Abstract
The high incidence of erectile dysfunction (ED) in diabetes highlights a need for effective treatment strategies. Resveratrol, an activator of silent information regulator 2-related enzymes 1 (sirtuin1, SIRT1), has received attention for its valuable effects in cancer, neurodegenerative diseases, longevity and cardiovascular disease. To explore the effects of resveratrol in diabetes-induced ED, resveratrol was administered to rats with streptozocin (65 mg kg−1)-induced diabetes. Erectile function, cavernous structure, tissue protein expression of silent information regulator 2-related enzymes 1 (sirtuin1, SIRT1), p53 and forkhead transcription factor O 3a (FOXO3a), superoxide dismutase (SOD) activity and malondialdehyde (MDA) levels in the corpora cavernosa were studied. We found that SIRT1 was expressed in cavernosal tissue, and it was downregulated in the corpora of diabetic rats. The administration of resveratrol upregulated the expression of SIRT1 and restored erectile function. In contrast, resveratrol downregulated the expression of p53 and FOXO3a, which regulate apoptosis and oxidative stress. Furthermore, the resveratrol-treated group showed an improvement in smooth muscle content, SOD activity and MDA levels when compared with the diabetic group. Therefore, the ability of resveratrol to improve diabetes-induced ED is likely related to its activation of SIRT1 expression, thus causing the suppression of apoptosis and resistance towards oxidative stress.
Keywords: apoptosis, erectile dysfunction, oxidative stress, resveratrol
Introduction
Erectile dysfunction (ED) is defined as the inability to sufficiently achieve and/or maintain penile erection to permit satisfactory sexual intercourse, and it is a common complication of diabetes mellitus. The incidence of ED in male diabetic patients is estimated to be between 35% and 75%, which is three times higher than the incidence in non-diabetic men.1 Multiple pathogenic mechanisms underlie diabetes-induced ED (including neural, vascular, endocrine and metabolic factors).2 In particular, recent studies have focused more on the role of apoptosis and oxidative stress in diabetes-induced ED. Experimental hyperglycemia has been shown to induce many of the pathological consequences observed in diabetics. Much of the neuronal and endothelial damage observed has been attributed to the effects of oxidative stress3 because hyperglycemia induces the overproduction of superoxide, which serves as an initiating event in the activation of the pathways involved in the pathogenesis of tissue damage in diabetes mellitus. Corporal apoptosis resulting from oxidative stress in penile tissues was found to be a major cause of erectile impairment in diabetic animals. Many treatments are used in diabetes-induced ED, including oral medications. The first line of treatment and the most commonly used oral medications are phosphodiesterase type 5 inhibitors.4 However, the efficacy of phosphodiesterase type 5 inhibitors in diabetic men with ED is lower than that observed in the general population.5
Combination therapies, which target the multiple pathogenic factors, may manage this difficult condition. Resveratrol is a polyphenolic compound that is found in foods, including red grapes and red wine. Recently, resveratrol has been shown to possess numerous important bioactivities including anti-apoptotic, antioxidant and anti-aggregation functions, and the ability to modulate lipoprotein metabolism.6 It has also shown chemopreventative properties in certain forms of cancer and cardiovascular disorders and to increase longevity.7,8,9 Moreover, resveratrol appears to protect against diabetes10 by inducing silent information regulator 2-related enzymes 1 (sirtuin1, SIRT1).11
In this study, we investigated the hypothesis that resveratrol improved erectile function in diabetes-induced ED by suppressing apoptosis and preventing oxidative stress in the corpus cavernosal and by upregulating SIRT1 in a streptozotocin (STZ)-induced diabetic rat model.
Materials and methods
Animals
All procedures in this study were approved by the institutional animal care committee. Sixty 12-week-old male Sprague–Dawley rats weighing 250–300 g were obtained from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). Forty-eight rats were administered an intraperitoneal injection of freshly prepared STZ (65 mg kg−1). Twelve rats were administered vehicle only (0.1 mol l−1 citrate/phosphate buffer, pH 4.5) and were used as a control group (n=12). All rats were housed under standard conditions at 25 °C with a 12-/12-h light–dark cycle and were given free access to food and water.
