Abstract
Prostaglandins control osteoblastic and osteoclastic function under physiological or pathological conditions and are important modulators of the bone healing process. The non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclooxygenase (COX) activity and consequently prostaglandins synthesis. Experimental and clinical evidence has indicated a risk for reparative bone formation related to the use of non-selective (COX-1 and COX-2) and COX-2 selective NSAIDs. Ketorolac is a non-selective NSAID which, at low doses, has a preferential COX-1 inhibitory effect and etoricoxib is a new selective COX-2 inhibitor. Although literature data have suggested that ketorolac can interfere negatively with long bone fracture healing, there seems to be no study associating etoricoxib with reparative bone formation. Paracetamol/acetaminophen, one of the first choices for pain control in clinical dentistry, has been considered a weak anti-inflammatory drug, although supposedly capable of inhibiting COX-2 activity in inflammatory sites.
Objective
The purpose of the present study was to investigate whether paracetamol, ketorolac and etoricoxib can hinder alveolar bone formation, taking the filling of rat extraction socket with newly formed bone as experimental model.
Material and methods
The degree of new bone formation inside the alveolar socket was estimated two weeks after tooth extraction by a differential point-counting method, using an optical microscopy with a digital camera for image capture and histometry software. Differences between groups were analyzed by ANOVA after confirming a normal distribution of sample data.
Results and conclusions
Histometric results confirmed that none of the tested drugs had a detrimental effect in the volume fraction of bone trabeculae formed inside the alveolar socket.
Keywords: Bone regeneration, Paracetamol, Ketorolac, Cyclooxygenase 1, Cyclooxygenase 2, Cyclooxygenase 1 inhibitors, Cyclooxygenase 2 inhibitors
INTRODUCTION
The cyclooxygenase enzymes COX-1 and COX-2 catalyze the conversion of arachidonic acid to prostaglandins, which, in addition to a variety of physiological functions, are also involved in several pathological processes. The non-steroidal anti- inflammatory drugs (NSAIDs) inhibit COX activity and consequently prostaglandin synthesis24. A wide array of NSAIDs is currently available, notably the conventional NSAIDs, which inhibit non-selectively both enzymes COX-1 and COX-2, and a new class of selective COX-2 inhibitors.
The skeleton is abundantly supplied with prostaglandins, mainly PGE2, which modulate osteoblastic and osteoclastic function under physiological or pathological conditions. The anabolic effect of PGE2 on bone occurs chiefly in response to mechanical forces and in the healing process24. Not surprisingly, considerable experimental and clinical evidence has indicated a risk for reparative bone formation related to the use of selective and non-selective NSAIDs in the orthopedic clinic, although an absence of this deleterious effect has also been reported16.
Even though the NSAIDs are among the most commonly used drugs for management of acute and chronic pain in clinical dentistry23, very few studies have been conducted to assess their effects on alveolar bone. A small number of experimental investigations have suggested the deleterious effect of conventional NSAIDs on alveolar bone healing2,30 and some clinical assessments have reported no significant difference in the rate of bone healing following administration of this kind of drug3,4,17.
Ketorolac is a conventional (non-selective) NSAID which, in low doses, has a preferential COX-1 inhibitory effect29 while etoricoxib is a new selective COX-2 inhibitor21,26. Literature data have suggested that ketorolac can interfere negatively with new bone formation in different experimental models (femur fracture healing, spinal fusion and ulna osteotomy in rats and rabbits)11,15 in opposition to one report stating that ketorolac does not interfere with tibia fracture repair in mouse18. To the best of our knowledge, there are no reports about the effects of etoricoxib on bone repair, except for few studies showing its beneficial action in experimental periodontitis.
Since the 1970s, paracetamol/acetaminophen has become one of the most popular and widely used drugs in the world for treatment of pain and fever6, and one of the first choices for pain control in clinical dentistry20. Although it is generally considered an ineffective or weak anti-inflammatory drug, paracetamol seems capable of inhibiting COX-2 activity in inflammatory sites12. In spite of this, treatment of rats with paracetamol did not hinder femur fracture healing5.
