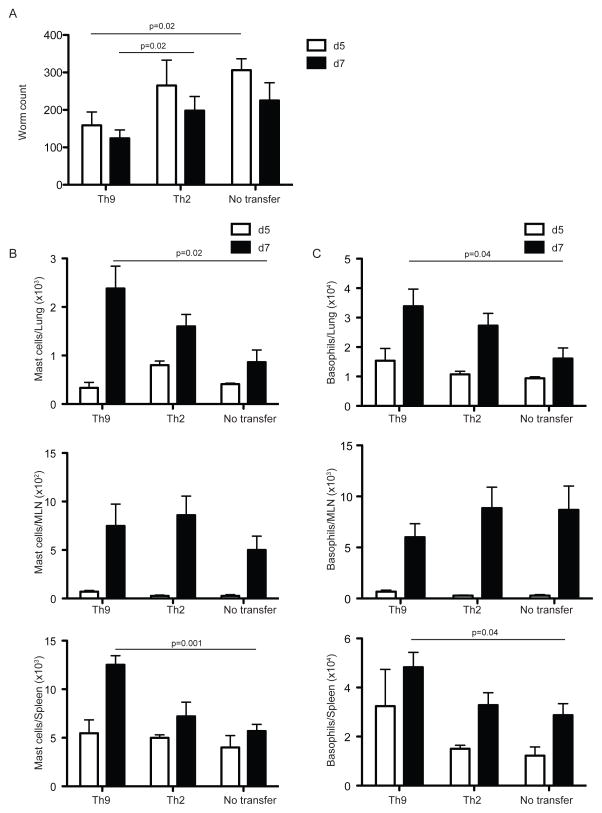
Based on our foregoing findings, we hypothesized that Th9 cells represent a protective subset of specialized effector Th cells in vivo. To characterize the function of Th9 cells, we sought to determine the role of these cells compared to classical Th2 cells during N. brasiliensis infection. To this end, we adoptively transfered 2 × 105 purified effector Th9 or Th2 cells generated in vitro, into immunodeficient Rag2 −/− compromised recipient mice and infected them 24 hrs later with N. brasiliensis. We analyzed the relevant tissues at days 5 and 7 p.i. To generate effector Th9 and Th2 cells, we sorted naïve CD4+ T cells from INFER or IL-4 reporter mice 4get (Mohrs et al., 2001) and polarized them towards Th9 or Th2 respectively for 5 days. Sorted effector Th9 or Th2 cells (measured as GFP expressing cells in these cultures), were used for the transfer experiments. Importantly, transfer of Th9 but not Th2 cells significantly decreased the worm burden in recipient mice starting at day 5 p.i, which was also observed by day 7 p.i (Figure 6A). Interestingly, IL-9 expression on T cells promoted better engraftment of the transferred cells observed in MLN by day 5 p.i that was also recapitulated in lung and spleen by day 7 p.i (Figure S5A). Despite increased eosinophilia observed in mice transferred with Th2 cells at day 7 p.i, we did not find important differences between groups in the numbers of this subset in vivo (Figure S5B), suggesting that the effect of Th9 cell transfer in worm expulsion was independent of eosinophils. Th9 cells promoted significantly increased mast cell and basophil numbers at day 7 p.i in lung and spleen but not MLN of recipient mice (Figure 6B and 6C), together with a significant concomitant increase on ILC2 cells in spleen (data not shown). It has been suggested, based on microarray data that ILC2 cells could directly respond to IL-9 (Price et al., 2010; Wilhelm et al., 2012), similarly IL-9 has been shown to act as a mast cell growth factor (Hultner et al., 1990). However, there is no report so far for IL-9R expression on basophils. To determine whether basophils could represent a direct target cell for IL-9 in vivo, we isolated lung infiltrating basophils from day 7 infected mice and analyze Il9r mRNA expression. Indeed, basophils express Il9r mRNA and this expression was lower compared to mast cells but higher compared to eosinophils isolated from the same tissue (Figure S6). Hence, the effect of IL-9 on basophilia could be mediated directly in vivo.
Figure 6. Adoptive transfer of Th9 cells but not Th2 cells improve worm expulsion.
