Abstract
Background:
Prenatal lead exposure could not only affect various organ systems of the mother, but also provide a plumbeous environment for the fetus and newborns, and may affect the fetus in a number of detrimental ways. The aim of this study was to adequately determine the interaction between these factors and risky behaviors such as smoking.
Methods:
Data from Nanjing Maternity and Child Health Care Hospital survey during the years of 2006–2011 were used (n = 4400) to evaluate the effections of age, parity, body mass index (BMI), race/ethnicity, pregnancy, iron (Fe) storage status and smoking status on the consumption of the levels of blood cadmium (Cd) and lead (Pb) of females aged 16–35yr old. The blood samples were sent to determine blood lead / cadmium concentration by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS). STATA 12.1 software (www.stata.com) was used to fit regression models for each of the two metals.
Results:
For both of the two metals, age was positively while BMI was negatively associated with the levels of these metals in blood. Smokers showed statistically significantly higher levels of Cd and Pb (P=0.007), while irrespective of race/ethnicity and Fe storage status as compared to nonsmokers.
Conclusion:
Novel to this study, pregnancy was found to be associated with significantly lower levels of Cd and Pb, while irrespective of race/ethnicity and Fe storage status as compared to non-pregnant females. It is conceivable that pregnancy could thus accelerate clearance of these metals in the blood.
Keywords: Cadmium, Lead, Pregnancy, Iron, Smoking, China
Introduction
The growing population, rapid industrialization and urbanization have increased the exposure to environmental pollutants of all living systems (1). Lead (Pb) and cadmium (Cd) are reported as two of the top 10 toxic metals in the Priority List of Hazardous Substances announced by the Agency for Toxic Substances and Disease Registry (2). Cd has caused neurotoxicologic and behavioral changes in both humans and experimental animal studies (3). Cd exposure may be implicated in some neurological disorders including hyperactivity and increased aggressiveness in humans (4). In the case of coronary risk with metal levels, Cd may be more important for females and copper (Cu) for elderly males (5). Cd was reported to damage bone microstructure (6), and Pb and Cd can negatively influence growth in newborns. Several studies have reported an inverse relationship between anthropometric measurements of the newborn and the placental or umbilical cord Pb/Cd levels (7–8). Pb and Cd exposure exerts inhibitory effects on testicular steroidogenesis (9). It is well known that lead could accumulate in the body for a long time; however, there is not a safe limit of lead exposure for children, while a great interest in the evaluation of the adverse effects of this metal in low concentrations has emerged in adults(10). The results indicated that exposure to Pb was associated with anxiety and depression (10). In addition; some researchers have shown that Pb exposure could influence the anthropometric measurements during the infancy period and childhood.
Lead can readily transferred to the fetus through the placenta. Thus, several studies focused on the issue of body burden of heavy metals on pregnant females (11–12). There are various risk factors which could contribute to the presence of higher heavy metal concentrations in humans, including: [1] Ability to store Fe is considered as a risk factor that has been studied often. Relatively higher Cd burden in pregnant females compared to males was reported due to low Fe status (13). In a study of never-smoking females aged 20–49 yr, an inverse relationship between urine and blood Cd level and ability to store Fe as reported Lee and Kim (14). In the research, they also noted an inverse association between blood Cd level and ability to store Fe as determined by serum ferritin levels both in males and females. [2] Smoking has been identified as one of the risk factors that are associated with increased levels of Cd and the consequences resulting in lung damage (15). Blood Cd levels of 0.7 μg/L among smokers and 0.13 μg/L among nonsmokers were obtained in their study of 6 hospital employees. Similar results were reported for urine Cd in a study on 300 copper smelters (16). There were increased levels of blood and urine Pb and Cd of those who smoked 20 cigarettes per day compared to those who did not smoke that amount or did not smoke at all. [3] Both blood and urinary Cd levels increased with age (17).
