Abstract
Background:
Diabetes type 2 is a world wide spread disease with a multifactorial pathogenetic evolution. Various factors like obesity, physical inactivity and poor lifestyle habits contribute to its development. The aim of this study was to verify if in young healthy sedentary male and female there is positive correlation between family history to type 2 diabetes and an increase in body weight and fat mass, or alterations in basal glycemia values.
Methods:
Totally183 male and 237 female healthy sedentary subjects were analysed in 2012, in Italy. They were divided in three groups: FH+ with first degree family history, FH++ with second degree family history and FH− with no family history. Anthropometrics, body composition and blood parameters were assessed.
Results:
Male had the highest BMI values (P<0.01). FH+ and FH++ had increased waist and hip circumferences and body weight (P<0.005 for men, P<0.0001 for women), body mass index (P< 0.0001 in both sexes), waist-hip ratio (P< 0.05 for men and women) and triceps skinfold (P< 0.0005 for both sexes). Obesity incidence was higher in FH+ and FH++ compared to control groups.
Conclusions:
The study confirms family history to diabetes type 2 as a risk factor for the development of the illness, mainly in a case of first degree of FH. Preventive interventions are necessary to promote significant life-style changes, such as increased physical activity and controlled quantity and quality of food intake.
Keywords: Lifestyle, Fasting glucose level, Type 2 diabetes, Family history, Body composition
Introduction
A deficit in insulin secretion and physiological tissutal action induces mellitus diabetes, causing chronic hyper-glycemia and various metabolic diseases (1). In occidental industrialized countries, type 2 diabetes (TD2) is the most widely spread disease, showing a constant increase of incidence also in young population. Moreover, genetic and environmental risk factors influence diabetes development: family history, age, obesity and physical inactivity (2). Maternal influence confirmed the hereditary role in the diabetes pathogenesis: women with positive family history to the illness presented major risks to develop gestational TD2, confirming the inter-generative transmission of this disease(3–11). Further studies showed the precocious influences of positive family history to TD2 on subjects’ phenotype, generating an increase in body weight and a tendency towards obesity and visceral adiposity (12–16).
Other studies showed metabolic disorders related to a positive family history to TD2: glucose metabolic disorders, insulin-resistance, an increase in blood pressure and a reduced glucose tolerance (9, 11, 14, 17–20).
Even energy expenditure showed a strong correlation with positive family history to TD2 in young subjects (21–23), and our experience suggests that familiarity induces a precocious increase in body weight for visceral deposit of body fat (21, 24–26), maybe for a reduction in basal metabolism. Further analysis also showed that subjects with diagnosis to diabetes have a lower energy expenditure level compared to subjects with negative diagnosis to this illness (22, 27, 28). Regular physical activity reduces body weight in these subjects, confirming the protective role of sport on people’s health (4, 29–31).
The aim of this study was to verify if in young healthy sedentary male and female there is positive correlation between family history to TD2 and an increase in body weight and fat mass, or alterations in basal glycaemia values. Moreover, according to our recent study (Bianco et al, 2013) (26), on that case we want to better understand how the degree (first or second) of family history may affect all measured parameters.
Materials and Methods
A cross-sectional study with a cohort of young adult people was performed by the University of Palermo in collaboration with the University of Padua. A number of 420 healthy sedentary subjects (183 male and 237 female) living in Palermo area were selected in 2012. All of them were Caucasians, with a middle-low socioeconomic status; the predominant diet regimen Sicily is the Mediterranean diet. These were then divided into three groups according on family history to diabetes type 2. First group (FH+) included all those who had a parent or first degree relative with type 2 diabetes (35 male and 44 female), second group (FH++) included all those subjects having second degree relatives with type 2 diabetes (32 male and 58 female) and third group (FH−) included those subjects with no family history to the illness; in this case we used FH− as control group. A proper six pages questionnaire was made up following the standards of the “MEDEOR clinics for metabolic disorders”. It contained three main sections: a) Demographic information, number of hours of physical activity (hours/week), diet regimen and anthropometrics characteristics; b) History of illness; c) Family history to type 2 diabetes mellitus. Afterwards, the questionnaire was administered to the volunteers in order to detect family history to type 2 diabetes mellitus (TD2) and previous cardiovascular diseases such as myocardial infarction, stroke, vascular peripheral disease, hospitalisation for coronary heart disease (32).
