Abstract
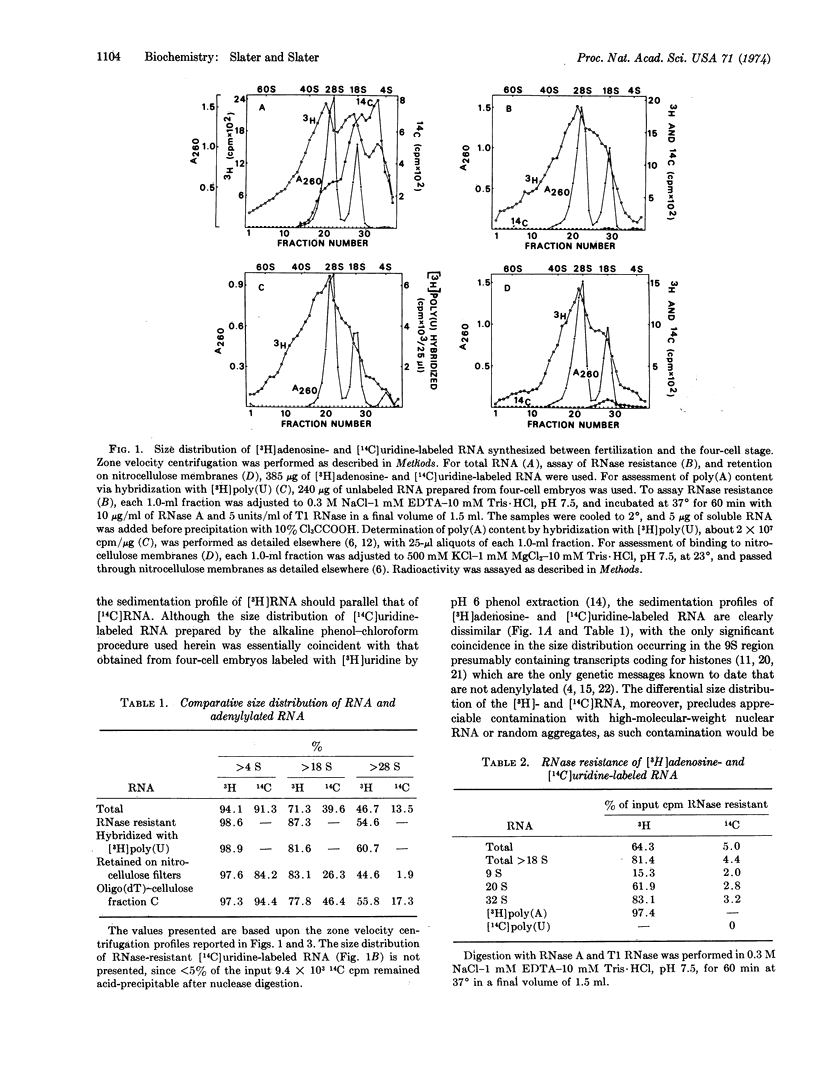
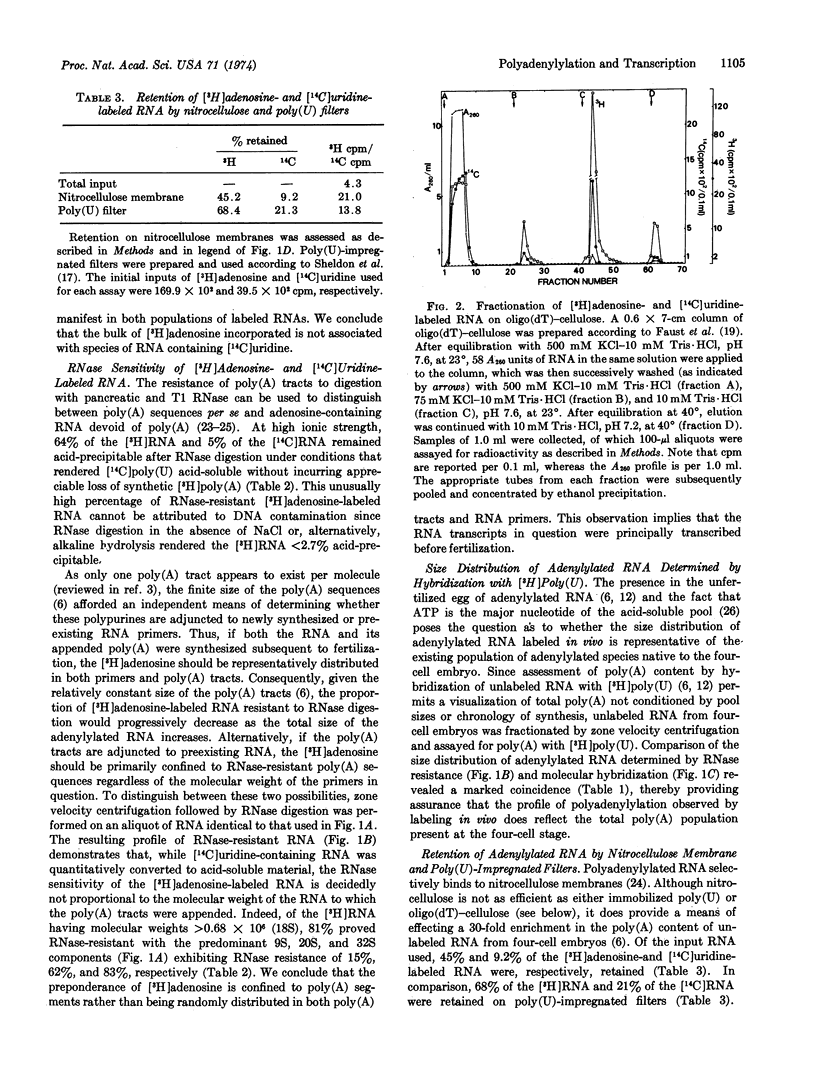
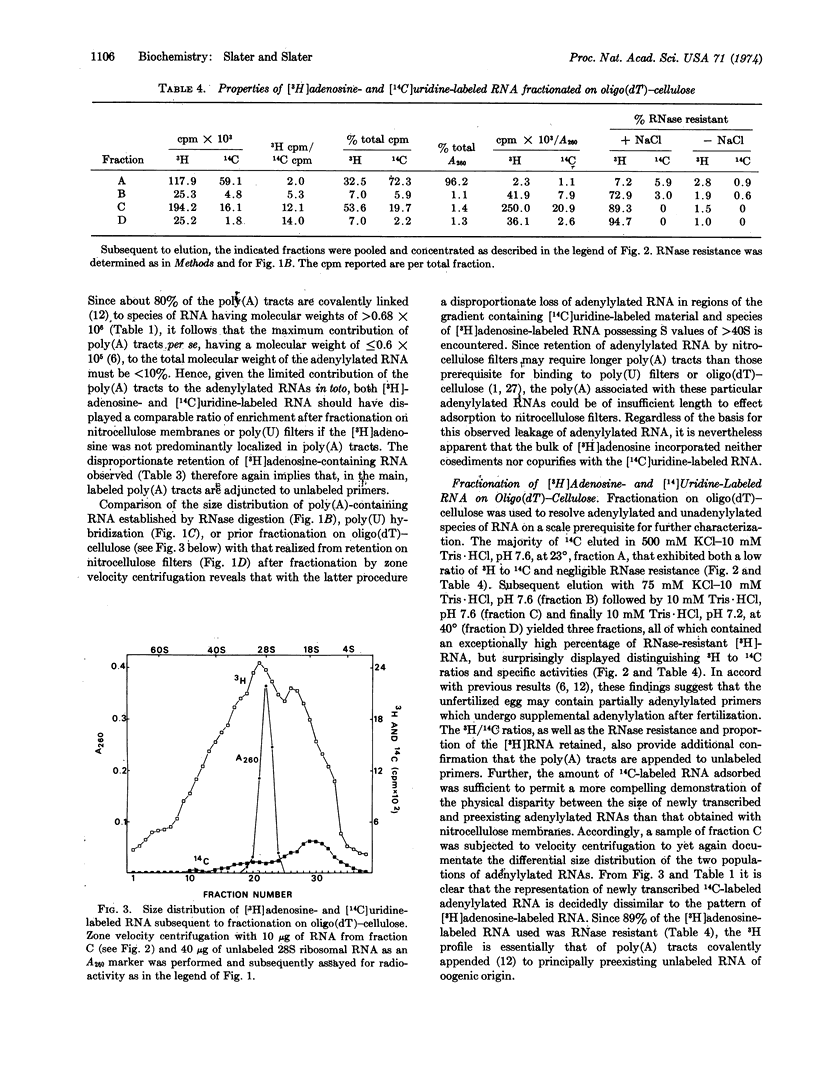
Polyadenylylated RNA from sea urchin embryos concomitantly labeled with [3H]adenosine and [14C]uridine between fertilization and the four-cell stage was used to determine whether the RNA primers prerequisite to the massive polyadenylylation known to occur after fertilization are synthesized during oogenesis or subsequent to fertilization. Characterization of this RNA and unlabeled RNA via retention on nitrocellulose membranes and poly(U)-impregnated filters, molecular hybridization with [3H]poly(U), RNase resistance, oligo(dT)-cellulose chromatography, and size-distribution studies indicates that the poly(A) tracts synthesized after fertilization are predominantly appended to preexisting cytoplasmic primers of oogenic origin. Hence, if polyadenylylation is involved in the selective editing of presumptive genetic messages, this process is not confined to the nucleus unless a given codogenic transcript can undergo more than one cycle of adenylylation.