While Cd levels were reported to decline over time, the trends were different between males and females (18). Lead and cadmium are trace elements which are present in the environment and which have no known biological role in the human organism. However, another case–control study showed an excess prostate cancer risk (19). A blood cadmium level of 0.38ug/L was associated with tubular impairment among women 53–64 years of age (20). and in the US, adults with mean blood cadmium of 0.41ug/L had a higher risk for albuminuria (21). Recently, the results of the III NHANES study performed on 13,958 subjects followed until December 2000 have shown that environmental cadmium exposure among the general population, has been associated with a higher risk of cancer, cardiovascular and overall mortality in men, but not in women (22). This survey targets a representative mix of population with adequate variations in genetics, culture, dietary behavior and risk-taking behavior. Thus, the aim of this study was to adequately determine the interaction of these factors with the risky behaviors such as smoking. This work is focused on how metal levels may vary and the potential influence of pregnancy status, thus the research objects were limited to pregnant and non-pregnant females. In addition, to limit the scope of this study, only blood metals, specifically Pb and Cd, were considered. Publicly available data from prenatal clinic over 3 cycles, Downloaded namely, 2006–2007, 2008–2009 and 2010–2011, were used.
Materials and Methods
Materials and Study Population
Publically available cross-sectional data is from Nanjing Maternity and Child Health Care Hospital Prenatal Clinic, during the years of 2006–2011 for a variety of variables including blood metals, specifically blood Cd and Pb, were downloaded totally.
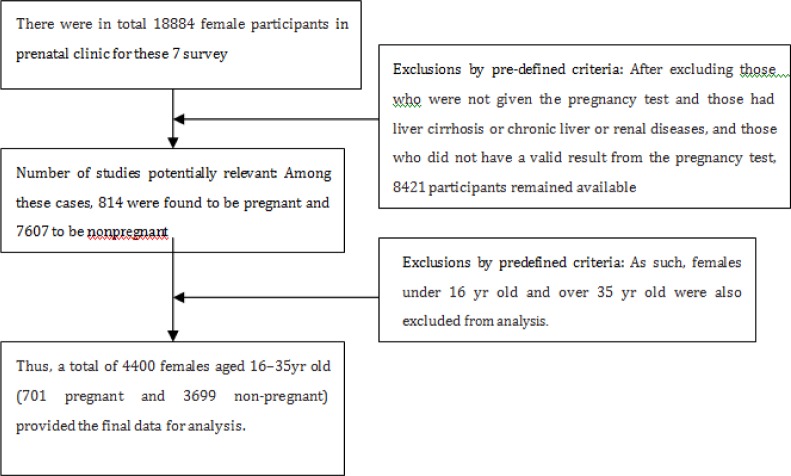
There were in total 18884 female participants in Nanjing Maternity and Child Health Care Hospital Gynecology and Obstetrics Clinic during the years of 2006–2011 for survey. Complex, stratified multistage probability sampling was used, designed as representative of the civilian, non-institutionalized Nanjing population based on age, gender, and race/ethnicity (Fig. 1).
Fig. 1:
Map of the Study area
Sampling weights are created in Gynecology and Obstetrics Clinic to account for the complex survey design, including oversampling, survey non-response, and post tratification. While pregnant females were over sampled before the 2007–2008 survey cycles, oversampling of pregnant females was discontinued starting with the 2007–2008 survey cycle. Female survey participants aged 7 yr and older were administered a urine pregnancy test. If the urine pregnancy test was positive on any female participants aged 8–17 yr, the result was confirmed using a serum test. After excluding those who were not given the pregnancy test and those had liver cirrhosis or chronic liver or renal diseases, and those who did not have a valid result from the pregnancy test, 8421 participants remained available for analysis. Among these cases, 814 were found to be pregnant and 7607 to be non-pregnant. Considering that the number of pregnant females under 16 yr (n = 16) and over 35 yr (n = 11) was quite small, thus these cases were also excluded from analysis. Finally, a total of 4400 females aged 16–35yr old (701 pregnant and 3699 non-pregnant) provided the final data for analysis (Fig. 2).
Fig. 2:
The screening flowsheet of health women
This study was approved by the Ethical Committee of Nanjing Maternity and Child Health Care Hospital. The Pregnancy mothers were informed about the purpose of the study and a written consent was obtained from all participants.
Methods and blood sample collection
Fasting blood samples were obtained from the antecubital vein of each pregnancy women during the pregnancy periods and the non-pregnant controls after a 30-min rest in a sitting position. Then, the blood samples were drawn into vacuum tubes (Becton-Dickinson, Franklin Lakes, NJ USA) containing heparin lithium and sent to determine blood lead concentration by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) within 1 h in the Department of Laboratory Medicine in Nanjing Maternity and Child Health Care Hospital. Internal quality controls were taken both before and after the detection.