Exclusion criteria
All those subjects resulting positive for the diseases above mentioned were excluded from the study. Moreover, we excluded from the study all people practicing more than 1 hour of physical activity per week and all people who declared were following intensive hypo-caloric diet regimen (a number of 22 subjects were excluded from the study). Height (Seca 709 ± 1g approximation, Hamburg – Germany), weight (Seca 220 ± 1mm approximation, Hamburg – Germany), shoulder, waist and hip circumferences (inelastic flexible meter with ± 1 mm approximation) were recorded. Body Mass Index (BMI, kg/m2), Waist-Hip Ratio (WHR), Body Surface Area (BSA, m2) were then calculated for each subject (33, 34). A bioelectrical impedance analysis (4 ways, 50 kHz frequency and 800 μA amplitude, Skylark, model BT-905 Taiwan, Korea) was then set for both male and female of all groups to assess Fat mass (FM) and Free Fat Mass (FFM) expressed both in kilograms (kg) and percentage (%). Fasting plasma glucose was finally measured for each subject with photometer Accuchek Active (Roche Diagnostic, Germany), with a range measure of 10–600 mg/dl (0.6–33.3 mmol/L). A bioelectrical impedance analysis was performed following standardised procedures: participants have not exercised or taken a sauna within 7 hours before the test; participants were not allowed to drink alcohol (12 hours prior to test); participants were not allowed to eat (4 hours prior to test); participants were not allowed to drink water (1 hour prior to test) participants did not have to be covered in sweat or soaked in urine (35). In order to evaluate body size (Shoulder Circum.), BSA and WHR we collected anthropometric measures. To evaluate body composition, for optimal accuracy and reliability we performed the BIA instead to use predictive equations.
Glucose levels were recorded through chemical reaction (mediator glucose-dehydrogenase pirrolochinolinechinone, GDH-PQQ), inducing colour translation on reactive zone. Ethical approval was established by the University of Palermo Ethics Committee (Commissione Etica del Dipartimento DISMOT) in Italy. The principles of the Italian data protection act (196/2003) were observed. All participants provided informed consent. The study was performed in compliance with the Helsinki Declaration.
Statistical Analysis
All recorded data were stored in excel format and were correlated when appropriate. The two-way ANOVA analysis of variance with Bonferroni post tests was used for statistical considerations through Instat-Graphpad Pri-sm 5 Software (San Diego, USA).P values were considered significant when <0.05.
Results
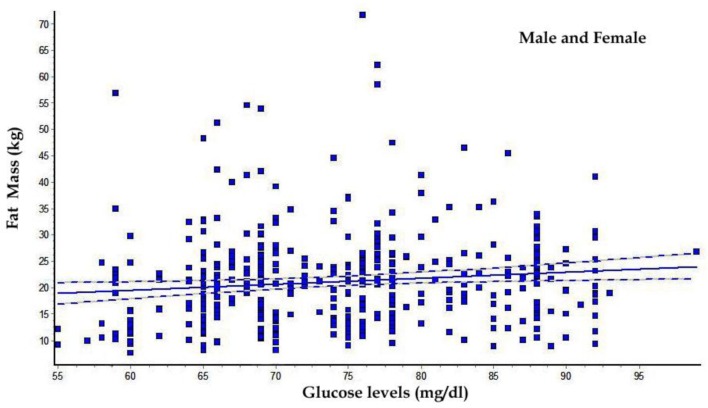
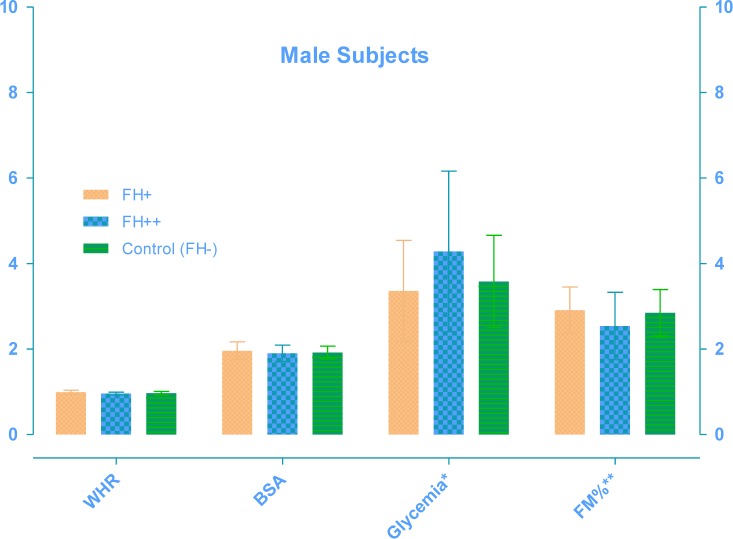
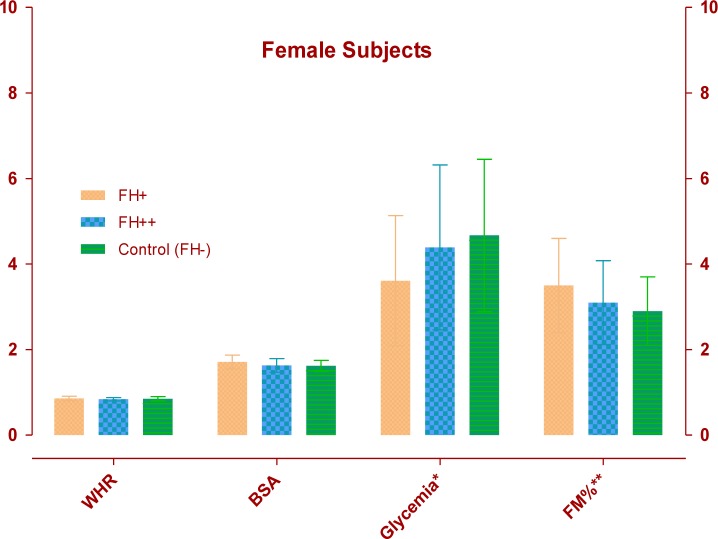