Blood glucose was measured 72 h after injection. In the 48 STZ-injected rats, those with serum glucose levels >300 mg dl−1 (16.6 mmol l−1) were included in this study (34 rats in total). These rats were divided randomly into a diabetic control group that were administered daily with intragastric normal saline (DED, n=17) and an experimental group that was administered with intragastric resveratrol (5 mg kg−1 day−1; Sigma-Aldrich, St Louis, MO, USA; Res, n=17). Blood glucose levels and body weight were monitored regularly throughout the study and prior to euthanasia. All rats were maintained for 8 weeks, except for two rats in the diabetic control group and one rat in resveratrol-treated group that died prior to the end of the study.
Erectile function evaluation and tissue procurement
Measurements of the intracavernous pressure (ICP) and the ratio of ICP/mean systemic arterial pressure (MAP) were used to assess erectile function. The ICP measurement, ICP/MAP calculation, and the method of cavernosal nerve stimulation have been described previously.12 Following the induction of anesthesia with ketamine (30 mg kg−1) and midazolam (5 mg kg−1), the major pelvic ganglion and cavernous nerve on either side of the prostate were exposed. The pressure was measured and recorded using a Windows computer program-controlled multiplying channel physiograph and analyzed using a RM6240B/C multichannel bio-signal collection processing system (Chengdu Implement Company, Chengdu, China). The nerve was stimulated at a frequency of 15 Hz and using a pulse width of 5 ms. Stimulations were performed at 5 V for 60 s with resting periods of 5 min between subsequent stimulations. After assessing ICP, rats were euthanized using an overdose of ketamine. The penises were removed and cleaned. Part of the proximal penis was fixed in 4% paraformaldehyde, and the remainder was stored in liquid nitrogen for further processing.
Masson's trichrome stain
Masson's trichome stain was used to evaluate the ratio between smooth muscle and collagen in the cavernosum. Tissues was fixed in formaldehyde overnight, and then stored in 70% alcohol at 4 °C until processed for paraffin-embedded tissue sectioning. Tissue sections were deparaffinized and hydrated. Nuclei were stained using Weigert's iron hematoxylin for 10 min. After washing with tap water, the samples were stained using 1% ponceau acetic acid solution for 5 min. They were then rinsed rapidly in water and differentiated in 1% phosphomolybdic acid for approximately 5 min. Finally, the slides were counterstained using fast green. The nuclei stained blue, the smooth muscle stained red, and the collagen stained green. The areas of smooth muscle and collagen were analyzed using Image Pro Plus 5.0 software (Media Cybernetics, Inc., Bethesda, MD, USA).
Immunohistochemistry for a-smooth muscle antigen
For immunohistochemistry, tissue was fixed in 4% paraformaldehyde overnight. Following deparaffinization and rehydration, sections (5 µm) were rinsed for 6 min using phosphate-buffered solution. Endogenous peroxidase activity was quenched using 0.3% H2O2 for 10 min. After 6 min of washing with phosphate-buffered solution, the tissue was blocked using 3% BSA for 30 min and then incubated with anti-a-smooth muscle antigen (Abcam Inc., Hong Kong, China; 1∶400) at 4 °C overnight. Sections were then incubated with biotinylated anti-mouse secondary antibodies (Boster, Wuhan, China; 1∶200) for 2 h at room temperature and then counterstained with hematoxylin. Sections incubated without primary antibodies were used as negative controls. Images were captured using a Nikon microscope with a Spot RT color digital camera, and digital histomorphometric analysis was performed using Image-Pro Plus 5.0 software (Media Cybernetics, Silver Spring, MD, USA).
Western blotting assay for SIRT1, FOXO3a and p53
Western blotting was used to determinate the protein expression of SIRT1, forkhead transcription factor O 3a (FOXO3a) and p53 in the cavernosum. The penile tissue that had been stored in liquid nitrogen was powdered and lysed in radioimmunoprecipitation assay buffer (phosphate-buffered solution, 1% NP-40, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and protease inhibitors). The samples were then homogenized on ice for 10 min and centrifuged at 12 000 g for 15 min at 4 °C. The supernatants were collected and stored at −80 °C. Equal amounts of proteins were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gels and then transferred to a nitrocellulose membrane. The membrane was blocked in Blotto-Tween (10 mmol l−1 Tris-HCl (pH 8.0), 150 mmol l−1 NaCl, 5% nonfat dry milk and 0.05% Tween-20) overnight at 4 °C. The membrane was then incubated with antibodies targeted against SIRT1 (Abcam Inc., Hong Kong, China; 1∶500), FOXO3a (Abcam Inc.; 1∶500), p53 (Abcam Inc.; 1∶500) or GAPDH (Abcam Inc.; 1∶1000) at room temperature for 4 h and then for 1 h at room temperature with anti-mouse (Chemicon, Temecula, CA, USA; 1∶1000) or anti-rabbit (Calbiochem, San Diego, CA, USA; 1∶2000) secondary antibodies. Detection was performed using enhanced chemiluminescence (Boster, Wuhan, China) followed by autoradiography. The densitometric results were quantified using Image Pro Plus 5.0.
