Abstract
Iron has been suggested to reduce the erosive potential of cola drinks in vitro.
Objective
The aim of this study was to evaluate in situ the effect of ferrous sulfate supplementation on the inhibition of the erosion caused by a cola drink.
Material and Methods
Ten adult volunteers participated in a crossover protocol conducted in two phases of 5 days, separated by a washout period of 7 days. In each phase, they wore palatal devices containing two human enamel and two human dentin blocks. The volunteers immersed the devices for 5 min in 150 mL of cola drink (Coca-ColaTM, pH 2.6), containing ferrous sulfate (10 mmol/L) or not (control), 4 times per day. The effect of ferrous sulfate on the inhibition of erosion was evaluated by profilometry (wear). Data were analyzed by paired t tests (p<0.05).
Results
The mean wear (±se) was significantly reduced in the presence of ferrous sulfate, both for enamel (control: 5.8±1.0 µm; ferrous sulfate: 2.8±0.6 µm) and dentin (control: 4.8±0.8 µm; ferrous sulfate: 1.7±0.7 µm).
Conclusions
The supplementation of cola drinks with ferrous sulfate can be a good alternative for the reduction of their erosive potential. Additional studies should be done to test if lower ferrous sulfate concentrations can also have a protective effect as well as the combination of ferrous sulfate with other ions.
Keywords: Tooth erosion, Demineralization, Iron, Dental enamel, Dentin, Soft drink
INTRODUCTION
Dental erosion is the loss of tooth substance by chemical processes not involving bacteria22. Although a multitude of factors seem to be involved in this process, the most important factors are dietary acids23,24 and intrinsic acids from the stomach3. Currently, the increased consumption of acidic foods and soft drinks is becoming an important factor for the development of erosive wear22,24. Thus, one preventive approach might be the reduction of the erosive potential of acidic beverages by ions supplementation.
The addition of calcium, fluoride and phosphate has been shown to reduce the erosive potential of pure acids and acidic drinks1,2,11-14,33. In situ studies have shown that ferrous sulfate reduces the demineralization of enamel in situations of high cariogenic challenge25,28. The possibility that ferrous sulfate could be also used to reduce erosive challenges was arisen based on studies using abiotic models, which showed that ferrous sulfate was effective on inhibition of hidroxyapatite4 and enamel powder5,17 dissolution. This prompted to an in situ study which was able to show that a 10 mmol/L ferrous sulfate rinse after an erosive attack followed or not by an abrasive episode, was able to significantly reduce the wear of dentin blocks30. From the practical point of view, however, it would be much easier if the soft drink were supplemented with ferrous sulfate, instead of rinsing with a ferrous sulfate solution after the consumption of a soft drink.
In this sense, it was conducted an in vitro study which demonstrated that the supplementation of a cola drink with ferrous sulfate at 10 mmol/L was able to reduce the wear of enamel blocks18. In the present study, an in situ model, which closely resembles the clinical situation, was chosen to investigate the effect of ferrous sulfate supplementation on the inhibition of the erosion of enamel and dentin blocks caused by a cola drink.
MATERIAL AND METHODS
This study was approved by the Institutional Review Board of Bauru School of Dentistry, University of São Paulo, Brazil (Process 130/2004). Ten adult volunteers (two male and eight female) with age range between 24 and 31 years old and normal stimulated salivary flow rate (>1 mL/min) took part in the study after signing an informed, written consent. The volunteers were not smokers, did not have active carious lesions, did not receive topical application of agents with high fluoride concentration at least two weeks prior to the beginning of the study, and did not have systemic diseases, such as xerostomia or gastroesophageal disorders15. Sample size was calculated based on a pilot study in order to provide an α-error of 5% and a power of 80%.
Experimental design
This study used a randomized design, performed in two crossover phases of five days. The factors under evaluation were experimental condition in two levels (Coca-ColaTM without ferrous sulfate - control; and Coca-ColaTM supplemented with ferrous sulfate at 10 mmol/L) and dental substrate in two levels (enamel and dentin). The volunteers wore acrylic palatal appliances each containing 2 dental slabs of each substrate. A new appliance was constructed for the volunteers in each phase. The response variable was depth of surface wear (µm).
Specimen preparation
Enamel and dentin slabs (4x4 mm) were obtained from recently extracted, caries free, unerupted human third permanent molars. All tooth surfaces were used for preparation of the specimens (crown and root for enamel and dentin, respectively). The enamel surface of the slabs was ground flat with water-cooled 320-, 600- and 1200-grit Al2O3 papers (Buehler, Lake Bluff, IL, USA) and polished with 1 µm diamond spray (Buehler). The same procedure was used for dentin surfaces, except for 320-grit Al2O3 papers. The complete removal of cementum was checked microscopically (40x magnification). For allocation of the samples to the groups, surface microhardness was determined by performing five indentations in different regions of the samples (Knoop diamond, 50 g, 10 s for enamel and 25 g, 5 s for dentin, HMV-2000; Shimadzu Corporation, Tokyo, Japan). The overall range of microhardness was 350-407 KNH for enamel and 59-71 KHN for dentin to select 40 enamel and 40 dentin specimens. Specimens were allocated to treatments by stratified randomization according to the mean surface microhardness. All groups presented similar mean microhardness values (around 388 KHN and 65 KHN, for enamel and dentin, respectively).