The FH+ males (Table 1) had a greater body mass index (P<0.01) with significant increase of waist and hip circumferences and consequently in WHR (P<0.01) than other groups. Table 2 shows that older FH+ females have an increase in body weight (P<0.01), body surface area (P<0.005), waist (P<0.005) and hip circumferences (P<0.05), greater than other groups. As showed in Table 3 and 4, FH+ subjects had an increase in FM, in relative (P<0.05 between males, P<0.005 between females) and absolute values (P<0.01 between males, P<0.005 between females), with worse FFM/FM ratio (P<0.01 between males, P<0.05 between females) compared to FH++ and the control group (FH−). Table 5 and 6 show blood glucose levels, of male and female subgroups, related to body parameters. Only FH+ males had a significant reduction in fasting glucose levels for kg FM unit (P<0.01). FH+ women showed lower levels of fasting glucose levels for kg of BW and FM unit (P<0.005) and for kg of BSA (P<0.01). Figure 1 shows the linear trend between the basal blood fasting glucose levels and body fat mass (r = 0.12; r2 = 0.014). Figure 2 and 3 are highlighting the results stratified by gender.
Table 1:
Anthropometric parameters of male subjects
| Variable | FH+ (n = 35) | FH++ (n = 32) | FH− (n = 116) | p |
|---|---|---|---|---|
| Age, years | 34.80 ± 12.03 | 25.50 ± 6.39 | 28.20 ± 10.41 | 0.0005 |
| Height, cm | 172.77 ± 6.57 | 174.27 ± 8.89 | 174.02 ± 6.33 | NS |
| Body weight, kg | 82.96 ± 18.51 | 75.63 ± 12.71 | 77.78 ± 11.58 | NS |
| BMI, kg/m2 | 27.72 ± 5.62 | 24.83 ± 3.20 | 25.67 ± 3.58 | 0.0078 |
| BSA, m2 | 1.96 ± 0.21 | 1.90 ± 0.19 | 1.92 ± 0.15 | NS |
| Shoulder circumference, cm | 113.73 ± 8.53 | 111.73 ± 9.91 | 114.00 ± 9.23 | NS |
| Waist circumferences. cm | 94.21 ± 15.47 | 84.17 ± 10.54 | 86.93 ± 10.47 | 0.0007 |
| Hip circumferences. cm | 95.21 ± 13.42 | 87.34 ± 9.10 | 89.44 ± 9.34 | 0.0360 |
| WHR | 0.99 ± 0.05 | 0.96 ± 0.03 | 0.97 ± 0.04 | 0.0079 |
NS: no significance
Table 2:
Anthropometric parameters of female subjects
| Variables | FH+ (n = 44) | FH++ (n = 58) | FH− (n = 135) | p |
|---|---|---|---|---|
| Age, years | 34.25 ± 12.31 | 27.62 ± 9.23 | 32.73 ± 11.71 | 0.0047 |
| Height, cm | 162.28 ± 6.27 | 160.18 ± 6.57 | 159.24 ± 5.09 | NS |
| Body weight, kg | 67.58 ± 14.67 | 61.51 ± 12.82 | 60.75 ± 10.89 | 0.0052 |
| BMI, kg/m2 | 25.77 ± 5.85 | 23.98 ± 4.85 | 23.96 ± 4.14 | NS |
| BSA, m2 | 1.71 ± 0.16 | 1.63 ± 0.16 | 1.62 ± 0.13 | 0.0015 |
| Shoulder circumferences, cm | 100.84 ± 8.87 | 98.02 ± 7.05 | 98.38 ± 6.00 | NS |
| Waist circumferences, cm | 82.82 ± 12.53 | 75.75 ± 10.79 | 77.46 ± 10.47 | 0.0040 |
| Hip circumferences. cm | 96.07 ± 13.57 | 90.55 ± 12.63 | 90.72 ± 11.35 | 0.0296 |
| WHR | 0.86 ± 0.05 | 0.84 ± 0.04 | 0.85 ± 0.05 | NS |
NS: no significance
Table 3:
Body composition (BIA) of male subjects
| Variables | FH+ (n = 35) | FH++ (n = 32) | FH− (n = 116) | P |
|---|---|---|---|---|
| H2O, litres | 39.06 ± 8.26 | 37.60 ± 7.50 | 36.95 ± 5.57 | NS |
| FM, % | 29.10 ± 5.41 | 25.46 ± 7.97 | 28.59 ± 5.43 | 0.0191 |
| FFM, % | 70.90 ± 5.41 | 74.54 ± 7.97 | 71.41 ± 5.43 | 0.0191 |
| FM, kg | 24.37 ± 7.83 | 19.23 ± 6.87 | 22.35 ± 5.85 | 0.0052 |
| FFM, kg | 58.59 ± 12.40 | 56.40 ± 11.25 | 55.43 ± 8.36 | NS |
| FFM/FM | 2.63 ± 1.10 | 3.51 ± 1.94 | 2.72 ± 1.20 | 0.0085 |
NS: no significance
Table 4:
Body composition (BIA) of female subjects
| Variables | FH+ (n = 44) | FH++ (n = 58) | FH− (n = 135) | P |
|---|---|---|---|---|
| H2O, litres | 28.33 ± 3.48 | 27.33 ± 3.20 | 27.96 ± 3.31 | NS |
| FM, % | 35.28 ± 11.12 | 31.76 ± 9.82 | 29.90 ± 8.32 | 0.0039 |