STATA 12.1 software (www.stata.com) was used to fit regression models for each of the two metals. Data for both two metals were log-transformed before fitting the models. The independent categorical variables used for the analysis were: pregnancy status (pregnant, non-pregnant), smoking status (smoker, deficient, replete).
In the first pass of the model for blood Cd, 4 of 6 interactions that were included in the model were found to be statistically significant at a = 0.05. These were race/ethnicity by smoking status, Fe storage status by smoking status, and Fe storage status by pregnancy status, which were kept in the final model. The un-weighted sample size for the model for blood Cd was 4111 and the adjusted R2 was 50%. For the final model for blood Pb, only the interactions between pregnancy and smoking status were found to be statistically significant at a = 0.05 and were kept in the final model. The un-weighted sample size of for the model for blood Pb was 4127, with R2 of 15.8%.
[1] Smoking status was determined based on the values of serum cotinine. Those with values of serum cotinine <10 ng/ml were classified as nonsmokers and those with serum cotinine values of ≥10 ng/ml were classified as smokers. This is the same classification as used by Jain and Wang (19).
[2] Those with serum ferritin levels of <16.5 ng/ml were defined as being Fe absent; those with ferritin levels of 16.5–26.5 ng/ml were defined as being Fe deficient; and those with ferritin levels of >26.5 ng/ml were defined as Fe replete.
Two way interactions between race/ethnicity, pregnancy status, smoking status, and Fe storage status were also included in each model. The independent continuous variables used were prenatal clinic survey cycle, parity defined as the number of live births, age, body mass index (BMI), serum albumin, serum creatinine, and serum hemoglobin. In the first pass of each model, interactions with statistically significant less than 5% were excluded from the final models. The weighted sample sizes and unweighted geometric means (UGM) with their 95% confidence intervals (CI) were listed in Table 1.
Table 1:
Unadjusted Geometric Means (UGM) with 95% Confidence Intervals for blood lead and cadmium, by race/ethnicity, pregnancy status, smoking status, and iron storage capacity for females aged 16–32 years in NANJING during 2006–2011
|
Blood Lead (ug/L) (ug/dL) |
Blood Cadmium | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Sample Size | 95%Conifedence Interval | |||||
| Geometric Mean | Lower Limit | Upper Limit | Geometric Mean | Lower Limit | Upper Limit | |||
| Total | Han nationality | 3778 | 3.913 | 3.880 | 3.937 | 1.434 | 1.401 | 1.834 |
| Others | 299 | 3.900 | 3.889 | 3.997 | 1.430 | 1.389 | 1.794 | |
| Pregnancy Status | Pregnant | 701 | 3.740 | 3.709 | 3.801 | 1.357 | 1.337 | 1.775 |
| Non-Pregnant | 3699 | 3.928 | 3.801 | 3.978 | 1.440 | 1.418 | 1.743 | |
| Smoking Status | Non-Smoker | 3533 | 3.856 | 3.821 | 3.894 | 1.345 | 1.337 | 1.742 |
| Smoker | 979 | 4.012 | 4.014 | 4.128 | 1.934 | 1.783 | 1.914 | |
| Iron Storage Capacity | Iron Absent | 627 | 3.962 | 4.014 | 4.115 | 4.503 | 1.414 | 1.541 |
| Iron Deficient | 460 | 3.916 | 3.879 | 3.951 | 1.478 | 1.449 | 1.501 | |
| Iron Replete | 3148 | 3.801 | 3.881 | 3.921 | 1.417 | 1.312 | 1.504 | |
Results
The descriptive analysis
Levels of both two metals rose significantly with increase in age at screening (P<0.01) (Table 2). Levels of both two metals decreased markedly with elevation of body mass index (BMI) (Table 2). Levels of Pb decreased significantly with each 2-yr survey cycle. A significant positive association with serum albumin was found only for blood Pb level (P=0.02). The levels of Cd and Pb were significantly elevated with increase in serum hemoglobin (P<0.01). In addition, there was a significant rise in concentration levels of blood Pb with the increase of serum creatinine (P<0.01). Main effects involving statistically significant interactions are not subject to interpretation. For Pb, Fe storage status was the only main effect that was interpretable and statistically significant (P<0.01) (Table 2).