(A) Sorted effector Th2 or Th9 (2 × 105) cells (CD45.2+GFP+) from day 5 in vitro cultures with naïve CD4+ T cells isolated from 4get or INFER mice were intravenously transferred into CD45.1+ Rag2−/− recipient mice. 24 hrs later, mice were subcutaneously infected with 625 L3 N. brasiliensis larvae. As a control a third group of Rag2−/− mice that did not received T cells was also infected. Worm counts in small intestine at days 5 (white bars) and 7 (black bars) p.i are shown.
(B) Total mast cell numbers in lung, MLN and spleen of mice described in (A) as determined by flow cytometry.
(C) Total basophil numbers in lung, MLN and spleen of mice described in (A) as determined by flow cytometry.
Data are represented as mean +/− SEM. The experiment was repeated three times with similar results. n= 4–5 mice per group. Statistics were calculated by unpaired two-tailed Student’s t test. The p values between groups are shown.
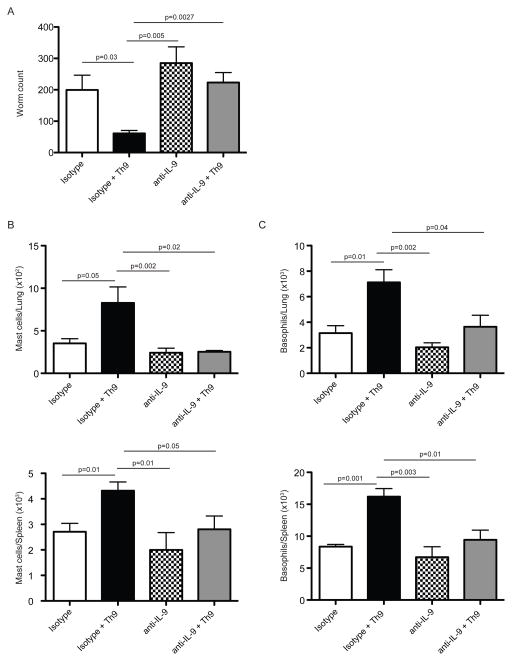
Finally, to definitively prove that IL-9 was the effector cytokine responsible for the Th9 induced responses in vivo, we performed transfer experiments in which we administered neutralizing antibodies against IL-9 during the course of the transfer experiment. IL-9 neutralization ablated the decrease in worm loads induced by Th9 cell transfers into Rag2−/− recipient mice (Figure 7A). In addition, Th9 cell-induced mast cell and basophil amplification in lung and spleen was severely impaired in mice treated with anti-IL-9 but not mice treated with isotype control that also received effector Th9 cells (Figure 7B and 7C). Thus we demonstrated that IL-9 was strictly required for Th9 cells to promote the phenotype observed when transferred into immunocompromised hosts. Altogether, these data indicate that Th9 cells, through the expression of the effector cytokine IL-9, are more efficient than classical Th2 cells in orchestrating anti-helminth responses in vivo.
Figure 7. IL-9 neutralization abrogates the Th9 induced phenotype in Rag2−/− recipient mice.
Sorted effector Th9 (2 × 105) cells (CD45.2+GFP+) from day 5 in vitro cultures with naïve CD4+ T cells isolated from INFER mice were intravenously transferred into CD45.1+ Rag2−/− recipient mice treated with anti-IL-9 mAb (gray bars) or isotype control (black bars). 24 hrs later, mice were subcutaneously infected with 625 L3 N.brasiliensis larvae. As additional controls, mice without transferred cells but treated with anti-IL-9 (checkered bars) or isotype control (white bars) were used.
(A) Worm counts in small intestine of mice described above at day 7 p.i.
(B) Total mast cell numbers in lung and spleen of mice described above as determined by flow cytometry.
(C) Total basophil numbers in lung and spleen of mice described above as determined by flow cytometry.
Data are represented as mean +/− SEM. The experiment was repeated three times with similar results. n= 4–5 mice per group. Statistics were calculated by unpaired two-tailed Student’s t test. The p values between groups are shown.