| FFM, % | 64.72 ± 11.12 | 68.24 ± 9.82 | 70.10 ± 8.32 | 0.0039 |
| FM, kg | 25.08 ± 13.86 | 20.51 ± 11.19 | 18.81 ± 8.49 | 0.0026 |
| FFM, kg | 42.50 ± 5.22 | 40.99 ± 4.80 | 41.94 ± 4.97 | NS |
| FFM/FM | 2.09 ± 0.90 | 2.43 ± 1.00 | 2.60 ± 0.97 | 0.0102 |
NS: no significance
Table 5:
Fasting blood glucose levels of male subject in absolute and relative values
| Glucose levels | FH+ (n = 35) | FH++ (n = 32) | FH− (n = 116) | P |
|---|---|---|---|---|
| mg/dl | 73.86 ± 10.14 | 71.72 ± 9.81 | 74.30 ± 9.31 | NS |
| mg/dl · kg BW−1 | 0.93 ± 0.24 | 0.97 ± 0.20 | 0.97 ± 0.18 | NS |
| mg/dl · kg FFM−1 | 1.32 ± 0.36 | 1.33 ± 0.34 | 1.37 ± 0.29 | NS |
| mg/dl · kg FM−1 | 3.36 ± 1.18 | 4.28 ± 1.88 | 3.58 ± 1.08 | 0.0076 |
| mg/dl · kg BSA−1 | 38.15 ± 6.81 | 38.15 ± 6.60 | 38.91 ± 5.59 | NS |
NS: no significance
Table 6:
Fasting blood glucose levels of female subject in absolute and relative values
| Glucose levels | FH+ (n = 44) | FH++ (n = 58) | FH− (n = 135) | P |
|---|---|---|---|---|
| mg/dl | 73.61 ± 8.93 | 73.09 ± 8.75 | 75.33 ± 9.29 | NS |
| mg/dl · kg BW−1 | 1.13 ± 0.23 | 1.24 ± 0.28 | 1.27 ± 0.23 | 0.0046 |
| mg/dl · kg FFM−1 | 1.75 ± 0.29 | 1.81 ± 0.32 | 1.82 ± 0.29 | NS |
| mg/dl · kg FM−1 | 3.61 ± 1.52 | 4.39 ± 1.93 | 4.67 ± 1.78 | 0.0031 |
| mg/dl · kg BSA−1 | 43.25 ± 6.40 | 45.22 ± 7.32 | 46.75 ± 6.30 | 0.0081 |
NS: no significance
Fig. 1:
Linear trend between glucose levels and fat mass (kg); (correlation Coefficient r = 0.1199; r2 = 0.01437)
Fig. 2:
Male Subjects variables: WHR (Waist–hip ratio); BSA (Body Surface Area, m2); Glycemia (* Glucose levels reported in mg/dl ·kg FM−1); FM% (** Body Fat Mass reported in % divided by 10)
Fig. 3:
Female Subjects variables: WHR (Waist–hip ratio); BSA (Body Surface Area, m2); Glycemia (* Glucose levels reported in mg/dl ·kg FM−1); FM% (** Body Fat Mass reported in % divided by 10)
Discussion
The development of TD2 during years has relevantly increased in occidental countries as shown by WHO (32, 36–38). This has demonstrated the multifactorial pathogenesis of this disease correlating its manifestation not only with genetic factors or predispositions but also with age, gender, un-healthy life styles, obesity and physical inactivity (10, 34, 39, 40). Our results are confirming the hypotheses that there are few anthropometrics and physiological variables strongly related to family history, predominantly linked to a first degree of familiarity. In addition, environmental factors are determinant for the pathogenesis of the illness (41, 42). This great variability of risk factors leads to a multiple therapeutic control: new drugs are now used to control this disease, such as incretins Gip (gastric polypeptide inhibitor) and Glp-1 (glucagone similar peptide) or similar drugs likesitagliptine and exenatide. Some studies have showed that TD2 patients produce lower levels of incretines, which normally promote insulin synthesis and release after food intake (43–48). Knowing phenotype, metabolic or blood parameters in subjects with positive family history to diabetes may prevent the development of this disease. It has been also shown that FH to TD2 can lead to unbalanced energy expenditure, so consequently may