Apoptosis assessment
To assess apoptosis, the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling assay was performed following the manufacturer's instructions (Roche Applied Science, Mannheim, Germany). Two slides from two different animals per group were selected randomly. Each slide was analyzed by counting cells in five non-overlapping zones of the entire section at ×400 magnification. The number of terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling-stained cells was expressed as a percent of the total number of cells and reported as the apoptotic index (AI).
Biochemical markers of oxidative stress
The superoxide dismutase (SOD) activity and malondialdehyde (MDA) levels were used to evaluate oxidative stress. The SOD and MDA levels in the corpus cavernosum was measured as described previously.13 All assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China).
Statistical analysis
The results were expressed as the mean±standard error of the mean. Statistical analysis was performed using one-way analysis of variance for multiple comparisons followed by post hoc comparisons using the least significant difference test. A P value <0.05 was considered statistically significant.
Results
General data
The body weights and blood glucose levels are shown in Table 1. The body weights of diabetic rats were significantly lower compared with controls after 8 weeks (P<0.01). The blood glucose in diabetic rats was significantly higher compared with controls (P<0.01). Resveratrol did not improve these changes.
Effect of resveratrol on erectile function in diabetic rats
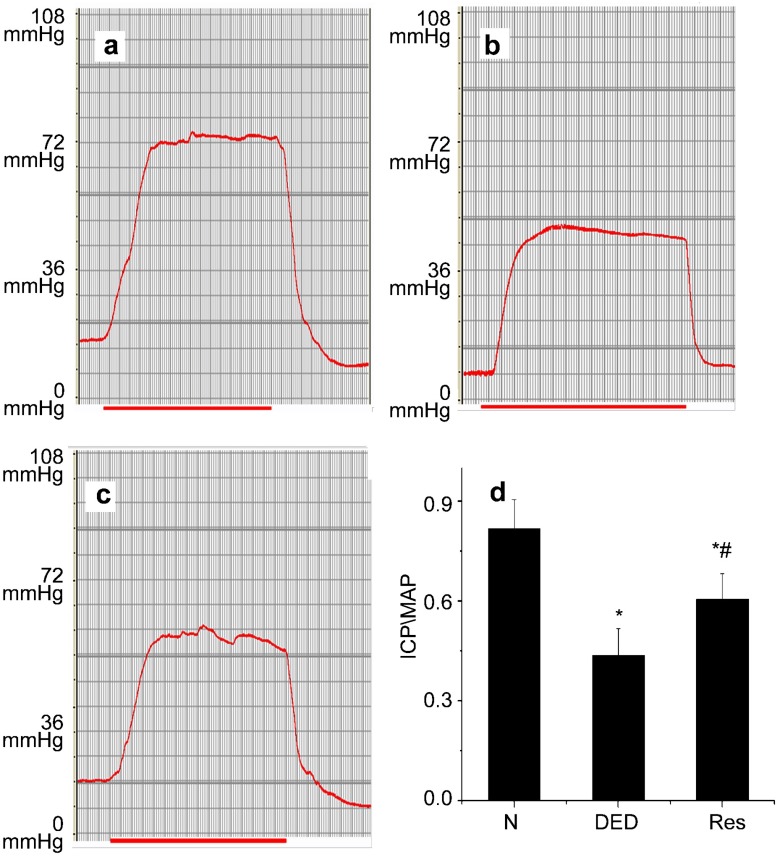
The ICP/MAP ratio is shown in Figure 1. When compared with normal rats, the mean ICP/MAP ratio in untreated diabetic rats decreased significantly (P<0.01). Following the administration of resveratrol for 8 weeks, the mean ICP/MAP ratio in diabetic rats increased (P<0.05).
Figure 1.