The purpose of the present study was to investigate whether paracetamol, ketorolac or etoricoxib may have a detrimental effect on alveolar bone formation, considering the filling of rat extraction socket with newly formed bone as experimental model.
MATERIAL AND METHODS
Male Wistar rats (about 250 g initial body weight) were anaesthetized with a solution of ketamine hydrochloride (Ketamina Agener, União Química Farmacêutica Nacional S/A, Embu-Guaçu, SP, Brazil; 75 mg/kg) and xylazine (Dopaser, Laboratórios Calier S/A, Barcelona, Spain; 10 mg/ kg) administered intraperitoneally, and had the upper right incisors extracted and the wounds sutured with mononylon 4-0 (Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil). A single dose (0.2 mL per rat, intramuscularly) of a polyvalent veterinary antibiotic (Pentabiótico Veterinário, Wyeth, São Bernardo do Campo, SP, Brazil) was administered immediately after surgery and 5 days later.
Treated rats received oral doses of 1 mL aqueous solution of paracetamol (Paracetamol; Biossintética Farmacêutica Ltda, São Paulo, SP, Brazil - 80 mg/ kg/rat/day), ketorolac (Toragesic; Sigma Pharma, Hortolândia, SP, Brasil - 4 mg/kg/day) or etoricoxib (Arcoxia; Merck do Brasil, São Paulo, SP, Brazil; 10 mg/kg/day) administered by gavage from the day of surgery until death, 2 weeks later. Control rats received tap water (1 mL/day by gavage). The animals were housed under climate-controlled environment (12 h light/12 h dark, 20-24ºC) with free access to standard laboratory chow and tap water. All procedures were conducted in compliance with ethical principles for animal research, as approved by institutional guidelines (Protocol CETEA 117/2006).
The animals were killed with an intraperitoneal overdose of sodium pentobarbital 2 weeks postoperatively and their heads were immersed in 10% formalin solution for 48 h. After fixation, the maxillae were dissected free, decalcified and processed for paraffin embedding. Semi-serial longitudinal 6-µm-thick sections of the hemimaxillae containing the alveolar sockets were cut at 60-µm intervals and stained with hematoxylin and eosin.
For histometric analysis, the degree of new bone formation inside the alveolar socket was estimated by a differential point-counting method, using an optical microscopy (Zeiss Axiostar Plus; Carl Zeiss, Jena, Germany) with a digital camera for image capture (Zeiss Axiocam IC; Carl Zeiss) and a public domain histometry software (Image J, version 1.38, Mental Health Institute, Bethesda, Maryland, USA). The healing process, which in this phase consists of a gradual replacement of connective tissue by bone trabeculae, was estimated by new bone volume fraction (% bone trabeculae relative to bone trabeculae plus connective tissue). A total of 1100-1300 points were counted in 8-9 histological sections per alveolus (final magnification ×100), the percentage of points lying on bone trabeculae being proportional to their volume density. The measurements were standardized in the cervical and apical alveolar thirds, in order to avoid interference of regional differences in the rate of bone healing. Differences between groups were analyzed by ANOVA (p<0.05 for statistical significance) after confirming a normal distribution of sample data (Kolmogorov-Smirnov normality test, p>0.10).