influence the body composition (21, 22, 24, 25). The present study are confirming what found in scientific literature and are showing, in addiction, that in subjects with first degree of FH there is an increase of body weight for an augment of body fat (this body composition modification may determine an alterations of energy expenditure) (22, 26, 28, 49). It is also confirmed that in sedentary man and women there is a strong correlation between FH TD2 and precocious increase in body weight (22, 50, 51).
These increases involve body fat mass, especially in FH+. Moreover when the same values are related to phenotype parameters a strong linear correlation with FM, in both sexes was shown even though, the results are more evident in women, maybe for the inter-generative transmission of illness predisposition (3–9, 51).
It is hypothesized that the muscle cells of these subjects undergo early alterations (genetically determined) in insulin-independent glucose transport, which could augment glucose uptake through insulin-dependent pathways, with the risk of hyper-insulinemia and peripheral insulin resistance (11). The most evident, direct factor contributing to such alterations could be a family history of diabetes, related to unknown factors thought to be linked to genes encoding altered poorly functioning proteins, inducing increase in body weight (hypertrophic effect of insulin) and particularly of body fat mass (lipogenic effect of insulin)(52–54). Coming up, the insulin-dependent compensatory mechanisms could deteriorate and break down, resulting in a clinical manifestation of the disease. In FH+, regular physical activity reduces the increase in glucose uptake through insulin-dependent pathways, stimulating the alternative, although defective, function of insulin-independent mechanisms, thus improving glucose control and reducing body mass, in particular body fat mass (55). The deficient insulin-dependent mechanisms could acts as a brake, impairing metabolic response and subsequent oxygen consumption during regular physical activity, which has also been demonstrated in studies conducted in normal conditions (22, 24).
Conclusion
Family history on TD2 especially in FH+ influences body composition and weight in healthy sedentary male and women. The multifactorial phatogenetic mechanism of TD2 makes this disease difficult to approach but it is also known the protective role of regular physical activity in order to maintain health and control body weight and blood glycemia. FH+ compared to other groups showed greater body mass and WHR. This important finding, highlighted for the first time on that study, is confirming the hypothesis that the first degree of FH is a strong indicator of precocious modifications of body composition.
Other studies are needed to confirm these encouraging results especially for unhealthy or athletes with family history to TD2.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
The study was supported by the MEDEOR Research Institute – Palermo, with the grant code AED-SELINO-S6/2011-2012. We are grateful to all participants for their contributions. The authors declare that there is no conflict of interests.