Table 2:
Final fitted regression models with f statistics for main and interaction effects regression slopes for continuous variables and their P values
| Parameter | log(Blood Cadmium) | log(Blood Lead) | ||
|---|---|---|---|---|
| F or β | P-value | F or β | P-value | |
| Race/Ethnicity | 7.45 | 0.04 | 30.12 | 0.05 |
| Pregnancy Status | 5.89 | <0.01 | 5.47 | <0.01 |
| Iron Storage Status | 8.14 | <0.01 | 5.14 | <0.01 |
| Smoking Status | 789.470 | <0.01 | 78.91 | <0.01 |
| Fish/Shellfish Consumption during the last 30 days (F/S) | 0.351 | 0.41 | N/A | 410.12 |
| Pregnancy X Iron Storage Status | 5.147 | <0.01 | NIM | |
| Pregnancy X Smoking Status | NIM | NIM | ||
| Iron Storage Status X Smoking Status | 8.478 | <0.01 | NIM | |
| Age | 0.006 | <0.01 | 0.007 | <0.01 |
| Body Mass Index | −0.004 | <0.01 | −0.006 | <0.01 |
| Parity | 0.004 | 0.25 | 0.005 | 0.91 |
| Survey cycle | 0.007 | 0.07 | −0.041 | <0.01 |
| Serum Albumin | −0.028 | 0.04 | 0.027 | 0.02 |
| Serum Hemoglobin | 0.020 | <0.01 | 0.021 | <0.01 |
| Serum Creatinine | 0.014 | 0.45 | 0.071 | <0.01 |
NIM: Not in the model
The results of regression
Blood Pb levels for those Fe was absent were significantly higher than those either deficient or replete (3.968 μg/dl vs. 3.804 μg/dl and 3.801 μg/dl, Table 3). The differences between those with deficient and replete Fe storage were not statistically significant (Table 3).
Table 3:
Adjusted Geometric Means (AGM) with 95% confidence intervals for blood lead, cadmium, and total mercury by race/ethnicity, pregnancy status, smoking status, and iron storage capacity for females aged 16–32 years in China during 2006–2011
| Variable | Group | Blood Cadmium (μg/L) | Blood Lead (μg/dl) | ||||
|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | ||||||
| AGM | Lower Limit | Upper Limit | AGM | Lower Limit | Upper Limit | ||
| Race/Ethnicity | Han nationality | 1.384 | 1.317 | 1.499 | 3.715 | 3.701 | 3.884 |
| Others | 1.378 | 1.307 | 1.457 | 3.724 | 3.703 | 3.901 | |
| Pregnancy Status | Pregnant | 1.398 | 1.384 | 1.407 | 3.849 | 3.811 | 3.924 |
| Non-Pregnant | 1.410 | 1.404 | 1.428 | 3.920 | 3.857 | 3.971 | |
| Smoking Status | Non-Smoker | 1.341 | 1.330 | 1.348 | 3.871 | 3.864 | 4.251 |
| Smoker | 1.884 | 1.857 | 1.974 | 4.012 | 4.010 | 4.620 | |
| Iron Storage Capacity | Iron Absent | 1.481 | 1.472 | 1.507 | 3.968 | 3.937 | 3.995 |
| Iron Deficient | 1.429 | 1.415 | 1.445 | 3.804 | 3.800 | 3.935 | |
| Iron Replete | 1.391 | 1.381 | 1.451 | 3.801 | 3.792 | 4.018 | |
The data of comparisons of AGM among different groups within each category were provided in Table 3. Irrespective of whether or not main effects were both interpretable. Smokers showed significantly higher Cd levels than nonsmokers for every Fe storage status category (P<0.01) (Table 4). In the case of Fe-deficient smokers, Cd levels were more than 3-fold higher of nonsmokers (Table 4, 1.353 μg/L vs. 1.926 μg/L). Iron-absent nonsmokers showed markedly higher Cd levels than either Fe-deficient or Fe replete nonsmokers. Pregnant females displayed significantly lower Cd levels than non-pregnant females irrespective of Fe storage status (P<0.01) (Table 5).