RESULTS
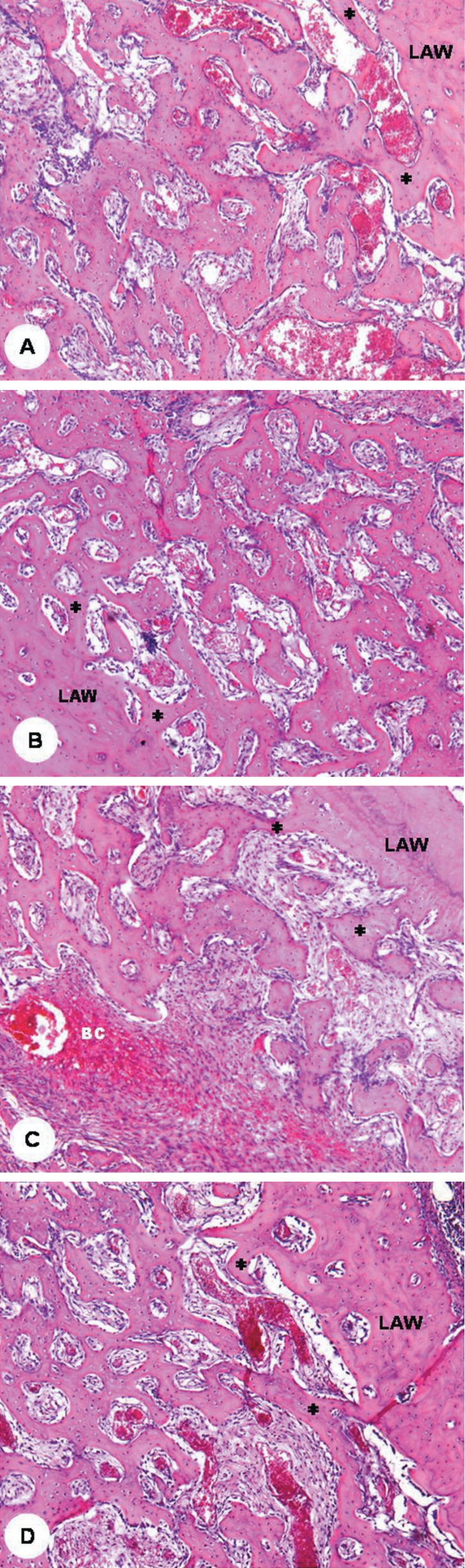
Histological examination showed the alveolar socket of control rats (Figure 1A) occupied by connective tissue and a network of delicate bone trabeculae lined with osteoblasts, forming from the inner surfaces of the alveolar walls. Blood clot remnants were still observed, mainly in the central region of the apical and middle alveolar thirds. In rats treated with paracetamol (Figure 1B), ketorolac (Figure 1C) or etoricoxib (Figure 1D), the bone healing process did not show any noticeable difference as compared to the controls.
Figure 1.
Rat alveolar sockets at 2 weeks after tooth extraction (hematoxylin and eosin; original magnification ×50). New bone trabeculae (*) forming from the inner surface of the lateral alveolar wall (LAW) in a control rat (A) and in rats treated with paracetamol (B), ketorolac (C) and etoricoxib (D); blood clot remnant (BC) interposed among new bone trabeculae in a ketorolac-treated rat
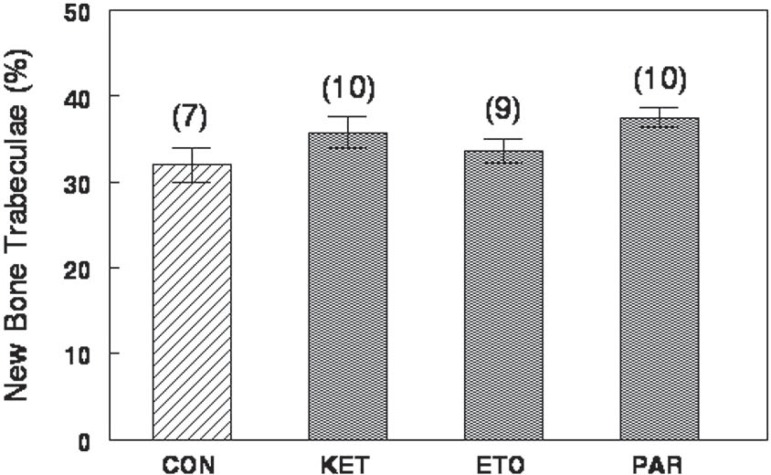
Histometric data confirmed histological observation that neither paracetamol nor ketorolac or etoricoxib affected significantly the volume fraction of bone trabeculae forming inside the alveolar socket (Figure 2).
Figure 2.