Keywords: sea urchin embryos, cytoplasmic adenylylation, maternal and embryonic primers
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Avadhani N. G., Battula N., Rutman R. J. Messenger ribonucleic acid metabolism in mammalian mitochondria. Origin of ethidium bromide resistant poly(adenylic acid) containing ribonucleic acid in Ehrlich ascites mitochondria. Biochemistry. 1973 Oct 9;12(21):4122–4129. doi: 10.1021/bi00745a015. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr Hydrolysis of polyadenylic acid by pancreatic ribonuclease. J Biol Chem. 1960 Aug;235:2393–2398. [PubMed] [Google Scholar]
- Chamberlain J. P., Metz C. B. Mitochondrial RNA synthesis in sea urchin embryos. J Mol Biol. 1972 Mar 14;64(3):593–607. doi: 10.1016/0022-2836(72)90085-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. N., McCarthy B. J. Histone mRNA in eggs and embryos of Strongylocentrotus purpuratus. Biochem Biophys Res Commun. 1973 Jul 17;53(2):515–522. doi: 10.1016/0006-291x(73)90692-x. [DOI] [PubMed] [Google Scholar]
- Faust C. H., Jr, Diggelmann H., Mach B. Isolation of poly(adenylic acid)-rich ribonucleic acid from mouse myeloma and synthesis of complementary deoxyribonucleic acid. Biochemistry. 1973 Feb 27;12(5):925–931. doi: 10.1021/bi00729a021. [DOI] [PubMed] [Google Scholar]
- Giudice G., Sconzo G., Ramirez F., Albanese I. Giant RNA is also found in the cytoplasm in sea urchin embryos. Biochim Biophys Acta. 1972 Mar 24;262(3):401–403. doi: 10.1016/0005-2787(72)90279-1. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Gross K. W., Jacobs-Lorena M., Baglioni C., Gross P. R. Cell-free translation of maternal messenger RNA from sea urchin eggs. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2614–2618. doi: 10.1073/pnas.70.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Gross P. R. Identification in cleaving embryos of three RNA species serving as templates for the synthesis of nuclear proteins. Nature. 1969 Sep 27;223(5213):1335–1339. doi: 10.1038/2231335a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Raff R. A., Colot H. V., Selvig S. E., Gross P. R. Oogenetic origin of messenger RNA for embryonic synthesis of microtubule proteins. Nature. 1972 Jan 28;235(5335):211–214. doi: 10.1038/235211a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Abrass I. B., Perkins L. A. Cleavage of the polyadenylate-rich region of polyadenylate-rich RNA. Biochem Biophys Res Commun. 1972 Oct 6;49(1):230–238. doi: 10.1016/0006-291x(72)90034-4. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D. W., Slater I., Gillespie D. Post-fertilization synthesis of polyadenylic acid in sea urchin embryos. Nature. 1972 Dec 8;240(5380):333–337. doi: 10.1038/240333a0. [DOI] [PubMed] [Google Scholar]
- Slater D. W., Spiegelman S. An estimation of genetic messages in the unfertilized echinoid egg. Proc Natl Acad Sci U S A. 1966 Jul;56(1):164–170. doi: 10.1073/pnas.56.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D. W., Spiegelman S. Template capabilities and size distribution of echinoid RNA during early development. Biochim Biophys Acta. 1968 Aug 23;166(1):82–93. doi: 10.1016/0005-2787(68)90493-0. [DOI] [PubMed] [Google Scholar]
- Slater D. W., Spiegelman S. Transcriptive expression during sea urchin embryogenesis. Biochim Biophys Acta. 1970 Jul 16;213(1):194–207. doi: 10.1016/0005-2787(70)90020-1. [DOI] [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater I., Slater D. W. DNA polymerase potentials of sea urchin embryos. Nat New Biol. 1972 May 17;237(72):81–85. doi: 10.1038/newbio237081a0. [DOI] [PubMed] [Google Scholar]
- Sullivan N., Roberts W. K. Characterization and poly(adenylic acid) content of Ehrlich ascites cell ribonucleic acids fractionated on unmodified cellulose columns. Biochemistry. 1973 Jun 19;12(13):2395–2403. doi: 10.1021/bi00737a005. [DOI] [PubMed] [Google Scholar]
- Terman S. A. Relative effect of transcription-level and translation-level control of protein synthesis during early development of the sea urchin. Proc Natl Acad Sci U S A. 1970 Apr;65(4):985–992. doi: 10.1073/pnas.65.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Isono N. Acid-soluble nucleotides in the sea urchin egg. I. Ion-exchange chromatographic separation and characterization. Embryologia (Nagoya) 1966 Oct;9(3):170–183. doi: 10.1111/j.1440-169x.1966.tb00222.x. [DOI] [PubMed] [Google Scholar]