Discussion
In this study we identify a central and non-redundant role for IL-9 in driving host protective Type 2 immunity against hookworm infection. Using newly generated IL-9 deficient mice, we found that IL-9 promotes IL-5 and IL-13 expression and increases basophilia, eosinophilia and mast cell numbers in parasite-targeted tissues, ultimately leading to efficient worm expulsion. Generation of IL-9 fluorescent reporter mice allowed us to identify Th9 cells and ILC2 cells as natural sources for IL-9 in vivo. Both, adaptive and innate IL-9-expressing subsets were rapidly induced within mucosal and lymphoid tissues upon N. brasiliensis infection. Furthermore, functional characterization of Th9 cells in adoptive transfer experiments, demonstrated that Th9 cells, compared to Th2 cells, are more efficient at driving basophil and mast cell amplification leading to worm expulsion. Our data also supports IL-9 as a critical effector cytokine for Th9 cell function. Taken together, this study centers IL-9 as an important regulator driving mucosal Type 2 immunity in vivo.
IL-9 has been reported as part of the Type 2 cytokine effector panel, however initial attempts to dissect its contribution in helminth infection did not demonstrate a significant role for this cytokine in worm clearance or Type 2 cytokine induction in vivo (Townsend et al., 2000). Contradictory results regarding IL-9 function in the immune system have been described, perhaps mainly due to differences in the mouse strain analyzed as well as the disease model and experimental approaches employed to neutralize or amplify IL-9 expression in vivo (Faulkner et al., 1998; Richard et al., 2000). In this report, we demonstrated that IL-9 works as an initiator and/or amplifier of Type 2 responses in vivo, activating innate subsets and promoting the expression of other Type 2 cytokines, which will further maintain an active response.
IL-9 was originally identified as a T cell derived cytokine (Schmitt et al., 1994; Schmitt et al., 1989). Although allergen specific induction of IL-9 in PBMC of atopic patients and in murine models of allergy have been reported (Chang et al., 2010; Jones et al., 2012; Yao et al., 2011), there is no report of IL-9 expression in T cells in vivo, without a requirement for ex-vivo restimulation on isolated T cell populations. Studies have identified Th9 cells as a new Th subset responsible for IL-9 expression in vitro (Dardalhon et al., 2008; Veldhoen et al., 2008). Previous attempts to identify Th9 in vivo using a fate mapping IL-9 reporter mice did not demonstrate IL-9 expressing T cells in a lung inflammation model, mainly due to incomplete reporter activity (Wilhelm et al., 2011). The generation of INFER mice in the present study enabled the tracking of active IL-9 expressing cells in real time. With this genetic tool we were able to identify for the first time Th9 cells generated in vivo without the requirement of additional manipulation for their detection.
We also found that N. brasiliensis infection induced a second population of IL-9 expressing cells identified as ILC2 cells. This innate subpopulation is an important source of IL-5 and IL-13 in vivo (Ikutani et al., 2012; Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Saenz et al., 2010). IL-9 expressing ILC2 cells have also been observed in a papain-induced lung inflammation model; however, this expression was transient and transitioned to IL-5 and/or IL-13 expression over time (Wilhelm et al., 2011). Our analysis of IL-9 expression in vivo, confirmed that this is a cytokine expressed early during nematode infection compared to other Type 2 cytokines (Faulkner et al., 1998); furthermore, IL-9 induction followed a strict temporal and compartmentalized regulation that might be important to avoid detrimental effects of chronic exposure as has been observed in transgenic models (Temann et al., 1998). Even with these intrinsic limitations related to the nature of IL-9 induction, we now demonstrated that both adaptive and innate sources of IL-9 are rapidly induced in response to helminth infection.
We further demonstrated that T cell derived IL-9 is sufficient to decrease worm loads and significantly increase IL-13 and IL-5 expression in small intestine. Moreover, CD4+ T cell-derived IL-9 induces significant increases in the levels of eosinophils and basophils in multiple organs. Interestingly, most of these parameters were only partially restored to wildtype levels upon Th9 adoptive transfer, suggesting that ILC2-derived IL-9 might be indispensable to amplify the immune response in infected mice. These results support the notion of a compartmentalized function for different sources of this cytokine in vivo; and the exact contribution of the innate IL-9+ compartment warrant further investigation. Nevertheless, the results presented here clearly demonstrate that IL-9 produced by T cells is sufficient to efficiently orchestrate anti-helminth responses in vivo.