Erectile function evaluation. Intracavernous pressure (ICP) and peak intracavernous pressure/mean systemi arterial pressure (ICP/MAP). (a) Normal control group. (b) Diabetic group. (c) Resveratrol-treated group. (d) Statistical chart of ICP/MAP ratio. Peak ICP/MAP values decreased significantly in diabetic rats. Resveratrol could partially restore the reduction, but could not bring erectile function to normal level. *P<0.01 vs. control group; #P<0.05 vs. diabetic group. N, normal control group; DED, diabetic group; Res, Resveratrol-treated group.
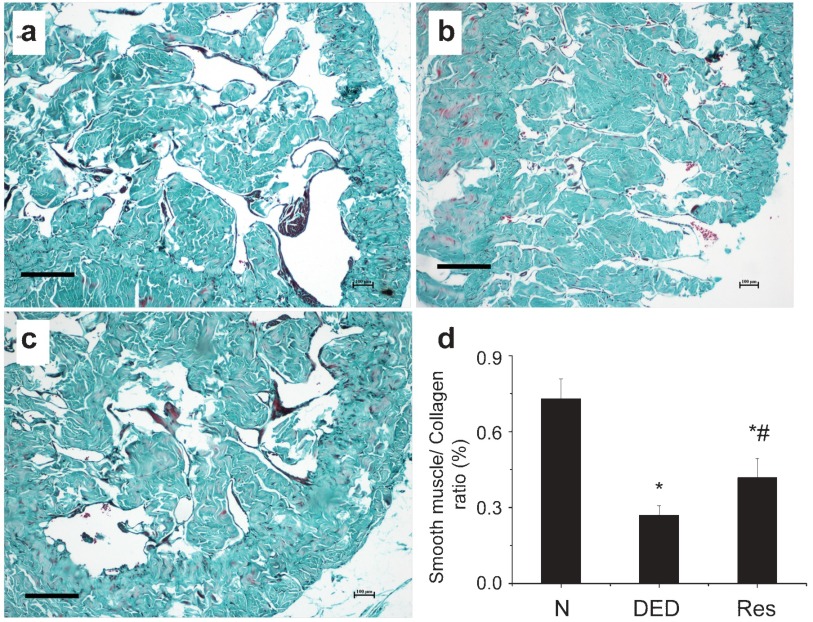
The effect of resveratrol on the smooth muscle/collagen ratio in the cavernosum
The ratio between smooth muscle and collagen was significantly reduced in the cavernosum corpus of diabetic rats (Figure 2b) compared with the controls (Figure 2a; P<0.01). Resveratrol improved this cavernous structural disorder in diabetic rats (P<0.05).
Figure 2.
Masson's trichome staining of cavernosum tissue. The smooth muscle/collagen ratio decreased significantly in diabetic group compared with normal control. Resveratrol contributed to the restoration of cavernous structure impairment in diabetes. (a) Normal control group. (b) Diabetic group. (c) Resveratrol-treated group. (d) Statistical chart of smooth muscle/collagen ratio; *P<0.01 vs. normal control group, #P<0.05 vs. diabetic group. N, normal control group; DED, diabetic group; Res, resveratrol-treated group. Scale bars=200 μm.
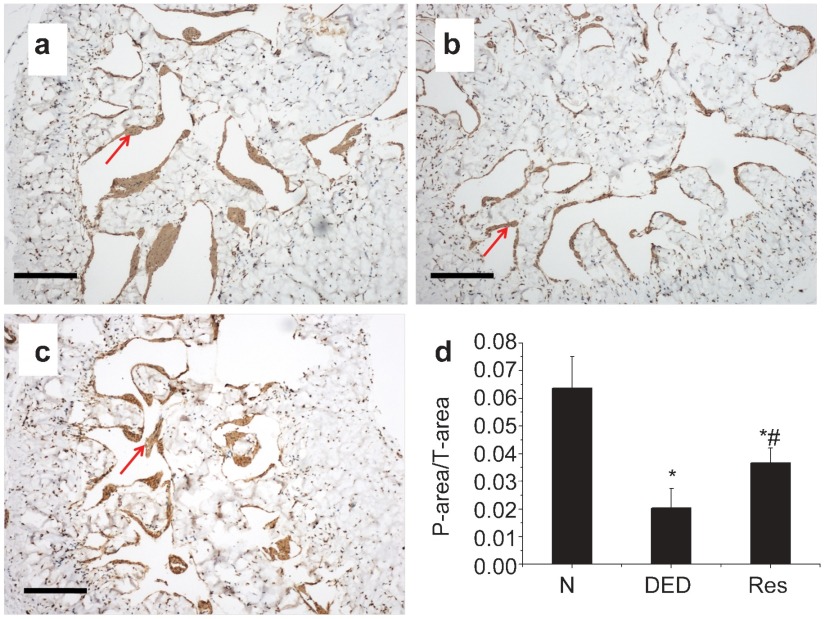
Effect of resveratrol on the smooth muscle content of the cavernosum
As shown in Figure 3, the smooth muscle content (as indicated by a-smooth muscle antigen) decreased significantly in diabetic rats (Figure 3b) compared with controls (Figure 3a; P<0.05). Eight weeks of resveratrol administration (Figure 3c) increased smooth muscle content significantly compared with diabetic rats (P<0.05).