Table 4:
Adjusted Geometric Means (AGM) for iron storage status by smoking status for blood cadmium and P values for the test between smoking and nonsmoking females
| Blood Cadmium (μg/L) | |||||
|---|---|---|---|---|---|
| 95% Confidence Interval | |||||
| Iron Storage Status | Smoking Status | AGM | Lower Limit | Upper Limit | P |
| Iron Absent | Non Smoker | 1.401 | 1.390 | 1.448 | <0.01 |
| Smoker | 1.947 | 1.869 | 2.014 | ||
| Iron Deficient | Non Smoker | 1.353 | 1.341 | 1.368 | <0.01 |
| Smoker | 1.926 | 1.747 | 1.991 | ||
| Iron Replete | Nonsmokers | 1.312 | 1.301 | 1.357 | <0.01 |
| Smoker | 1.866 | 1.821 | 1.933 | ||
Table 5:
Adjusted Geometric Means (AGM) for iron storage status by pregnancy status for blood cadmium (μg/l) and p values for the test between pregnant and non-pregnant females
| Iron Storage Status | Pregnancy Status | 95% Confidence Interval | P | ||
|---|---|---|---|---|---|
| AGM | Lower Limit | Upper Limit | |||
| Iron Absent | Pregnancy | 1.435 | 1.390 | 1.474 | <0.01 |
| Non-Pregnant | 1.501 | 1.407 | 1.620 | ||
| Iron Deficient | Pregnancy | 1.399 | 1.361 | 1.499 | <0.01 |
| Non-Pregnant | 1.416 | 1.405 | 1.476 | ||
| Iron Replete | Pregnancy | 1.390 | 1.361 | 1.520 | <0.01 |
| Non-Pregnant | 1.411 | 1.324 | 1.674 | ||
In addition, for pregnant females, Cd levels did not show markedly difference among the groups of Fe absent, Fe deficient, and Fe replete. However, non-pregnant females in Fe-absent group showed significantly higher Cd levels than non-pregnant females in Fe-deficient and Fe-replete.
Discussion
Iron intake is insufficient among the China general population, particularly in women, which is possibly because of the poor oral intake of major iron sources such as liver, soybeans, and spinach and/or because of menstrual blood loss. Blood Pb levels for those Fe was absent were significantly higher than those either deficient or replete (3.968 μg/dl vs. 3.804 μg/dl and 3.801 μg/dl, Table 3). The present findings of high blood lead concentrations in iron deficiency have a toxicological implication. The results indicate the importance of assessing the iron and hematologic status when addressing occupational or environmental exposure to lead and related toxic effects. Epidemiological observations should be collected on iron deficiency as an important susceptibility factor, considering the high prevalence of iron deficiency in the general population (23), especially in environmental lead exposure-related health risk assessment
In this study the levels of blood Pb and Cd were found to increase with age (Table 2), which might be indicative of continuous cumulative environmental exposure over time, which was similar as the previous report (24). A significant association was noted with BMI (Table 2), while urine Cd excretion rates were reported to have declined over 1988–2008(16). this reduction for blood Cd was not observed (Table 2). From 1988 to 2006 in the United States, Urinary Cd decreased form 0.58ug/g to 0.41 ug/g creatinine in males but in females only from 0.71 to 0.63 ug/g creatinine (25). However, blood Pb levels were observed to have significantly fallen over time during 2006–2011. An interesting interaction was noted between race/ethnicity and pregnancy status for both blood Pb and Cd. While unadjusted geometric means (UGM) were significantly lower of the pregnant group compared to non-pregnant group for both Pb and Cd levels (Table 1). However, the spread between pregnant and non-pregnant AGM was markedly less than that was for UGM. While the difference of UGM between pregnant and non-pregnant groups for Pb was 0.18 μg/dL (Table 1). Increased mobilization of Pb from maternal skeleton during pregnancy may indicate that Pb levels are higher for pregnant than non-pregnant females (26). The relative levels between pregnant and non-pregnant females were still in the same direction when interaction between pregnancy status and Fe storage status was considered (Table 5). However, as expected based on previous studies (14), Cd levels increased with decrease in ability of storage of Fe for both pregnant and non-pregnant females. For pregnant females, Cd levels rose from 0.25 μg/L to 0.30μg/L, corresponding with Fe was replete to absent (Table 4). In addition, the difference range (0.06 μg/L) between levels of pregnant versus non-pregnant females was largest when Fe was absent. The difference range at Fe replete was 0.001 μg/L. These results was confirmed when Fe was either deficient or replete (Table 4). Evidence suggested that body Fe as a better indicator of Fe deficiency (27) was used often rather than serum ferritin. However, the equation developed by Cook et al. (28) computed body Fe from serum ferritin and serum transferrin receptor, which was based on a small sample of 14 healthy subjects, none of whom was pregnant female.
