Abstract
Lifestyle factors, such as weight and nutritional status may affect male fertility, including sperm fertilization ability. The objective of this retrospective study was to evaluate the association between body mass index (BMI) and sperm–zona pellucida binding ability assessed according to the zona binding (ZB) test, which has been described to be a relevant diagnostic tool for the prediction of in vitro fertilization (IVF) ability. Three hundred and six male patients from couples diagnosed with primary idiopathic or mild male factor infertility were included. Correlations between BMI and semen parameters according to ZB test indices were assessed, together with frequencies of positive and negative tests across the BMI categories. In this selected population, BMI was not related to conventional semen parameters or sperm quality assessed according to the ability of spermatozoa to bind to the zona pellucida. The previously described poor outcomes of IVF procedures in cases of male obesity could be due to other sperm defects, such as alterations of sperm capacitation or acrosome reaction. The link between male BMI and biological outcomes during IVF procedures, such as fertilization rates, should be further evaluated.
Keywords: body mass index (BMI), fertilization ability, obesity, semen quality, zona binding test
Introduction
Many lifestyle or environmental factors may have deleterious quantitative or qualitative effects on spermatozoa, leading to a negative influence on male fertility. Among those factors, a recent and growing interest has been placed on weight and nutritional status, particularly as overweight and obesity concern more than half of all men.
Although controversial, recent data seem to confirm an association between increased body mass index (BMI) and altered sperm parameters.1 However, conventional quantitative sperm parameter values are not sufficient to predict sperm fertilization ability, as unexplained fertilization failure can be observed with a completely normal semen analysis.2
Recently, data obtained from animal models suggest that male BMI could have an impact on sperm function and fertilization ability, including altered sperm binding to the zona pellucida (ZP),3 which is reversible in the presence of diet or exercise.4 In humans, only one publication has shown a significant negative correlation between BMI and hyaluronic acid (HA) binding scores in men from infertile couples.5 However, the population was not selected, and included all types and causes of infertility, and the HA binding test only provides an indirect assessment of the ability of spermatozoa to bind to the ZP.
The sperm–zona binding (ZB) test allows the assessment of the first steps of fertilization, recognition and binding of spermatozoa to the oocyte's ZP. The test compares the binding ability of spermatozoa from the studied population to those from a fertile control population.6 Data in the literature confirm that it is a relevant diagnostic tool for the prediction of in vitro fertilization (IVF) ability,7,8 especially in case of idiopathic or mild male factor infertility.2
The aim of this study was to evaluate whether BMI was related to sperm–ZP binding ability, as assessed by the ZB test.
Materials and methods
Patients
This retrospective observational study included 306 patients between January 2005 and April 2012. Men came from couples diagnosed with primary idiopathic or primary mild male factor infertility. They presented normal or subnormal semen parameters according to the 2010 World Health Organization (WHO) reference values.6 Men with cancer, vasectomy reversal or varicocele were excluded, as were men from couples presenting with a female cause of infertility. In our centre, a ZB test is routinely performed after the failure of three cycles of ovulation induction or intrauterine insemination in idiopathic or mild male factor indications.
Age, tobacco use (smoking/non smoking) and BMI were recorded at the time of the ZB test. Subjects were grouped according to the following WHO BMI categories: 18.5–24.9 kg m−2 (normal weight), 25.0–29.9 kg m−2 (overweight), ≥30.0 kg m−2 (obese).9 The reference group was defined by a BMI between 18.5 kg m−2 and 24.9 kg m−2.
Semen analysis
Semen samples were collected at the laboratory after 2–5 days of sexual abstinence. Semen volume, sperm concentration, sperm motility were analyzed according to the WHO guidelines,6 and percentage normal morphology was assessed according to David's modified criteria.10 The analyses were performed in one single laboratory, which is ISO9001 certified.