References
- 1.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T, mellitus Cot J, Sotdcod D. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Erasmus RT, Blanco Blanco E, Okesina AB, Mesa Arana J, Gqweta Z, Matsha T. Importance of family history in type 2 black South African diabetic patients. Postgrad Med J. 2001;77:323–5. doi: 10.1136/pmj.77.907.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crispim D, Canani LH, Gross JL, Tschiedel B, Souto KE, Roisenberg I. Familial history of type 2 diabetes in patients from Southern Brazil and its influence on the clinical characteristics of this disease. Arq Bras Endocrinol Metabol. 2006;50:862–8. doi: 10.1590/s0004-27302006000500006. [DOI] [PubMed] [Google Scholar]
- 5.Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes care. 2005;28:1457–62. doi: 10.2337/diacare.28.6.1457. [DOI] [PubMed] [Google Scholar]
- 6.Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes care. 2002;25:16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Bjørnholt JV, Erikssen G, Liestøl K, Jervell J, Thaulow E, Erikssen J. Type 2 diabetes and maternal family history: an impact beyond slow glucose removal rate and fasting hyperglycemia in low-risk individuals? Results from 22.5 years of follow-up of healthy nondiabetic men. Diabetes care. 2000;23:1255–9. doi: 10.2337/diacare.23.9.1255. [DOI] [PubMed] [Google Scholar]
- 8.Bjørnholt JV, Erikssen G, Liestøl K, Jervell J, Erikssen J, Thaulow E. Prediction of Type 2 diabetes in healthy middle-aged men with special emphasis on glucose homeostasis. Results from 22.5 years' follow-up. Diabet Med. 2001;18:261–7. doi: 10.1046/j.1464-5491.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 9.Grill V, Persson G, Carlsson S, Norman A, Alvarsson M, Ostensson CG, Svanström L, Efendic S. Family history of diabetes in middle-aged Swedish men is a gender unrelated factor which associates with insulinopenia in newly diagnosed diabetic subjects. Diabetologia. 1999;42:15–23. doi: 10.1007/s001250051106. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Fennoy I, Accacha S, Altshuler L, Carey DE, Holleran S, Rapaport R, Shelov SP, Speiser PW, Ten S, Bhangoo A, Boucher-Berry C, Espinal Y, Gupta R, Hassoun AA, Iazetti L, Jacques FJ, Jean AM, Klein ML, Levine R, Lowell B, Michel L, Rosenfeld W. Racial/ethnic Differences in Clinical and Biochemical Type 2 Diabetes Mellitus Risk Factors in Children. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carstens MT, Goedecke JH, Dugas L, Evans J, Kroff J, Levitt NS, Lambert EV. Fasting substrate oxidation in relation to habitual dietary fat intake and insulin resistance in non-diabetic women: a case for metabolic flexibility? Nutr Metab (Lond) 2013;10:8. doi: 10.1186/1743-7075-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissén M, Ehrnström BO, Forsén B, Snickars B, Lahti K, Forsblom C, Saloranta C, Taskinen MR, Groop LC. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–7. doi: 10.2337/diabetes.48.1.150. [DOI] [PubMed] [Google Scholar]
- 13.Hamaguchi K, Kimura A, Kusuda Y, Yamashita T, Yasunami M, Takahasi M, Abe N, Yoshimatsu H. Clinical and genetic characteristics of GAD-antibody positive patients initially diagnosed as having type 2 diabetes. Diabetes Res Clin Pract. 2004;66:163–71. doi: 10.1016/j.diabres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Morales LM, Semprún-Fereira M, Ryder E, Valbuena H, Rincón E, Fernandez V, Flórez H, Campos G, Gómez ME, Raleigh X. Improved triglyceride control with low glycaemic index-high carbohydrate modified-lipid diet in a hypertriglyceridaemic child. Acta Paediatr. 1997;86:772–4. doi: 10.1111/j.1651-2227.1997.tb08586.x. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26:650–5. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- 16.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan SR, Elkasabani A, Dalferes ER, Bao W, Berenson GS. Characteristics of young offspring of type 2 diabetic parents in a biracial (black-white) community-based sample: the Bogalusa Heart Study. Metabolism. 1998;47:998–1004. doi: 10.1016/s0026-0495(98)90358-4. [DOI] [PubMed] [Google Scholar]
- 18.Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissén M, Ehrnström BO, Forsén B, Isomaa B, Snickars B, Taskinen MR. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes. 1996;45:1585–93. doi: 10.2337/diab.45.11.1585. [DOI] [PubMed] [Google Scholar]