Figure 3.
Immunohistochemical staining of a-SMA. Positive staining was noted in penile vascular smooth muscle (red arrow). (a) Normal control group. (b) Diabetic group. (c) Resveratrol-treated group. (d) Statistical chart of a-SMA density among groups; *P<0.01 vs. normal control group; #P<0.05 vs. diabetic group. N, normal control group; DED, diabetic group; Res, resveratrol-treated group. Scale bars=100 μm.
Protein levels of SIRT1, FOXO3a and p53 in cavernous tissue
As demonstrated in Figure 4, the expression of SIRT1 protein decreased in the diabetic controls when compared with normal controls, but the expression of FOXO3a and p53 (P<0.01) increased. After 8 weeks of resveratrol treatment, FOXO3a and p53 proteins decreased (P<0.05). In contrast, the expression of SIRT1 increased in the resveratrol-treated diabetic group when compared with the diabetic rats that were not treated (P<0.05).
Figure 4.
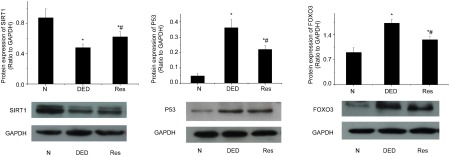
The protein level of SIRT1, FOXO3a, P53 in penile tissues. SIRT1 protein decreased in diabetic group and increased in resveratrol-treated group. On the other hand, the FOXO3a and P53 protein increased in the diabetic controls compared with the normal control, and decreased by the resveratrol treatment. *P<0.01 vs. control group; #P<0.05 vs. diabetic group. N, normal control group; DED, diabetic group; Res, resveratrol-treated group; GAPDH, glyceraldehyde 3-dehydrogenase.
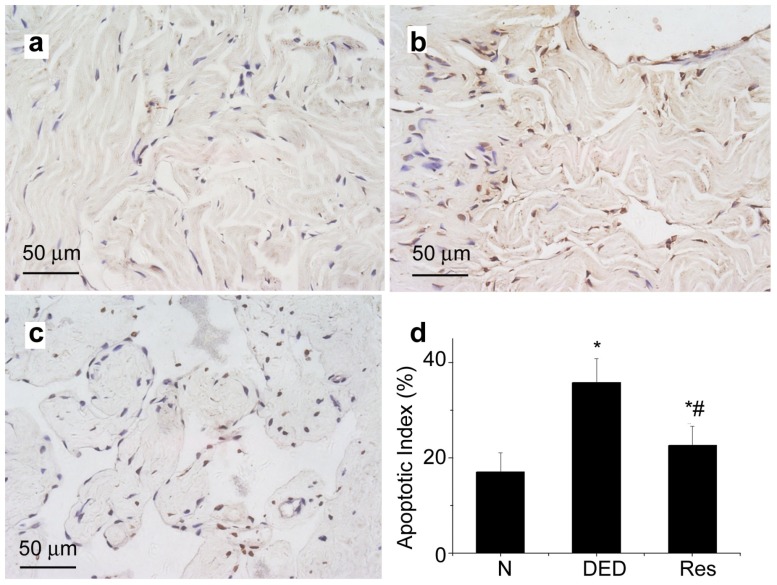
Apoptosis analysis
In Figure 5, we show that the diabetic group treated with resveratrol demonstrated a significant reduction in apoptosis within the corporal tissue (Figure 5c) with a mean AI of 22.61% compared with an AI of 35.81% in the diabetic group (P<0.05; Figures 5b and d). In comparison, the AI value in the normal group was 17.04% (Figures 5a and d), which was lower than the resveratrol group (P<0.05).
Figure 5.