ZB test
Semen samples of proven fertile donors, known to have fertilized a pregnancy within 2 years were also collected and used as controls for the ZB test. Discontinuous PureSperm (Nidacon International, Gothenburg, Sweden) gradient centrifugal separation was performed on control and patient semen samples with two layers of density gradients (90% and 45%). Following two centrifugations (20 min at 300g and 10 min at 600g), the pellet was resuspended in Ferticult Hepes (FertiPro, Beernem, Belgium). Salt-stored human oocytes were used: these were either metaphase I oocytes or metaphase II oocytes that were unfertilized after intracytoplasmic sperm injection. The ZB test was performed as previously described.2 Briefly, four intact ZP were rinsed and incubated separately in 20 µl culture media droplets. Individual ZP were inseminated with a mixture of 4000 FITC-stained control and 4000 unstained test motile spermatozoa under classical IVF culture conditions (i.e. in equilibrated drops of appropriate culture media at 37 °C in a 6% CO2 atmosphere). After 18 h incubation, each ZP was washed to remove loosely bound sperm cells and mounted on a glass slide in a 5-µl 5% glycerol–PBS droplet. Each ZP was observed at ×400 magnification: first under a fluorescence microscope (Axiophot; Zeiss, Le Peck, France) to count the number of FITC-stained bound control spermatozoa, and second under a phase-contrast microscope (Optiphot-2; Nikon, Champigny sur Marne, France) to determine the number of total bound spermatozoa resulting from both controls and patients. A sperm–ZP binding index (the number of spermatozoa from patients bound per zona divided by the number from controls ×100) was calculated. As previously published,2 ZB was considered negative when the index was <70% or when grade ‘a' motility was <5%, and positive when the index was ≥70% and associated with a grade ‘a' motility ≥5%.
Statistical analysis
Sperm parameters, including the ZB index, are given as the median (25th and 75th percentiles) and comparisons among the BMI categories were analyzed using the Kruskall–Wallis test. ZB was considered a binary variable, and logistical regression was used to compare negative and positive test frequencies across the BMI categories. Adjustment for age and tobacco use was made. Statistical analyses were performed using the R statistical software version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
There were 159 men with normal BMI, 120 overweight and 27 obese (respectively 52%, 39% and 9%). In this specific population, conventional semen parameter values were similar across BMI categories. No significant difference between the groups was observed regarding to semen volume, sperm concentration, sperm motility and morphology (Table 1).
Table 1. Relationship between male BMI and semen quality.
| BMI categories | P | |||
|---|---|---|---|---|
| Normal (n=159) | Overweight (n=120) | Obese (n=27) | ||
| Age (year) | 35.3 (31.2; 38.5) | 35.9 (31.9; 39.3) | 35.4 (32.5; 38.6) | 0.58 |
| Semen volume (ml) | 2.9 (2.1; 4.2) | 3.0 (2.0; 4.3) | 2.7 (2.1; 4.0) | 0.81 |
| Sperm concentration (×106 ml−1) | 36.2 (19.9; 71.2) | 37.2 (21.9; 68.3) | 48.0 (22.0; 82.2) | 0.55 |
| Sperm progressive motility (a+b) (%) | 40 (30; 50) | 40 (30; 45) | 40 (35; 50) | 0.27 |
| Normal sperm morphology (%) | 19 (13; 26.5) | 19.5 (12.7; 27) | 19 (18; 27) | 0.51 |
| ZB index (%) | 87.5 (57.3; 176.7) | 88.4 (54.4; 154.5) | 103.7 (70.3; 200.7) | 0.45 |
Abbreviations: BMI, body mass index; ZB, zona binding.
Medians (25th and 75th percentiles) for age, semen parameters and ZB indices are presented according to BMI category.
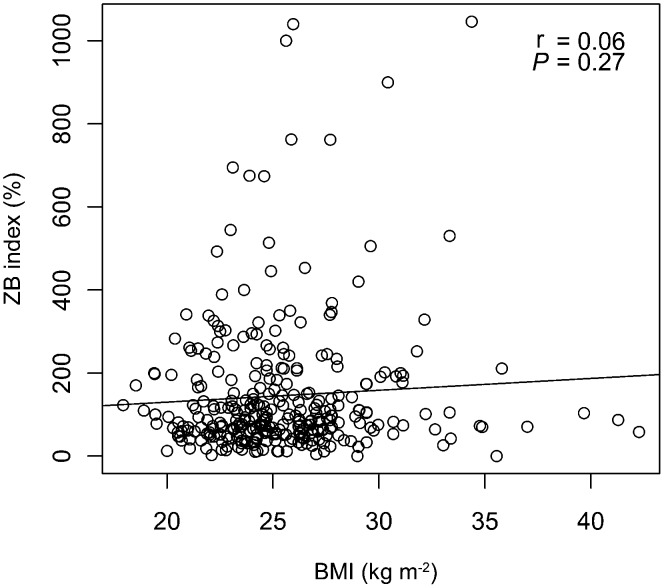
No statistically significant association was observed between BMI and the results of the ZB index (Table 1). When considered as continuous variables, there was no correlation between BMI and the ZB index (r=0.06; P=0.27) (Figure 1). Frequencies of positive and negative tests were similar across the BMI categories, without or with adjustment for age and tobacco use (Table 2).