- 19.Florez H, Ryder E, Campos G, Fernandez V, Morales LM, Valbuena H, Rincón E, Gómez ME, Raleigh X. Women relatives of Hispanic patients with type 2 diabetes are more prone to exhibit metabolic disturbances. Invest Clin. 1999;40:127–42. [PubMed] [Google Scholar]
- 20.Valdez R. Detecting undiagnosed type 2 diabetes: family history as a risk factor and screening tool. J Diabetes Sci Technol. 2009;3:722–6. doi: 10.1177/193229680900300417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravante G, Pomara F, Angelomè C, Russo G, Truglio G. The basal energy expenditure of female athletes vs. sedentary women as related to their family history of type 2 diabetes. Acta Diabetol. 2001;38:63–70. doi: 10.1007/s005920170015. [DOI] [PubMed] [Google Scholar]
- 22.Bianco A, Pomara F, Jemni M, Paoli A, Petrucci M, Bellafiore M, Palma A. Influence of family history of NIDDM on basal metabolic rate in sedentary and active women. Panminerva Med. 2011;53:253–9. [PubMed] [Google Scholar]
- 23.Wareham NJ. Epidemiological studies of physical activity and diabetes risk, and implications for diabetes prevention. Appl Physiol Nutr Metab. 2007;32:778–82. doi: 10.1139/H07-032. [DOI] [PubMed] [Google Scholar]
- 24.Pomara F, Russo G, Amato G, Gravante G. Familiar history and predictive risk factors to type 2 diabetes: a cross sectional study in young Sicilian subjects of both sexes. Panminerva Med. 2005;47:259–64. [PubMed] [Google Scholar]
- 25.Pomara F, Russo G, Gravante G. Influence of family history to type 2 diabetes on the body composition and homeostasis model assessment: a comparison between young active and sedentary men. Minerva Med. 2006;97:379–83. [PubMed] [Google Scholar]
- 26.Bianco A, Pomara F, Raccuglia M, Bellafiore M, Battaglia G, Filingeri D, Paoli A, Palma A. The relationship between type 2 diabetes family history, body composition and blood basal glycemia in sedentary people. Acta Diabetol. 2013 2013 Jul 1; doi: 10.1007/s00592-013-0502-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Olive JL, Ballard KD, Miller JJ, Milliner BA. Metabolic rate and vascular function are reduced in women with a family history of type 2 diabetes mellitus. Metabolism. 2008;57:831–7. doi: 10.1016/j.metabol.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Machac S, Prochazka M, Radvansky J, Slaby K. Validation of Physical Activity Monitors in Individuals with Diabetes: Energy Expenditure Estimation by the Multisensor SenseWear Armband Pro3 and the Step Counter Omron HJ-720 Against Indirect Calorimetry During Walking. Diabetes Technol Ther. 2013;15:413–8. doi: 10.1089/dia.2012.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colucci A, Bianco A, Pomara F, Petrucci M, Grosso F, De Vita A, Adamo V, Palma A. Disease management of type 2 diabetes: a follow-up analysis in a sanitary district of Sicily. Minerva Gastroenterol Dietol. 2011;57:241–6. [PubMed] [Google Scholar]
- 30.Shahid A, Saeed S, Rana S, Mahmood S. Family history of diabetes and parental consanguinity: important risk for impaired fasting glucose in south east Asians. West Indian Med J. 2012;61:219–23. doi: 10.7727/wimj.2011.072. [DOI] [PubMed] [Google Scholar]
- 31.Bianco A, Pomara F, Bellafiore M, Petrucci M, Cacciola F, Battaglia G, Palma A. Family history to type 2 diabetes influence on body parameters of young soccer players. It Jour of Sport Sciences. 2011;15:32–35. [Google Scholar]
- 32.Xu F, Wang Y, Ware RS, Tse LA, Dunstan DW, Liang Y, Wang Z, Hong X, Owen N. Physical activity, family history of diabetes and risk of developing hyperglycaemia and diabetes among adults in Mainland China. Diabet Med. 2012;29:593–9. doi: 10.1111/j.1464-5491.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 33.Montomoli M, Gonnelli S, Giacchi M, Mattei R, Cuda C, Rossi S, Gennari C. Validation of a food frequency questionnaire for nutritional calcium intake assessment in Italian women. Eur J Cli Nutr. 2002;56:21–30. doi: 10.1038/sj.ejcn.1601278. [DOI] [PubMed] [Google Scholar]
- 34.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 35.Going S, Nichols J, Loftin M, Stewart D, Lohman T, Tuuri G, Ring K, Pickrel J, Blew R, Stevens J. Validation of bioelectrical impedance analysis (BIA) for estimation of body composition in Black, White and Hispanic adolescent girls. Int J Body Compos Res. 2006;4:161–167. [PMC free article] [PubMed] [Google Scholar]
