Abstract
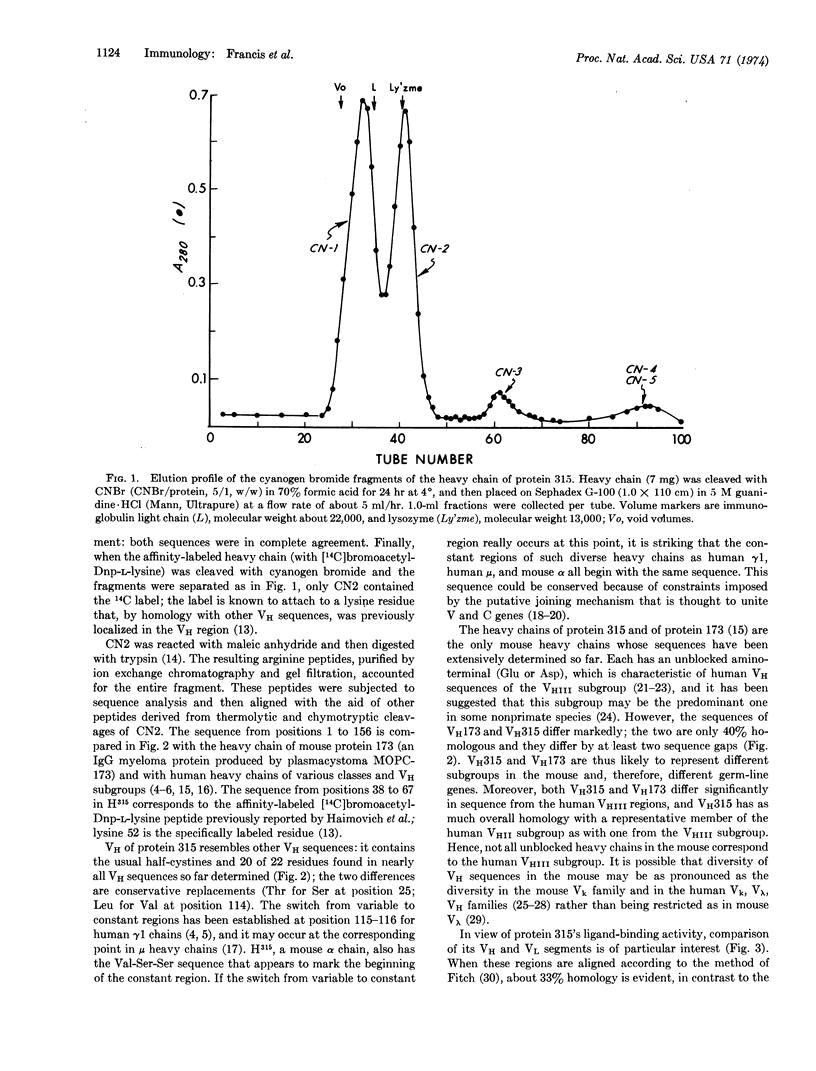
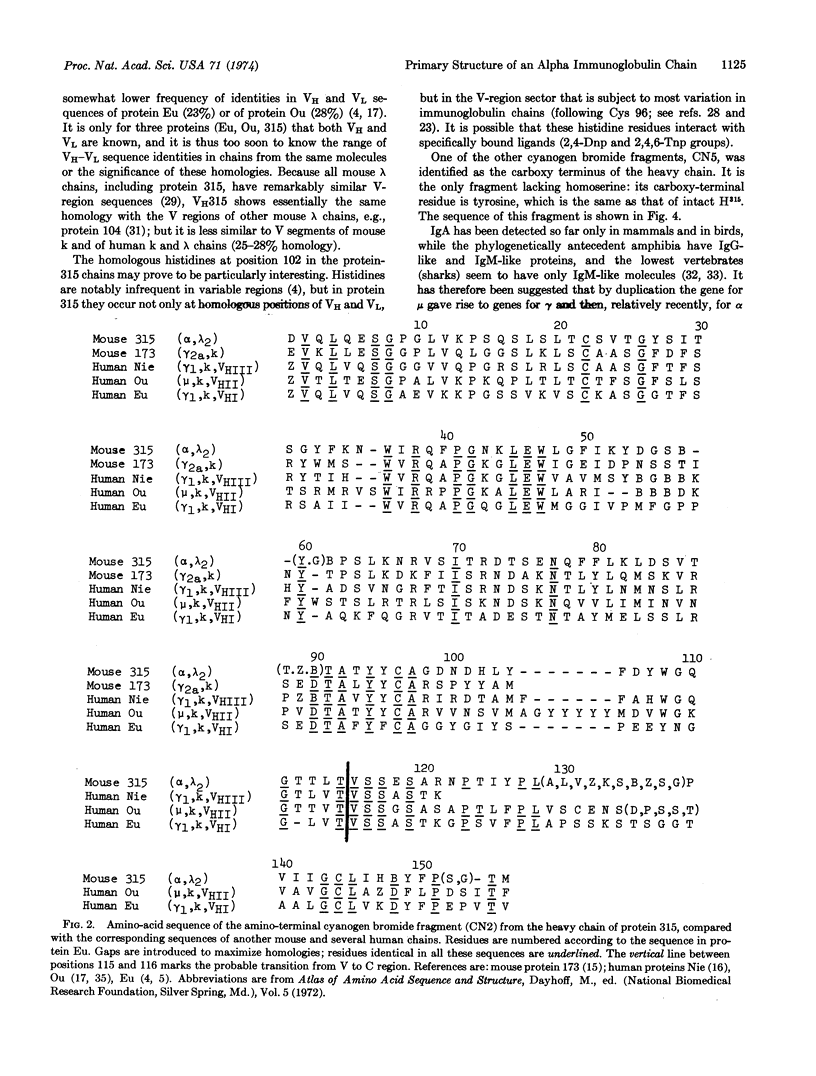
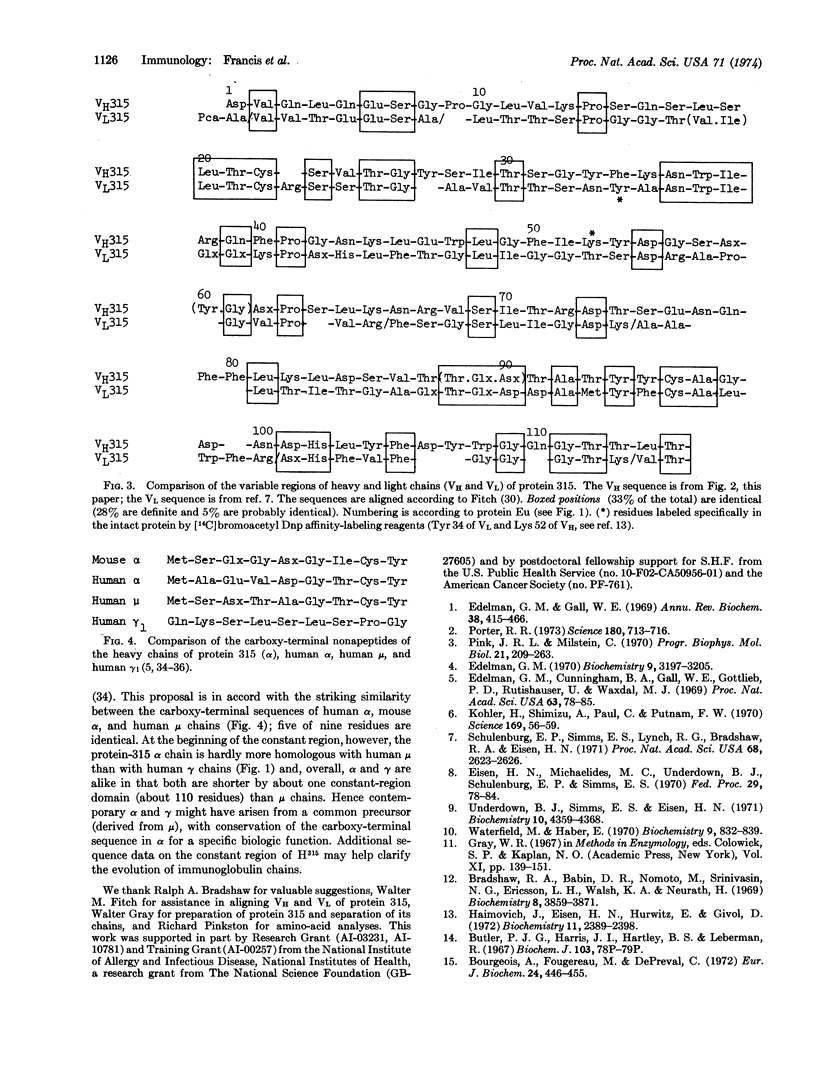
Cyanogen bromide cleavage of the heavy (alpha) chain of protein 315 (an immunoglobulin A mouse myeloma protein with anti-dinitrophenyl activity) yielded five fragments of which one (CN2), with 156 residues, contained the chain's entire variable region. Determination of the amino-acid sequence of CN2 showed that: (1) the variable region has appreciable homology (about 33% identities) with the variable region of the light chain from the same molecule; and (2) the constant-region sequence immediately following the probable transition from variable to constant domains is the same in the protein-315 α as in human γ1 and μ chains (-Val-Ser-Ser-). The sequence of the cyanogen bromide octapeptide (CN5) from the carboxy terminus of the protein-315 heavy chain closely resembles the corresponding segments of human α and μ chains.
Keywords: CNBr cleavage, alpha immunoglobulin chains, anti-dinitrophenyl activity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgois A., Fougereau M., De Preval C. Sequence of amino acids of the NH 2 -terminal region of a mouse-clonal immunoglobulin heavy chain. Eur J Biochem. 1972 Jan 21;24(3):446–455. doi: 10.1111/j.1432-1033.1972.tb19705.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Babin D. R., Nomoto M., Srinivasin N. G., Ericsson L. H., Walsh K. A., Neurath H. The amino acid sequence of bovine carboxypeptidase A. II. Tryptic and chymotryptic peptides of the cyanogen bromide fragment F-III. Biochemistry. 1969 Sep;8(9):3859–3871. doi: 10.1021/bi00837a052. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Leberman R. Reversible blocking of peptide amino groups by maleic anhydride. Biochem J. 1967 Jun;103(3):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- Cesari I. M., Weigert M. Mouse lambda-chain sequences. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2112–2116. doi: 10.1073/pnas.70.7.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem L. W., Small P. A., Jr Phylogeny of immunoglobulin structure and function. I. Immunoglobulins of the lemon shark. J Exp Med. 1967 May 1;125(5):893–920. doi: 10.1084/jem.125.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. XI. Functional implications. Biochemistry. 1970 Aug 4;9(16):3197–3205. doi: 10.1021/bi00818a012. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Michaelides M. C., Underdown B. J., Schulenburg E. P., Simms E. S. Experimental approaches to homogenous antibody populations. Myeloma proteins with antihapten antibody activity. Fed Proc. 1970 Jan-Feb;29(1):78–84. [PubMed] [Google Scholar]
- Fitch W. M. An improved method of testing for evolutionary homology. J Mol Biol. 1966 Mar;16(1):9–16. doi: 10.1016/s0022-2836(66)80258-9. [DOI] [PubMed] [Google Scholar]
- Fudenberg H. H., Wang A. C., Pink J. R., Levin A. S. Studies of an unusual biclonal gammopathy: implications with regard to genetic control of normal immunoglobulin synthesis. Ann N Y Acad Sci. 1971 Dec 31;190:501–506. doi: 10.1111/j.1749-6632.1971.tb13559.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M. Phylogeny of immunoglobulins. Adv Immunol. 1969;10:51–104. doi: 10.1016/s0065-2776(08)60415-0. [DOI] [PubMed] [Google Scholar]
- Haimovich J., Eisen H. N., Hurwitz E., Givol D. Localization of affinity-labeled residues on the heavy and light chain of two myeloma proteins with anti-hapten activity. Biochemistry. 1972 Jun 20;11(13):2389–2398. doi: 10.1021/bi00763a001. [DOI] [PubMed] [Google Scholar]
- Hood L. E. Two genes, one polypeptide chain--fact or fiction? Fed Proc. 1972 Jan-Feb;31(1):177–187. [PubMed] [Google Scholar]