Percent of new bone trabeculae (mean±standard error of mean) inside the alveolar socket, 2 weeks after tooth extraction, in control (CON) rats and in rats treated with ketorolac (KET), etoricoxib (ETO) and paracetamol (PAR)
DISCUSSION
In the present study, the intra-alveolar healing process in the control and treated groups closely resembled literature data. Briefly, after tooth extraction, the socket is filled with blood clot, which is progressively invaded by capillary sprouting and fibroblasts originating from periodontal ligament remnants; in the sequence, the coagulum is gradually absorbed and replaced by immature connective tissue, the amount of inflammatory cells and blood vessels decreases and the osteoblasts become evident. The osteoblasts initially synthesize an immature bone matrix that is further mineralized by calcium deposition as hydroxyapatite crystals. New alveolar bone formation takes place from the apical and lateral walls towards the center, and the healing process culminates with filling of the dental socket by trabecular bone7,8. It has been confirmed that, in rats, the major proportion of bone formation13 and the maximum mineral bone density8 take place by the end of the 2nd week after tooth extraction, which was the period elected for bone healing measurement in the present study.
In the present study, neither NSAIDs, ketorolac and etoricoxib, interfered with rat alveolar bone repair two weeks after tooth extraction.
Studies regarding the effect of COX inhibition on alveolar bone healing are scarce and refer only to non-selective NSAIDs10. A histological study was conducted to investigate the effects of long-term treatment with aspirin cones placed in the extraction sockets of dogs2. The authors reported delayed alveolar bone formation in the 2-week samples, but not after long-term treatment, and admitted limitations in the experimental model that allowed the drug to stay in the sockets only for 2-3 days. In rats, short-term treatment (4 days) with diclofenac also delayed alveolar socket healing30. Clinical investigations are equally rare and pertain to short-term administration of NSAIDs to control edema and pain after removal of impacted third molars. Based on clinical postoperative assessment, some surgeons have reported no significant difference in the rate of bone healing following administration of ibuprofen17 aspirin, diclofenac4 and flurbiprofen3.
Contrarily, a wide range of experimental and clinical data have confirmed that a non-selective inhibition of COX-1 and COX-2 by the conventional NSAIDs (indomethacin, ibuprofen, naproxen, ketorolac) can interfere negatively with long bone fracture healing and spinal fusion rate, although an absence of this deleterious effect has also been reported. Many experimental and clinical studies were also conducted to verify the effects of selective COX-2 NSAIDs and, despite the importance of COX-2 and PGE2 for bone formation, the results are also controversial. In addition to evidence showing that the coxibs (rofecoxib, celecoxib and parecoxib) inhibit long bone repair in rats and rabbits, there are some reports stating that they do not interfere with fracture repair and spinal fusion rate, both in rodents and humans16.
Factors such as dosage and duration of treatment, as well as age, intra- and inter-species differences regarding sensitivity to drugs, compensatory local and systemic factors, pharmacokinetics of drugs in laboratory animals compared to humans and rate of bone remodeling9,18,24 have been considered responsible for the controversies in the experimental results about the effects of COX inhibitors on bone healing.
In rats, ketorolac used in small doses is considered a preferential COX-1 inhibitor (3 mg/ kg/day inhibits 95% of COX-1 without a significant inhibition of COX-2 activity)28, and can hinder femur fracture healing11. A recent in vitro study investigated the effect of ketorolac (among seven COX-1 and COX-2 inhibitors) on the marrow stem cell (MSC) potential for proliferation and differentiation towards the osteogenic and chondrogenic lineages. Although none of the drugs affected significantly MSC proliferation and osteogenic differentiation, some of them (mostly ketorolac) inhibited bone formation via blockage of MSC chondrogenic differentiation, which is an important intermediate phase in endochondral bone formation, such as in bone fracture healing22.
Etoricoxib is a selective COX-2 inhibitor (can block COX-2 activity 106 times28 or 344 times21 more than COX-1 activity). In the same dose as that used in the present study, it can control hiperalgesia26 and reduce bone loss in periodontal disease1, in rats. In 2008, the Brazilian Agency of Sanitary Surveillance (ANVISA) suspended commercialization of 120 mg pills of etoricoxib, but allowed the sale of 60 and 90 mg pills provided the retention of medical prescription and inclusion of adverse effects warning in the bulla were done. Thus, remains the interest of the present study regarding long-term use of this drug for controlling chronic pain associated to musculoskeletal disorders and rheumatoid diseases as well as an experimental selective COX-2 inhibitor.