Since there has been recent controversy regarding the function of T cells expressing IL-9, we focused our study on whether Th9 cells represented a bona fide protective T cell subset in N. brasiliensis infection. Although growing evidence suggests that an IL-9 expressing T cell could account for diverse immune responses in vivo, functional characterization of purified Th9 cells has not been thoroughly addressed. Phenotypic characterization of Th9 cells in vivo has been done with heterogeneous populations of naïve CD4+ T cells polarized towards Th9 in vitro (Dardalhon et al., 2008; Purwar et al., 2012; Staudt et al., 2010), where contaminating IL-9 non-expressing cells are unavoidable. Our newly generated mouse model enabled us to study a pure population of IL-9 expressing T cells and its function in a physiological setting in vivo. With this approach, we now demonstrated that Th9 cells provide better protection to N. brasiliensis infection when compared to classical Th2 cells, supporting a specialized role for this Th subset in vivo (Jones et al., 2012; Staudt et al., 2010).
Increased basophilia in Rag2−/− mice transferred with T cells during nematode infection has been previously reported (Min et al., 2004). We now demonstrate that this characteristic phenotype is dependent on IL-9 expression by CD4+ T cells. Similarly, we demonstrated that the phenotype observed in Th9 recipient mice on mast cell amplification was also dependent on IL-9, supporting the concept that IL-9 is a potent mast cell growth factor (Camberis et al., 2003; Hultner et al., 1990; Jones et al., 2012). In the future, the use of novel murine models to analyze the specific role of basophils in vivo, will be important to determine the contribution of IL-9 production by Th9 cells to basophil expansion and activation during nematode infection (Ohnmacht et al., 2010; Wada et al., 2010). Similarly, the development of new experimental tools will be critical to dissect the effects of IL-9 on other cell types such as ILC2 cells as well as in non-hematopoietic cells such as mucosal epithelium (Gounni et al., 2004). Our present findings regarding ILC2 cell amplification induced by Th9 transfer is particularly relevant since ILC2 and T cells seem to work in concert to potentiate and induce robust N. brasiliensis elimination (Neill et al., 2010). An interesting observation is that IL-9R is one of the genes most highly expressed on the ILC2 subpopulation (Price et al., 2010). Based on this observation, we propose that T cell derived IL-9 could potentially account for the increased ILC2 cell numbers observed in Rag2−/− recipients transferred with Th9 cells.
In conclusion, our work indicates that IL-9 from both innate and adaptive sources orchestrates and amplifies Type 2 immunity. Importantly Th9 but not Th2 cells promote effective anti-helminth responses in vivo, supporting specialized functions of Th9 cells, strictly dependent on IL-9, that are not shared with other T helper subsets. Future studies monitoring IL-9 expression with the INFER mice will facilitate identification of the relevant sources and functions of IL-9 in vivo not only in different Type 2 immune responses such as asthma, but also in other IL-9-driven inflammatory disorders.