TUNEL assessment of apoptosis. Reveratrol therapy decreased apoptosis. Compared with the normal control group (a), diabetic group (b) increased apoptosis in penile (brown-stained nuclei; magnified×400); Resveratrol treatment (c) reduced apoptosis in penile compared with diabetic group (b). Apoptotic indices (AI) is presented as ratio of apoptotic nuclei(brown-stained nuclei) to total number of nuclei counted. A reduced AI percentage for resveratrol therapy was found compared to diabetic group (P<0.05), although still higher than the normal group (P<0.05) (d). *P<0.05 vs. control group; #P<0.05 vs. diabetic group. N, normal control group; DED, diabetic group; Res, resveratrol-treated group; GAPDH, glyceraldehyde 3-dehydrogenase. Scale bars=50 μm.
MDA and SOD activity in the cavernosum
The MDA levels and SOD activity in the corpus cavernosum are shown in Table 2. Decreased SOD activity and increased MDA levels were found in the corpus cavernosum of diabetic rats compared with controls (P<0.05). Following treatment with resveratrol, SOD activity increased, and MDA levels decreased (P<0.05).
Discussion
This study shows that treatment with resveratrol restored ED in the STZ-induced diabetic rat. Resveratrol can improve the structural impairment in the cavernosum, activate SIRT1 and suppress apoptosis and oxidative stress by downregulating FOXO3a and p53 expression.
Recent studies have demonstrated that resveratrol (trans-3,5,4′-trihydroxy stilbene), which is found in red grapes and blue berries, lowers blood sugar, improves lipid profile14 and prevents developmental delays in embryos of diabetic rats.15 Several clinical trials have been conducted to study the metabolic effects of resveratrol in diabetics. Although these trials have used different subject groups (e.g., type 2 diabetics or older adults with glucose intolerance) and different resveratrol doses, they have indicated that resveratrol may improve insulin sensitivity.16,17,18 Moreover, other studies have shown that resveratrol can treat organic injuries to tissues including the retina and liver by resisting oxidative stress.19,20
To investigate the effects of resveratrol in diabetes-induced ED, we used a model of STZ-induced diabetes in rats. ICP/MAP was used to assess erectile function, which decreased significantly in diabetic rats. Resveratrol restored ICP and increased the ratio of ICP/MAP.
These results are consistent with Fukuhara's research. In their study, they demonstrated that resveratrol led to SIRT1 activation in SMCs and subsequently activated eNOS, which led to enhanced cyclic guanosine monophosphate synthesis via the nitric oxide/cyclic guanosine monophosphate pathway.21 SIRT1, a mammalian ortholog of the yeast silent information regulator 2, has increasingly been referenced as a longevity protein or an ageing regulator because increasing evidence has indicated that the silent information regulator 2 family of proteins mediates lifespan extension in yeast, worms, flies, and mammals. In mammals, SIRT1 is a member of a small gene family of seven members, designated Sirtuin 1 through 7. Among them, SIRT1 is by far the best characterized. In vitro, it has been demonstrated that resveratrol increases SIRT1 mRNA levels and protein levels. In addition, several research groups have shown that resveratrol activates SIRT1 deacetylase activity.22,23,24 SIRT1 has been shown to deacetylate both histones and a wide range of non-histone proteins; thus it is involved in a wide range of functions including endothelial homeostasis,25,26 apoptosis,27,28 cell-cycle regulation, transcriptional silencing and oxidative stress.29,30,31 Several pieces of evidence suggest that SIRT1 acts as a longevity factor in vascular tissue; in particular, its critical role in endothelial homeostasis by regulating endothelial nitric oxide synthase (eNOS) activity. In calorie-restricted rats, it has been reported that SIRT1 promotes endothelial-dependent vasodilation by targeting eNOS for deacetylation, thereby leading to enhanced nitric oxide production in the aorta.32
Although Fukuhara's research demonstrated that resveratrol improved erectile function in STZ-induced diabetic rats by elevating the level of cyclic guanosine monophosphate, other pathophysiological factors underlying diabetes-induced ED, such as apoptosis and oxidative stress, were not reported. In this study, we demonstrated that resveratrol improved diabetes-induced ED by preventing oxidative stress and suppressing apoptosis.