- Hood L., McKean D., Farnsworth V., Potter M. Mouse immunoglobulin chains. A survey of the amino-terminal sequences of kappa chains. Biochemistry. 1973 Feb;12(4):741–749. doi: 10.1021/bi00728a026. [DOI] [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann N Y Acad Sci. 1971 Dec 31;190:382–393. doi: 10.1111/j.1749-6632.1971.tb13550.x. [DOI] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Localization of two additional hypervariable regions in immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2019–2021. doi: 10.1073/pnas.68.9.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Sequence relationships among the variable regions of immunoglobulin heavy chains from various mammalian species. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2052–2055. doi: 10.1073/pnas.69.8.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H., Shimizu A., Paul C., Putnam F. W. Macroglobulin structure: variable sequence of light and heavy chains. Science. 1970 Jul 3;169(3940):56–59. doi: 10.1126/science.169.3940.56. [DOI] [PubMed] [Google Scholar]
- Köhler H., Shimizu A., Paul C., Moore V., Putnam F. W. Three variable-gene pools common to IgM, IgG and IgA immunoglobulins. Nature. 1970 Sep 26;227(5265):1318–1320. doi: 10.1038/2271318a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J Exp Med. 1965 Sep 1;122(3):601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Pink J. R. Structure and evolution of immunoglobulins. Prog Biophys Mol Biol. 1970;21:209–263. doi: 10.1016/0079-6107(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Nisonoff A., Fudenberg H. H., Wilson S. K., Hopper J. E., Wang A. C. Individual antigenic specificity in immunoglobulins: relationship to biosynthesis. Fed Proc. 1972 Jan-Feb;31(1):206–209. [PubMed] [Google Scholar]