Experimental procedures
Mice
To generate IL-9 deficient mice, a genomic region containing exons 1–4 of the Il9 gene was deleted and substituted for the neomycin resistance gene (neo). The targeting construct contained homology arms of 1.2 and 2.8 kb flanking the neo cassette. Downstream of the 3′ homology arm the thymidine kinase gene was inserted for additional screening (Figure S2). The linearized vector was electroporated into BALB/c embryonic stem cells. G418-resistant, gancyclovir-sensitive clones were screened and injected into C57BL/6 blastocysts. Chimeric males in which germline transmission was successful were bred to BALB/c mice for more than 10 generations. Screening of Il9 deficient mice with the primers: 5′-CAGGATGATTGTACCACACCG-3′ and 5′-CTCATTTGCTTGGATGTGAGC-3′ or 5′-TCGCCTTCTTGACGAGTTCT-3′ and 5′-CCAACAGGTGGAGAGAAGACA-3′ amplifies a 239 bp wildtype allele or a 450 bp targeted allele respectively. INFER (Interleukin Nine Fluorescent Reporter) mice were generated with standard cloning strategy. In brief, the targeting construct was generated by standard subcloning techniques using appropriate restriction enzymes and the parental vectors pEasyflox and pMCSV2.2. The targeting construct contained two homology arms of 1.9 and 4.4 kb. The IRES-GFP was introduced immediately after the stop codon to avoid disruption of cis-regulatory-elements in the 3′UTR (Untranslated Region) and the poly-adenylation signal. In addition, the construct contained a neo gene flanked by loxP sites driven by a promoter that allowed for positive selection in both bacterial and mammalian cells (Figure 3). The targeting construct was electroporated in JM8 embryonic stem cells derived from the purebred C57BL/6 background and individual clones were tested for successful homologous recombination by PCR. Embryonic stem cells carrying the targeted allele were injected into BALB/c blastocysts, which were then implanted in CD1 pseudopregnant foster mothers. Male chimeras were bred with the EIIa Cre germline deleter (C57BL/6) strain (Jackson Laboratories) to remove the neo cassette and screen for germline-transmitted offspring. Mice bearing the targeted allele were screened by PCR. Primers for wildtype allele: 5′-GGGACGTTCCAGAAGACAGA-3′ and 5′-CCAAAAGCAACTGTGAAGCA-3′ amplifies a 179 bp product. Primers for targeted allele: 5′-GGGACGTTCCAGAAGACAGA-3′ and 5′-GGAAAGACCCCTAGGAATGC-3′ amplifies a bp product. For transfer experiments into IL-9−/− mice, donor cells from INFER mice backcrossed at least 8 generations (N8) into BALB/c background were used.
C57BL/6 and BALB/c mice were purchased from National Cancer Institute, Rag2 −/− on the C57BL/6 background from Taconic and IL-4 reporter 4get mice (Mohrs et al., 2001) from Jackson Laboratories were backcrossed for more than 10 generations to the C57BL/6 background in house.
All mouse experiments were approved by Institutional Animal Care and Use Committee of Yale University.
Helminth Infection
Individual mice were inoculated subcutaneously with 625 viable third-stage N. brasiliensis larvae. Animals were sacrificed at different time points post infection, harvested tissues were snap frozen for mRNA analysis or processed for flow cytometry staining. Worm burdens were determined as described (Camberis et al., 2003) and serum was collected.
Lymphocyte Preparation and Flow Cytometry
Spleen, mediastinal and mesenteric lymph nodes were removed from euthanize mice, place into 5% FCS supplemented RPMI media and passed through a cell strainer (70 μm). For isolation of lung infiltrates, cells were collected after 1 hr digestion in RPMI supplemented with 5% FCS, 1 mg/ml Collagenase D (Roche) and 10 μg/ml DNAse I (Invitrogen) at 37°C. Homogenates were passed through a cell strainer and infiltrates separated with a 27.5%, Optiprep gradient (Axis-Shield) by centrifugation at 2750 rpm for 20 min. Cells were removed from the interface and ACK lysis buffer was used. Cells were acquired on an LRSII flow cytometer (BD Biosciences) and data analyzed with FlowJo software (Treestar).
For intracellular IL-9 staining, day 5 Th9 cultures were restimulated for 4 hrs with PMA (20ng/ml) and ionomicin (1μg/ml) in the presence of Brefeldrin A (1μg/ml).
Antibodies
All antibodies for flow cytometry analysis used were from eBioscience. For the lin− cocktail biotin coupled antibodies against CD4, CD8, CD11b, CD11c, CD19, NK1.1, B220, Gr-1, Ter119, TCRγδ and TCRβ were used. Fluorescent coupled streptavidin or antibodies against CD4, CD8, CD11b, CD25, CD44, CD45, CD45.1, CD45.2, CD49b, CD62L, Thy1.2, Sca-1, c-kit, Ly6G, FcεR, Siglec-F were used. For intracellular staining anti IL-9-PE (BD) and anti-GFP (Invitrogen) were used. For ELISA, purified and biotinylated antibodies (BD Biosciences) against IFNγ, IL-4, IL-9 and IL-17 were used.