As a supplement to Fukuhara's research, we demonstrated that the expression of SIRT1 was decreased significantly in the corpus cavernosum of diabetic rats and that resveratrol upregulated SIRT1 expression in the cavernosum. In addition, we observed that the expression of p53 and FOXO3a, which are the most important SIRT1 substrates, increased in the corpus cavernosum of diabetic rats, whereas they decreased after the administration of resveratrol. P53 is a transcription factor that targets many genes and micro RNAs in response to cellar stress. The major functions of p53 are the regulation of growth arrest and apoptosis.33 SIRT1 binds to p53 tightly both in vitro and in vivo and specifically deacetylates p53, thereby impairing its DNA binding activity.28,34 Undoubtedly, the negative regulation of DNA binding reduces p53-mediated apoptosis in cultured cells in response to DNA damage and oxidative stress. Moreover, SIRT1 attenuates p53-dependent apoptosis specifically because it does not affect p53-independent Fas-mediated apoptosis.35
In addition to p53, four members of the FOXO subgroup (FOXO1/FKHR, FOXO3/FKHRL1, FOXO4/AFX and FOXO6) have been identified in mammals, and their participation in the regulation of apoptosis,36,37 cell-cycle arrest, DNA repair and oxidative stress resistance38 has been demonstrated. SIRT1 may deacetylate p300 and repress p300-mediated activation of FOXO3.39 It has been reported that SIRT1 represses FOXO3-dependent transcription from various promoters in HeLa cells, and an increased level of SIRT1 represses FOXO3-dependent apoptosis in non-neuronal cells.40 Thus, SIRT1 can attenuate FOXO-induced apoptosis under stress conditions.
As shown in previous studies,41,42 both apoptosis and oxidative stress induce smooth muscle cell and endothelial cell damage in the corpus cavernosum. In this study, administration of resveratrol significantly increased the amount of smooth muscle and the smooth muscle/collagen ratio and suppressed apoptosis. Furthermore, to investigate oxidative stress in the corpus cavernosum, we measured SOD activity and MDA levels. SOD represents a major cellular defense against superoxide. MDA is a reactive aldehyde and one of the many reactive electrophile species that can cause toxic stress in cells. The results showed that the administration of resveratrol inhibited oxidative stress in diabetes-induced ED.
The animal model used in this study modeled type 1 diabetes. This model has been used in numerous studies and is known to mimic the clinical characteristics of type 1 diabetes more accurately than those of type 2 diabetes. Thus, one limitation of this study is that we used STZ-induced diabetic rats. Another limitation is that no positive control, i.e., medicine that has been used for ED patients, such as phosphodiesterase type 5 inhibitors, was used. Further studies will combine resveratrol with other medicines.
Conclusion
Resveratrol restored the impaired erectile function in the cavernous tissue of STZ-induced diabetic rats. Diabetes-induced ED is a disease that involves multiple pathogenic pathways, including endothelial dysfunction, apoptosis and oxidative stress.43 Resveratrol not only improved endothelial function by activating eNOS,21 but also suppressed apoptosis and prevented oxidative stress in the corpus cavernosum. Calorie restriction is another effective method of upregulating SIRT1,44,45 and it is also the most common treatment for diabetic patients.46 Thus, resveratrol should be considered in combination with calorie restriction or other treatments to obtain better outcomes for diabetes-induced ED.
Author contributions
WY and ZW performed the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. XFQ conducted the immunoassays. WY and YC participated in the design of the study and performed the statistical analyses. YTD conceived of the study and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Table 1. Body weight and blood glucose(mean±s.d.).
| Control group | Diabetic group | Resveratrol-treated group | |
|---|---|---|---|
| Intial | |||
| BW(g) | 250.86±30.43 | 253.42±34.28 | 269.08±30.97 |
| BG (mg dl−1) | 75.9±11.9 | 486.1±50.6 | 528.2±38.5 |
| After 8 weeks | |||
| BW(g) | 481±31.0 | 203.40±18.07* | 216.73±37.25* |
| BG(mg dl−1) | 69.9±7.7 | 477.5±49.3* | 505.4±73.6* |
P<0.01 compared with control group. BW, body weight, BG, blood glucose.
Table 2. Malondialdehyde (MDA) and superoxide dismutase (SOD) activity in corpus cavernosum (mean±s.d.).
| Control group | Diabetic group | Resveratrol-treated group | |
|---|---|---|---|
| SOD | 127.45±19.7 | 69.3±7.62* | 102.44±8.79# |
| MDA | 2.69±0.44 | 5.45±0.49* | 4.06±0.56** |
P<0.01 vs. control group;
P<0.05 vs. diabetic group;
P<0.05 vs. diabetic group.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81170563).