Paracetamol is a potent analgesic and antipyretic with a central mechanism of action still not completely understood. It has been claimed that this drug, unlike the NSAIDs, devoid a significant inhibition of peripheral prostanoids, presenting thus a weak or ineffective anti-inflammatory effect6. However, a recent study carried out with human volunteers showed that paracetamol promotes more than 80% inhibition of peripheral COX-2, which is comparable to selective NSAIDs14.
Studies carried out in the late 1990s revealed a second peak of COX-2 occurring in the resolution phase of the inflammatory process, and showed that this COX-2 has a reduced sensibility to NSAIDs but is highly sensitive to inhibition with paracetamol27. Inhibition of this late-appearing COX-2 with NSAIDs delayed resolution of the inflammatory process, suggesting that this enzyme may present a proinflammatory action during the early phases, but may contribute to resolution of the inflammatory reaction and the healing process at a later phase20. It was recently confirmed that prostaglandins produced by activation of COX-2 are important factors that mediate resolution of the inflammatory process25.
All things considered, it could be supposed that paracetamol, inhibiting locally the late-appearing COX-2, would delay the resolution of the inflammatory process elicited by tooth extraction and hinder the subsequent bone healing. However, the supposed detrimental effect of paracetamol on alveolar bone healing was not confirmed in the present study.
A dose of paracetamol (80 mg/kg/day) compatible to human therapy and in the range of investigations carried out in laboratory animals was used in the present study. Experimental studies dealing with the analgesic effect of paracetamol have employed a single dose (acute treatment) of 850 mg/kg19 and investigations about its effect on the healing of soft and mineralized tissues have used dosages of 60 to 300 mg/kg/day, for up to 14 days (prolonged treatment)5. In the same way as observed in the present study, treatment of rats with paracetamol/ acetaminophen (60 or 300 mg/kg/day for 10 days) had no negative effect on femur fracture healing, as observed 8 weeks later by radiographic and histological examination as well as by mechanical testing. It is worth mentioning, however, that no assessment was made immediately after treatment completion5. Acetaminophen administered in standard concentrations in the food at an average dosage of 60 mg/kg/day during 14 days also had no detrimental effect on the healing of rat patellar tendon8.
CONCLUSION
Histometric data showed that prolonged treatment (2 weeks) with ketorolac (exerting a preferential COX-1 inhibition), etoricoxib (a selective COX-2 inhibitor) or paracetamol (a weak NSAID supposedly capable of inhibiting COX-2 activity in inflammatory sites) did not interfere negatively with alveolar bone healing, in rats.
REFERENCES
- 1.Azoubel MC, Menezes AM, Bezerra D, Oriá RB, Ribeiro RA, Brito GA. Comparison of etoricoxib and indomethacin for the treatment of experimental periodontitis in rats. Braz J Med Biol Res. 2007;40:117–125. doi: 10.1590/s0100-879x2007000100015. [DOI] [PubMed] [Google Scholar]
- 2.Baratieri A, Deli R. The effect on bone repair of aspirin cones placed in extraction sockets in dogs: a histopathologic study. J Oral Pathol. 1979;8:198–206. doi: 10.1111/j.1600-0714.1979.tb01886.x. [DOI] [PubMed] [Google Scholar]
- 3.Battisti N. The evaluation of the analgesic and anti-inflammatory effects of flurbiprofen mouthwash and 100-mg tablets in oral medicine. Minerva Stomatol. 1994;43:141–144. [PubMed] [Google Scholar]
- 4.Bailey BM, Zaki G, Rotman H, Woodwards R. A double-blind comparative study of soluble aspirin and diclofenac dispersible in the control of postextraction pain after removal of impacted third molars. Int J Oral Maxillofac Surg. 1993;22:238–241. doi: 10.1016/s0901-5027(05)80645-9. [DOI] [PubMed] [Google Scholar]
- 5.Bergenstock M, Min W, Simon AM, Sabatino C, O'Connor JP A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–723. doi: 10.1097/01.bot.0000184144.98071.5d. [DOI] [PubMed] [Google Scholar]