For neutralization experiments anti murine IL-9 (9C1) and isotype control (C1.18.4) from BioXcell were used.
In vitro T cell differentiation
CD4+ T cells from spleen, axillary and inguinal lymph nodes of C57BL/6, INFER or 4get mice were MACS enriched using anti-CD4 beads (Milteny Biotech). Then, naive CD4+ T cells (CD25−CD62L+CD44−) were purified by flow cytometry sorting and cultured in Clicks medium (Sigma-Aldrich) suplemented with 10% FCS, L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) and β-mercaptoethanol (40 nM) in the presence of plate bound anti-CD3 (2C11 μg/ml for Th2 and Th9, 5 μg/ml for Th1 and Treg, 10 μg/ml for Th17) and anti-CD28 (37N51 10 μg/ml for Th2 and Th9, 2 μg/ml for Th1 and Treg, 2 μg/ml for Th17 soluble). 0.75 × 10 6 cells/ml were cultured for 5 days under Th1, Th2, Th9, Th17 or Treg polarizing conditions. Cytokines for effector differentiation were as follows, Th1: IL-12 (10 ng/ml Peprotech) anti-IL-4 (11B11, 10 μg/ml), IL-2 (20 U/ml Peprotech), Th2: IL-4 (20 ng/ml Peprotech) anti-IFNγ (XMG1.2, 10 μg/ml), IL-2 (20 U/ml), Th9: IL-4 (10 ng/ml), TGF-β (1 ng/ml R&D), IL-1β (10 ng/ml Peprotech), IL-25 (20 ng/ml R&D), TSLP (50 ng/ml Peprotech), anti-IFNγ (10 μg/ml), Th17: TGF-β (0.5 ng/ml), IL-6 (20 ng/ml), IL-23 (50 ng/ml), anti-IL-4 (10 μg/ml), anti-IFNγ (10 μg/ml), anti-CD28 (5 μg/ml), Treg: TGF-β (2 ng/ml).
Adoptive transfer experiments
Naïve CD4+ T cells (CD25−CD62L+CD44−) from 4get or INFER mice were culture in Th2 or Th9 skewing conditions, respectively. On day 5, in vitro generated effector cells were sorted by flow cytometry based on GFP expression. 2 × 105 IL-4 GFP+ Th2 or IL-9 GFP+ Th9 cells were transferred intravenously into CD45.1 Rag2−/− recipient mice. CD45.2 expression on transferred cells was used to identify donor cells. One day after transfer mice were infected with N. brasilienisis as described above and euthanized for analysis 5 to 7 days p.i.
Transfer experiments with neutralizing antibodies against IL-9 were performed as described above with Th9 cells. Mice were i.v (first two doses) or i.p injected with 200μg/dose of antibody every other day starting at day -2. Mice were euthanized for analysis at day 7 p.i.
For transfer experiments into IL-9−/− mice, 5 × 105 sorted Th9 cells (GFP+) from day 5 cultures were used. One day after transfer mice were infected with N. brasilienisis as described above and euthanized for analysis at day 7 p.i.
Real-time RT-PCR
RNA from cells or organs was extracted with Trizol reagent (Invitrogen) and reverse-transcribed with Superscript II (Invitrogen) and oligo dT according to the manufacturer’s protocol. cDNA was semi-quantitated with commercially available TaqMan primer and probe sets using an ABI-PRISM 7500 sequence-detection system (Applied Biosystems). Expression was calculated with the ΔΔCτ method normalized to Hprt expression.
Cytokine and mMCPT-1 measurements
Cells from day 5 T helper cultures in vitro were harvested and directly re-cultured or cultured after sort based on GFP expression in anti-CD3 (10 μg/ml) plate bound culture dishes. Cells were resuspended at 1–2 × 106 cells/ml in Click’s Media and supernatants were collected to determine cytokine concentration by ELISA. For mMCPT-1 quantification, serum from N. brasilienisis infected mice was collected and analyzed according to manufacturer’s protocol (eBioscience).