Figure 1.
Correlation between male BMI and ZB index. BMI, body mass index; ZB, zona binding.
Table 2. Relationship between male BMI and the results of ZB tests.
| BMI categories | P | |||||
|---|---|---|---|---|---|---|
| Normal (n=159) | Overweight (n=120) | Obese (n=27) | Univariate model | Adjusted model* | ||
| ZB test | Negative | 38.4% | 38.3% | 25.9% | 0.43 | 0.43 |
| Positive | 61.6% | 61.7% | 74.1% | |||
Abbreviations: BMI, body mass index; ZB, zona binding.
*Adjusted for age and tobacco use. Frequencies of negative or positive ZB tests across the BMI categories are presented.
Discussion
In the present study, we found no association between BMI and conventional semen parameter values or the ability of sperm to bind to the ZP in a population of men from subfertile couples diagnosed with idiopathic or mild male factor infertility.
The link between BMI and sperm quality remains controversial. Defects in spermatozoa associated with abnormal BMI have been described,11,12 including decreases in sperm concentration or total sperm count, a decrease in sperm motility, and an increase in the percentage of morphological abnormalities. Others have failed to document this association13,14 and our results are consistent with these studies. However, conventional semen parameters are not sufficient to evaluate sperm function. In order to address the potential link between BMI and sperm fertilizing ability, we used the ZB assay, which is a proven indirect indicator of sperm defects6 and has previously been described as a relevant diagnostic tool for the prediction of IVF ability.7,8
To our knowledge, this is the first study to directly assess the ZB ability of spermatozoa in relation to BMI. We could not find any significant association between BMI and the results of the ZB test. Our findings are in contrast with previous results obtained from animal models,3 suggesting that male BMI could have an impact on sperm function and fertilization ability. In mice, a high-fat diet induces significant decreases of sperm capacitation and binding to the ZP of oocytes, resulting in significantly lower fertilization rates.3 This perturbed sperm function is reversible after dietary change or exercise, with a 1.4-fold increase in sperm binding.4 Moreover, we could not confirm the only available publication in men5 that studied the ability of spermatozoa from 107 non-selected infertile men to bind to HA. Indeed, Wegner et al.5 showed a negative correlation between BMI and the HA binding score, and significantly lower scores in overweight and obese men compared to those of normal weight men. However, motile sperm concentration and percentage normal morphology were also positively correlated to HA binding in this non-selected population, which includes all types and causes of infertility, so these altered sperm parameters could have confounded the interpretation. Furthermore, the HA binding test does not appear in the 2010 WHO laboratory manual (World Health Organization, 2010), and only provides an indirect assessment of the sperm–ZP binding ability. Finally, recent studies have reported the low relevance of the HA binding test, which fails to provide a prognostic threshold for fertilization rate during IVF procedures.15,16,17
A limitation of our study is that the group of obese men was relatively small. Moreover, our population consisted of men from subfertile couples diagnosed with idiopathic or mild male factor infertility, so our results cannot be extended to other infertility causes. Finally, BMI may not be the best indicator of adiposity, as suggested by the questions about its thresholds and its inability to distinguish body composition or fat distribution.18,19 On the other hand, our study has several strengths, including the use of the sperm–ZB assay in a population for which it has been described to be relevant.2 Fertilization failure during IVF procedures is known to be much more frequent in cases of idiopathic or mild male factor infertility than for female indications, such as tubal defects or endometriosis.2 Lack of sperm penetration into the oocyte has been shown to be the major cause of those fertilization failures.20 This suggests the existence of sperm defects that are not evaluated by conventional semen analysis, but that are detectable by the ZB test. Our results indicate that these defects may not be linked with obesity.
Finally, recent data suggest that increased male BMI can affect reproductive outcomes,21,22 including blastocyst development, clinical pregnancy and live birth rates during conventional IVF attempts,21 IVF and intracytoplasmic sperm injection.22 These poor reproductive outcomes could be due to alterations in sperm functions, such as sperm capacitation or acrosome reaction. This hypothesis is supported by results obtained from animal models.3,23 These show that sperm capacitation is decreased in diet-induced obese mice3 and that diet-induced hypercholesterolemia adversely affects capacitation and the acrosome reaction in rabbits, probably due to sperm membrane defects linked to an increase in intramembranous cholesterol.23
In conclusion, in our population, BMI was not associated with any deleterious conventional semen parameter values or sperm quality assessed by the ability of spermatozoa to bind to the ZP. The link between male BMI and biological outcomes during IVF procedures, such as fertilization rates, should be further evaluated.