The authors declared that there are no competing financial interests.
References
- Richardson D, Vinik A. Etiology and treatment of erectile failure in diabetes mellitus. Curr Diab Rep. 2002;2:501–9. doi: 10.1007/s11892-002-0120-4. [DOI] [PubMed] [Google Scholar]
- Moore CR, Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8:675–84. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Jones RA, Koupparis AJ, Hotston M, Persad R, et al. Reactive oxygen species and erectile dysfunction: possible role of NADPH oxidase. Int J Impot Res. 2007;19:265–80. doi: 10.1038/sj.ijir.3901523. [DOI] [PubMed] [Google Scholar]
- Safarinejad MR. Oral sildenafil in the treatment of erectile dysfunction in diabetic men: a randomized double-blind and placebo-controlled study. J Diabetes Complications. 2004;18:205–10. doi: 10.1016/S1056-8727(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Vickers MA, Satyanarayana R. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus. Int J Impot Res. 2002;14:466–71. doi: 10.1038/sj.ijir.3900910. [DOI] [PubMed] [Google Scholar]
- Frei B. Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J Nutr. 2004;134:3196S–8S. doi: 10.1093/jn/134.11.3196S. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–61. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- Shankar S, Nall D, Tang SN, Meeker D, Passarini J, et al. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial–mesenchymal transition. PLoS ONE. 2011;6:e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timperio AM, D'Alessandro A, Fagioni M, Magro P, Zolla L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol Biochem. 2011;50:65–71. doi: 10.1016/j.plaphy.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278–83. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Qiu X, Lin H, Wang Y, Yu W, Chen Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li XX, Lin HC, Qiu XF, Gao J, et al. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J Androl. 2012;14:616–20. doi: 10.1038/aja.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do GM, Jung UJ, Park HJ, Kwon EY, Jeon SM, et al. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282–91. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- Singh CK, Kumar A, Hitchcock DB, Fan D, Goodwin R, et al. Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res. 2011;55:1186–96. doi: 10.1002/mnfr.201000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–9. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–12. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK.Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinologye-pub ahead of print 9 March 2013. Doi: 10.1159/000350435. [DOI] [PubMed]
- Soufi FG, Mohammad-Nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress–nuclear factor kappaB–apoptosis pathway. Pharmacol Rep. 2011;64:1505–14. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Tsujimura A, Okuda H, Yamamoto K, Takao T, et al. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat: preliminary findings. J Sex Med. 2011;8:1061–71. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–81. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Song MY, Song EK, Kim EK, Moon WS, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–51. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–9. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–58. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–96. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, et al. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Xu J, Qu W, Peng X, Xin P, et al. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J Nutr Biochem. 2012;23:1410–6. doi: 10.1016/j.jnutbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Kairisalo M, Bonomo A, Hyrskyluoto A, Mudo G, Belluardo N, et al. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci Lett. 2011;488:263–6. doi: 10.1016/j.neulet.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Akar F, Pektas MB, Tufan C, Soylemez S, Sepici A, et al. Resveratrol shows vasoprotective effect reducing oxidative stress without affecting metabolic disturbances in insulin-dependent diabetes of rabbits. Cardiovasc Drugs Ther. 2011;25:119–31. doi: 10.1007/s10557-010-6255-7. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–48. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–81. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–65. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, et al. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–82. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Tarhan F, Demirel GY, Kuyumcuoglu U, Faydaci G, Eryildirim B. Apoptosis of corpus cavernosum in patients with organic erectile dysfunction. World J Urol. 2009;27:235–40. doi: 10.1007/s00345-008-0332-6. [DOI] [PubMed] [Google Scholar]
- Li XX, Qiu XF, Yu W, Zhu WD, Chen Y, et al. Mechanisms of oxidative stress-induced damage and its protection in cavernous mitochondria of diabetic rats. Beijing Da Xue Xue Bao 43189–93.Chinese. [PubMed] [Google Scholar]
- Trent JT, Falabella A, Eaglstein WH, Kirsner RS. Venous ulcers: pathophysiology and treatment options. Ostomy Wound Manage. 2005;51:38–54; quiz 5–6. [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–67. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaviani R, Soewondo P, Semiardji G, Sudoyo AW. The influence of calorie restriction during the Ramadan fast on serum fructosamine and the formation of beta hydroxybutirate in type 2 diabetes mellitus patients. Acta Med Indones. 2004;36:136–41. [PubMed] [Google Scholar]