In addition, since Fe storage is affected during pregnancy as noted in this study, it seems not appropriate to use body Fe measurements (28). However, with additional research and development of an equation that can also be used for pregnant females, body Fe might serve as a more reliable predictor of Fe deficiency than serum ferritin. The results presented in this study need to be viewed with caution because of the cross-sectional nature of the data provided by prenatal clinic. As previously report (29), in order to truly understand the effect of pregnancy, one needs to have longitudinal data starting from the pre pregnancy period through the entire pregnancy and possibly through the lactation period. In order to evaluate the contribution of pregnancy toward the levels of blood metals, it is almost essential to obtain the pre pregnancy values. In addition, while prenatal clinic does provide a representative sample of China general population by age, race/ethnicity, and gender, it does not necessarily provide a representative sample of pregnant females. Unfortunately, a longitudinal study of the size of China would be prohibitively expensive statuses.
Conclusion
Pregnant females showed lower levels of blood Pb and Cd, and the magnitude of decrease varied across different race/ethnicities and Fe storage status. Body mass index was negatively correlated with levels of Pb and Cd. In addition, reduction in smoking habits should be encouraged. To raise national health levels, the lead levels of pregnant women should be monitored and lead prevention should be prioritized in health care. Health care organizations and government bodies should carry out a series of measures to reduce lead exposure.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
We thank all the Pregnancy women who were taken part in the study and also the research staff involved. We thank Dr. Li Xia and Wenjun Cheng of Medical department of Nanjing/Maternity and Child Health Care Hospital and School of Public Health of An hui Medical University with great effort in revising the paper in English. The author(s) declare that they have no competing interests.
References
- 1.Counter SA, Buchanan LH, Ortega F. Neurocognitive screening of lead exposed Andean adolescents and youngadults. J Toxicol Environ Health A. 2009;72(10):625–632. doi: 10.1080/15287390902769410. [DOI] [PubMed] [Google Scholar]
- 2.ATSDR CERCLA Priority List of Hazardous Substances. Atlanta: Agency for Toxic Substances and Disease Registry; 2007. [Google Scholar]
- 3.Counter SA, Buchanan LH, Ortega F. Neurocognitive screening of lead exposed Andean adolescents and young adults. J Toxico Environ Health A. 2009;72(10):625–632. doi: 10.1080/15287390902769410. [DOI] [PubMed] [Google Scholar]
- 4.Matés JM, Segura JA, Alonso FJ, Márquez J. Role of dioxins and heavy metals in cancer and neurological diseases by ROS-mediated mechanisms. Free Radic Biol Med. 2010;49(9):1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Olsen L, Lind PM, Lind L. Gender differences for associations between circulating levels of metals and coronary risk in he elderly. Int JHyg Environ Health. 2012;215(3):411–417. doi: 10.1016/j.ijheh.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhu G, Shao C, Jin T, Tan M, Gu S, et al. Effects of cadmium on bone microstructure and erum tartrate-resistant acid phosphatase 5b in male rates. Exp Biol Med(Maywood) 2011;236(11):1298–1305. doi: 10.1258/ebm.2011.011104. [DOI] [PubMed] [Google Scholar]
- 7.Llanos MN, Ronco AM. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod Toxicol. 2009;27(1):88–92. doi: 10.1016/j.reprotox.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 8.Ronco AM, Urrutia M, Montenegro M, Llanos M. Cadmium exposure during pregnancy reduces birth weight and increases maternal and foetal glucocorticoids. Toxicol Lett. 2009;188(3):186–191. doi: 10.1016/j.toxlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Pillai P, Pandya C, Bhatt N, GuPta S. Biochemical and reproductive effects of gestational/lactational exposure to lead and cadmium with respect to testicular steroidogeneis, antioxidant system, endogenous sex steroid and cauda epididymal functions. Andrologia. 2012;44(2):92–101. doi: 10.1111/j.1439-0272.