Statistical Analysis
Statistical analyses were calculated in Prism 5.0 (Graphpad Software) using two tailed Student’s t test or one way ANOVA, p ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank T. Strowig and N. Palm for critical reading of the manuscript and helpful discussion. J. Stein and L. Evangelisti for help in the generation of mouse strains. T. Taylor and G. Tokmoulina for help with cell sorting; and C. Lieberman for help in submitting the manuscript. R.A.F is an investigator of the Howard Hughes Medical Institute. P.L.L was supported by a postdoctoral fellowship from PEW Charitable Trust: PEW Latin American Fellow Program in Biomedical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nature reviews. Immunology. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers--a review. International journal for parasitology. 2010;40:1137–1144. doi: 10.1016/j.ijpara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberis M, Le Gros G, Urban J., Jr . Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. In: Coligan John E., editor. Current protocols in immunology. Unit 19. Chapter 19. 2003. p. 12. [DOI] [PubMed] [Google Scholar]
- Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature immunology. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JS, Lum E, Goulding J, Muir R, Van Snick J, Openshaw PJ. IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. J Immunol. 2009;183:7006–7013. doi: 10.4049/jimmunol.0900085. [DOI] [PubMed] [Google Scholar]
- Dong Q, Louahed J, Vink A, Sullivan CD, Messler CJ, Zhou Y, Haczku A, Huaux F, Arras M, Holroyd KJ, et al. IL-9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. European journal of immunology. 1999;29:2130–2139. doi: 10.1002/(SICI)1521-4141(199907)29:07<2130::AID-IMMU2130>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Smith P, Richardson EJ, Jones FJ, Faulkner HC, Van Snick J, Renauld JC, Grencis RK, Dunne DW. Expression of interleukin-9 leads to Th2 cytokine-dominated responses and fatal enteropathy in mice with chronic Schistosoma mansoni infections. Infection and immunity. 2000;68:6005–6011. doi: 10.1128/iai.68.10.6005-6011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. European journal of immunology. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infection and immunity. 1998;66:3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. The Journal of experimental medicine. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, Shan L. IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J Immunol. 2004;173:2771–2779. doi: 10.4049/jimmunol.173.4.2771. [DOI] [PubMed] [Google Scholar]
- Gounni AS, Nutku E, Koussih L, Aris F, Louahed J, Levitt RC, Nicolaides NC, Hamid Q. IL-9 expression by human eosinophils: regulation by IL-1beta and TNF-alpha. The Journal of allergy and clinical immunology. 2000;106:460–466. doi: 10.1067/mai.2000.109172. [DOI] [PubMed] [Google Scholar]
- Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, Dormer P, Van Snick J. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) European journal of immunology. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- Hultner L, Kolsch S, Stassen M, Kaspers U, Kremer JP, Mailhammer R, Moeller J, Broszeit H, Schmitt E. In activated mast cells, IL-1 up-regulates the production of several Th2-related cytokines including IL-9. J Immunol. 2000;164:5556–5563. doi: 10.4049/jimmunol.164.11.5556. [DOI] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. The Journal of allergy and clinical immunology. 2012;129:1000–1010. e1003. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops L, Louahed J, Van Snick J, Renauld JC. IL-9 promotes but is not necessary for systemic anaphylaxis. J Immunol. 2005;175:335–341. doi: 10.4049/jimmunol.175.1.335. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nature reviews. Immunology. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, et al. Th9 cells promote antitumor immune responses in vivo. The Journal of clinical investigation. 2012;122:4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. The Journal of experimental medicine. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. The Journal of allergy and clinical immunology. 2010;125:469–476. e462. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nature reviews. Immunology. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nature medicine. 2012 doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- Schmitt E, Van Brandwijk R, Van Snick J, Siebold B, Rude E. TCGF III/P40 is produced by naive murine CD4+ T cells but is not a general T cell growth factor. European journal of immunology. 1989;19:2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. The Journal of allergy and clinical immunology. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. The Journal of experimental medicine. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, Moritz RL, Simpson RJ. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40) The Journal of experimental medicine. 1989;169:363–368. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. The Journal of clinical investigation. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nature immunology. 2012;13:637–641. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. The Journal of allergy and clinical immunology. 2011;128:1357–1360. e1355. doi: 10.1016/j.jaci.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.