Author contributions
NS, CD, MB, CS, RL participated in study conception and design. NS, CD, CF, ICD participated in acquisition of data. NS, CD, PCP, CS, RL participated in interpretation and analysis of data. MB performed statistical analyses. NS and CD drafted the manuscript. CF, MB, ICD, PCP, CS and RL participated in critical revision of the manuscript for important intellectual content. RL supervised the study. All authors read and approved the manuscript.
The authors declare that they have no competing financial interests.
References
- Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S, et al. Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med. 2012;172:440–2. doi: 10.1001/archinternmed.2011.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifer C, Sasportes T, Barraud V, Poncelet C, Rudant J, et al. World Health Organization grade ‘a' motility and zona-binding test accurately predict IVF outcome for mild male factor and unexplained infertilities. Hum Reprod. 2005;20:2769–75. doi: 10.1093/humrep/dei118. [DOI] [PubMed] [Google Scholar]
- Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–10. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab. 2012;302:E768–80. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- Wegner CC, Clifford AL, Jilbert PM, Henry MA, Gentry WL. Abnormally high body mass index and tobacco use are associated with poor sperm quality as revealed by reduced sperm binding to hyaluronan-coated slides. Fertil Steril. 2010;93:332–4. doi: 10.1016/j.fertnstert.2009.07.970. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Cambridge; Cambridge University Press; 2010. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 5th ed. [Google Scholar]
- Liu DY, Baker HW. High frequency of defective sperm–zona pellucida interaction in oligozoospermic infertile men. Hum Reprod. 2004;19:228–33. doi: 10.1093/humrep/deh067. [DOI] [PubMed] [Google Scholar]
- Oehninger S, Mahony M, Ozgur K, Kolm P, Kruger T, et al. Clinical significance of human sperm–zona pellucida binding. Fertil Steril. 1997;67:1121–7. doi: 10.1016/s0015-0282(97)81449-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization Obesity: preventing and managing the global epidemicIn: Roa W, editor. Technical report series no. 894. Geneva; World Health Organization; 2000 [PubMed] [Google Scholar]
- Auger J, Eustache F. Standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie. 2000;10:358–73. [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, et al. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–5. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Paasch U, Grunewald S, Kratzsch J, Glander HJ. Obesity and age affect male fertility potential. Fertil Steril. 2010;94:2898–901. doi: 10.1016/j.fertnstert.2010.06.047. [DOI] [PubMed] [Google Scholar]
- Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile. Fertil Steril. 2008;90:619–26. doi: 10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- Duits FH, van Wely M, van der Veen F, Gianotten J. Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. 2010;94:1356–9. doi: 10.1016/j.fertnstert.2009.05.075. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Kovats T, Sajgo A, Szollosi J, Matyas S, et al. The role of hyaluronic acid binding assay in choosing the fertilization method for patients undergoing IVF for unexplained infertility. J Assist Reprod Genet. 2010;28:49–54. doi: 10.1007/s10815-010-9479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs M, Creemers E, Cox A, Franssen K, Janssen M, et al. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod Biomed Online. 2009;19:671–84. doi: 10.1016/j.rbmo.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Tarozzi N, Nadalini M, Bizzaro D, Serrao L, Fava L, et al. Sperm-hyaluronan-binding assay: clinical value in conventional IVF under Italian law. Reprod Biomed Online. 2009;19 Suppl 3:35–43. doi: 10.1016/s1472-6483(10)60282-9. [DOI] [PubMed] [Google Scholar]
- Akpinar E, Bashan I, Bozdemir N, Saatci E. Which is the best anthropometric technique to identify obesity: body mass index, waist circumference or waist-hip ratio. Coll Antropol. 2007;31:387–93. [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–7. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- Miyara F, Aubriot FX, Glissant A, Nathan C, Douard S, et al. Multiparameter analysis of human oocytes at metaphase II stage after IVF failure in non-male infertility. Hum Reprod. 2003;18:1494–503. doi: 10.1093/humrep/deg272. [DOI] [PubMed] [Google Scholar]
- Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, et al. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27:539–44. doi: 10.1007/s10815-010-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95:1700–4. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, et al. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010;5:e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]