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 10.Scinicariello F, Abadin HG, Murray HE. Association of low-level blood lead and blood pressure in NHANES 1999–2006. Environ Res. 2011;111(8):1249–1257. doi: 10.1016/j.envres.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sale I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int J Hyg Environ Health. 2011;214(2):79–101. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S, Nieboer E, Sandanger TM, Wilsgaard T, Thomassen Y, Veyhe AS, et al. Changes in maternalblood concentrations of selected essential and toxic elements during and after pregnancy. J Environ Monit. 2011;13(8):2143–2152. doi: 10.1039/c1em10051c. [DOI] [PubMed] [Google Scholar]
- 13.Akesson A, Berglund M, Schutz A, Schütz A, Bjellerup P, Bremme K, et al. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92(2):284–287. doi: 10.2105/ajph.92.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BK, Kim Y. Iron deficiencyis associated with increased levels of blood cadmium in the Korean general population:analysis of 2008–2009 Korean National Health and Nutrition Survey Data. Environ Res. 2012;112:155–163. doi: 10.1016/j.envres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Estecha M, Trasobares E, Fuentes M, Martínez MJ, Cano S, Vergara N, et al. Blood lead and cadmium levels in a six hospital employee population PEAS study, 2009. J Trace ElemMed Biol Suppl. 2011;1:S22–S29. doi: 10.1016/j.jtemb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Muntner P, Guallar E, et al. Reduction in cadmium exposure in the United States population, 1988–2008: The contribution of declining smoking rates. Environ Health Perspect. 2012;120(2):204–209. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda M, Ohashi F, Fukui Y, Sakuragi S, Moriguchi J, et al. Closer correlation of cadmium in urine than that of cadmiumin blood with tubular dysfunction markers in urine among general women population in Japan. Int Arch Occup Environ Health. 2011;84(2):121–129. doi: 10.1007/s00420-010-0527-1. [DOI] [PubMed] [Google Scholar]
- 18.Ferraro PM, Stumiol oA, Naticchia A, D’Alonzo S, Gambaro G, et al. Temporal trend of cadmium exposure in the United States population suggests gender specificities. Intern Med J. 2012;42(6):691–697. doi: 10.1111/j.1445-5994.2011.02627.x. [DOI] [PubMed] [Google Scholar]
- 19.Vinceti M, Venturelli M, Sighinolfi C, Trerotoli P, Bonvicini F, Ferrari A, et al. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ. 2007;373(1):77–81. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170(9):1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among US adults. Environ Health Perspect. 2009;117(2):190–6. doi: 10.1289/ehp.11236. ealth 24(Supplemental 1):1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Benoist B, McLean E, Egli I, Cogswell M. Worldwide Prevalence of Anaemia. World Health Organization; Geneva: 2008. [DOI] [PubMed] [Google Scholar]
- 24.Huang M, Choi SJ, KiM DW, Kim NY, Bae HS, Yu SD, et al. Evaluation of factors associated with cadmium exposure and kidney function in the general population. Environ Toxicol. 2011 doi: 10.1002/tox.20750. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro PM, Stumiolo A, Naticchia A, D’Alonzo S, Gambaro G. Temporal trend of cadmium exposure in the United States population suggests gender specificities. Intern Med J. 2012;42(6):691–697. doi: 10.1111/j.1445-5994.2011.02627.x. [DOI] [PubMed] [Google Scholar]
- 26.Gulson BL, Mizon KJ, Plamer JM, Korsch MJ, Taylor AJ, Mahaffey KR. Blood lead changes during pregnancy and postpartum with calcium supplementation. Environ Health Perspect. 2004;112(15):1499–1507. doi: 10.1289/ehp.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher CM, Chen JJ, Kovach JS. The relationship between body iron stores and blood and urine cadmium concentration sin U.S. never-smoking, nonpregnant women aged 20–49 years. Environ Res. 2011;111(5):702–707. doi: 10.1016/j.envres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 29.Wang RY, Jain RB, Wolkin AF, Rubin CH, Needham LL, et al. Serum concentrations of selected persistent organic pollutantsin a sample of pregnant females and changes in their concentrations during gestation. Environ Health Perspect. 2009;117(8):1244–1249. doi: 10.1289/ehp.0800105. [DOI] [PMC free article] [PubMed] [Google Scholar]