- 36.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12:14. doi: 10.1186/2251-6581-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhowmik B, Afsana F, My Diep L, Binte Munir S, Wright E, Mahmood S, Khan AK, Hussain A. Increasing prevalence of type 2 diabetes in a rural bangladeshi population: a population based study for 10 years. Diabetes Metab J. 2013;37:46–53. doi: 10.4093/dmj.2013.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 39.Gagliardino JJ, Lapertosa S, Pfirter G, Villagra M, Caporale JE, Gonzalez CD, Elgart J, Gonzalez L, Cernadas C, Rucci E, Clark C, Jr, the P Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR) Diabet Med. 2013 2013 May 13; doi: 10.1111/dme.12230. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft AJ, Carpena-Ruiz M, Montero-Errasquin B, Sanchez-Castellano C, Sanchez-Garcia E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61:734–8. doi: 10.1111/jgs.12215. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Xu W, Dahl AK, Xu Z, Wang HX, Qi X. Relation of socio-economic status to impaired fasting glucose and Type 2 diabetes: findings based on a large population-based cross-sectional study in Tianjin, China. Diabet Med. 2013;30:e157–62. doi: 10.1111/dme.12156. [DOI] [PubMed] [Google Scholar]
- 42.Juren AJ, Sarwal G, Al-Sarraf A, Vrablik M, Chan D, Humphries KH, Frohlich JJ. Low prevalence of type 2 diabetes mellitus among patients with high levels of high-density lipoprotein cholesterol. J Clin Lipidol. 2013;7:194–8. doi: 10.1016/j.jacl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Thongtang N, Sriwijitkamol A. Incretins: the novel therapy of type 2 diabetes. J Med Assoc Thai. 2008;91:943–54. [PubMed] [Google Scholar]
- 44.Knop FK, Vilsbøll T, Holst JJ. Incretin-based therapy of type 2 diabetes mellitus. Curr Protein Pept Sci. 2009;10:46–55. doi: 10.2174/138920309787315158. [DOI] [PubMed] [Google Scholar]
- 45.Hare KJ, Knop FK. Incretin-based therapy and type 2 diabetes. Vitam Horm. 2010;84:389–413. doi: 10.1016/B978-0-12-381517-0.00015-1. [DOI] [PubMed] [Google Scholar]
- 46.Krishna R, Herman G, Wagner JA. Accelerating drug development using biomarkers: a case study with sitagliptin, a novel DPP4 inhibitor for type 2 diabetes. AAPS J. 2008;10:401–9. doi: 10.1208/s12248-008-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woehl A, Evans M, Tetlow AP, McEwan P. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom. Cardiovasc Diabetol. 2008;7:24. doi: 10.1186/1475-2840-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24:381–7. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- 49.Butte NF, Puyau MR, Vohra FA, Adolph AL, Mehta NR, Zakeri I. Body size, body composition, and metabolic profile explain higher energy expenditure in overweight children. J Nutr. 2007;137:2660–7. doi: 10.1093/jn/137.12.2660. [DOI] [PubMed] [Google Scholar]
- 50.Ackermann RT, Holmes AM, Saha C. Designing a natural experiment to evaluate a national health care-community partnership to prevent type 2 diabetes. Preven Chron Disease. 2013;10:E12. doi: 10.5888/pcd10.120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Organization WH . Preventing Chronic Diseases: a Vital Investment: WHO Global Report. 2005. ed. © WHO. [Google Scholar]
- 52.Bener A, Yousafzai MT, Al-Hamaq AO, Mohammad AG, Defronzo RA. Parental transmission of type 2 diabetes mellitus in a highly endogamous population. World J Diabetes. 2013;4:40–6. doi: 10.4239/wjd.v4.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhattarai MD. Three patterns of rising type 2 diabetes prevalence in the world: need to widen the concept of prevention in individuals into control in the community. JNMA, Jour of the Nepal Med Assoc. 2009;48:173–9. [PubMed] [Google Scholar]
- 54.Pambianco G, Costacou T, Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care. 2007;30:1248–54. doi: 10.2337/dc06-2053. [DOI] [PubMed] [Google Scholar]
- 55.Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, Yang BT, Lang S, Parikh H, Wessman Y, Weishaupt H, Attema J, Abels M, Wierup N, Almgren P, Jansson PA, Ronn T, Hansson O, Eriksson KF, Groop L, Ling C. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61:3322–32. doi: 10.2337/db11-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]