- Pink J. R., Buttery S. H., De Vries G. M., Milstein C. Human immunoglobulin subclasses. Partial amino acid sequence of the constant region of a gamma 4 chain. Biochem J. 1970 Mar;117(1):33–47. doi: 10.1042/bj1170033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Schwarz J., Reichel W., Hilschmann N. Die Primärstruktur eines monoklonalen gamma-1-Immunoglobulins (Myelomprotein NIE). I. Aminosäuresequenz des variablen Teils der H-Kette, Subgruppen variabler Teile. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1591–1594. [PubMed] [Google Scholar]
- Porter R. R. Structural studies of immunoglobulins. Science. 1973 May 18;180(4087):713–716. doi: 10.1126/science.180.4087.713. [DOI] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Florent G., Paul C., Shinoda T., Shimizu A. Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science. 1973 Oct 19;182(4109):287–291. doi: 10.1126/science.182.4109.287. [DOI] [PubMed] [Google Scholar]
- Schulenburg E. P., Simms E. S., Lynch R. G., Bradshaw R. A., Eisen H. N. Amino acid sequence of the light chain from a mouse myeloma protein with anti-hapten activity: evidence for a third type of light chain. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2623–2626. doi: 10.1073/pnas.68.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Putnam F. W., Paul C., Clamp J. R., Johnson I. Structure and role of the five glycopeptides of human IgM immunoglobulins. Nat New Biol. 1971 May 19;231(20):73–76. doi: 10.1038/newbio231073a0. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Simms E. S., Eisen H. N. Subunit structure and number of combining sites of the immunoglobulin A myeloma protein produced by mouse plasmacytoma MOPC-315. Biochemistry. 1971 Nov 23;10(24):4359–4368. doi: 10.1021/bi00800a002. [DOI] [PubMed] [Google Scholar]
- Waterfield M., Haber E. Amino acid sequence analysis with methyl isothiocyanate. Resolution of the methylthiohydantoins by gas-liquid partition chromatography. Biochemistry. 1970 Feb 17;9(4):832–839. doi: 10.1021/bi00806a016. [DOI] [PubMed] [Google Scholar]