- 6.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho TL, Bombonato KF, Brentegani LG. Histometric analysis of rat alveolar wound healing. Braz Dent J. 1997;8:9–12. [PubMed] [Google Scholar]
- 8.Elsubeihi ES, Heersche JN. Quantitative assessment of post- extraction healing and alveolar ridge remodelling of the mandible in female rats. Arch Oral Biol. 2004;49:401–412. doi: 10.1016/j.archoralbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing of rat patellar tendon. Am J Sports Med. 2007;35(8):1326–1333. doi: 10.1177/0363546507301584. [DOI] [PubMed] [Google Scholar]
- 10.Fracon RN, Teófilo JM, Satin RB, Lamano T. Prostaglandins and bone: potential risks and benefits related to the use of nonsteroidal anti-inflammatory drugs in clinical dentistry. J Oral Sci. 2008;50:247–252. doi: 10.2334/josnusd.50.247. [DOI] [PubMed] [Google Scholar]
- 11.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg. 2007;89:114–125. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- 12.Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Guglielmotti MB, Cabrini RL. Alveolar wound healing and ridge remodeling after tooth extraction in the rat: a histologic, radiographic and histometric study. J Oral Maxillofac Surg. 1985;43:359–364. doi: 10.1016/0278-2391(85)90257-5. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–390. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- 15.Ho ML, Chanq JK, Wang GJ. Antiinflammatory drug effects on bone repair and remodeling in rabbits. Clin Orthop Relat Res. 1995;313:270–278. [PubMed] [Google Scholar]
- 16.Lamano Carvalho TL. Effect of conventional and COX-2 selective non-steroidal antiinflammatory drugs on bone healing. Acta Ortop Bras. 2007;15:166–168. [Google Scholar]
- 17.Lökken P, Olsen I, Bruaset I, Norman-Pedersen K. Bilateral surgical removal of impacted lower third molar teeth as a model for drug evaluation: a test with ibuprofen. Eur J Clin Pharmacol. 1975;8:209–216. doi: 10.1007/BF00567117. [DOI] [PubMed] [Google Scholar]
- 18.Mullis BH, Copland ST, Weinhold PS, Miclau T, Lester GE, Bos GD. Effect of COX-2 inhibitors and non-steroidal anti-inflammatory drugs on a mouse fracture model. Injury. 2006;37:827–837. doi: 10.1016/j.injury.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Parente L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: is two better than one? J Rheumatol. 2001;28:2375–2332. [PubMed] [Google Scholar]
- 21.Patrignani P, Capone ML, Taconelli S. Clinical pharmacology of etoricoxib: a novel selective COX-2 inhibitor. Expert Opin Pharmacoter. 2003;4:265–284. doi: 10.1517/14656566.4.2.265. [DOI] [PubMed] [Google Scholar]
- 22.Pountos I, Giannoudis PV, Jones E, English A, Churchman S, Field S, et al. NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01006.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poveda Roda R, Bagán JV, Jiménez Soriano Y, Gallud Romero L. Use of nonsteroidal antiinflammatory drugs in dental practice. A review. Med Oral Patol Oral Cir Bucal. 2007;12(1):E10–E18. [PubMed] [Google Scholar]
- 24.Radi ZA, Khan NK. Effects of cyclooxygenase inhibition on bone, tendon and ligament healing. Inflamm Res. 2005;54:358–366. doi: 10.1007/s00011-005-1367-4. [DOI] [PubMed] [Google Scholar]
- 25.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Mol Interv. 2006;6:199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 26.Rindeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol exp Ther. 2001;296(2):558–566. [PubMed] [Google Scholar]
- 27.Simmons DL, Botting RM, Robertson PM, Madsen ML, Vane JR. Induction of an acetaminophen-sensitive cyclooxygenase with reduced sensitivity to nonsteroid antiinflamatory drugs. Proc Natl Acad Sci USA. 1999;96:3275–3280. doi: 10.1073/pnas.96.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID- induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 29.Warner TD, Giuliano F, Voinovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yugoshi LI, Sala MA, Brentegani LG, Lamano Carvalho TL. Histometric study of socket healing after tooth extraction in rats treated with diclofenac. Braz Dent J. 2002;13:92–96. doi: 10.1590/s0103-64402002000200003. [DOI] [PubMed] [Google Scholar]