Palatal device preparation
Custom-made acrylic palatal devices were made with four sites (6x6x3 mm) recessed into the polished surface of each appliance. One slab was randomly assigned to each of the four sites and fixed with wax, totaling two enamel and two slabs. The position of each slab in the device was randomly determined for each volunteer. In order to maintain reference surfaces for lesion depth determination, two layers of nail polish (Risqué, Niasi, Taboão da Serra, SP, Brazil) were applied on the surfaces of half of the specimens. To minimize the contact between the tongue and the specimens, these were positioned posterior to the incisive papilla.
Intraoral phase
A 1-week lead-in period was used. During this period, and throughout the experimental phase, the volunteers brushed their teeth with a fluoride-free dentifrice. In this crossover protocol, the volunteers were randomly allocated to the treatments and participated in two phases. In the first 12 h of each intraoral phase, specimens were not subjected to erosive treatment to allow the formation of a salivary pellicle10. On the following 5 days, erosive challenges were made extraorally 4 times a day after the mean meals and before sleeping. In each challenge, the volunteers were instructed to remove the appliance and immerse it in a cup containing 150 mL of regular Coca-ColaTM (pH 2.6, Cia de Bebidas Ipiranga, Ribeirão Preto, SP, Brazil)29,30, containing or not ferrous sulfate at 10 mmol/L (as FeSO4.7H20, Merck, Darmstadt, Germany), depending on the phase. For supplementation of Coca-ColaTM with ferrous sulfate, the salt was added to a 600 mL bottle of freshly opened regular Coca-ColaTM once per day, just before the first challenge. After each challenge, the volunteers were instructed to take one sip of the beverage and to immediately return the appliance into the mouth. This was done to simulate the clinical condition of pH drop after consumption of acidic beverages. In addition, it is also probable that the low pH of the drink induces the activation of dentin-derived matrix metalloproteinases (MMPs) and saliva-derived MMPs, when the volunteers drink one sip of the beverage32.
The volunteers were instructed not to consume acidic foods and to wear the appliances continuously for 24 h but to remove them during meals (4 times a day, 1 h each). In this period, the appliance was stored in wet gauze. They were also instructed to not touch the appliance with the tongue in order to avoid abrasion of the samples9.
Wear analysis
At the end of day 6, the volunteers stopped wearing the palatal devices. The nail polish over the reference surfaces was carefully removed and the slabs were removed from the device. The dentin specimens were maintained wet until the analysis to avoid shrinkage of collagen fibrils. The enamel and dentin wear was determined in relation to the reference surfaces by profilometry using a profilometer (Hommel Tester T 1000, Hommelwerke, VS, Schwenningen, Germany). Five readings were performed on each slab and the average wear depth was calculated. These profilometric traces were taken from the reference surface, across the exposed surface. The average wear depth of an experimental unit was computed by using the 10 readings: two slabs X five readings.
Statistical analysis
GraphPad InStat software version 3.0 for Windows (Graph Pad Software Inc., San Diego, CA, USA) was used. The assumptions of equality of variances and normal distribution of errors were checked, using Bartlett and Kolmogorov-Smirnov tests, respectively. Since the assumptions were satisfied, data were analyzed by paired t tests. The significance level was set at 5%.
RESULTS
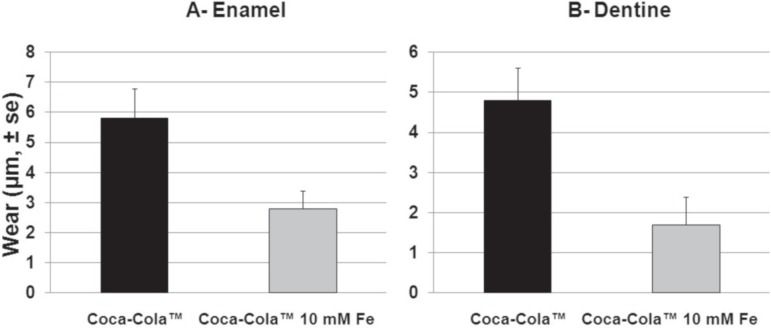
The Figure 1 shows the mean wear of enamel (A) and dentin (B) blocks submitted to erosive challenge by Coca-ColaTM or Coca-ColaTM supplemented with ferrous sulfate. For enamel, supplementation of Coca-ColaTM with ferrous sulfate (10 mmol/L) led to a mean wear (±s.e.) of 2.8±0.6 µm, which was significantly lesser (t=2.327, p=0.045) than the one observed for the control group (5.8± 1.0 µm). The same results were obtained for dentin. In the presence of ferrous sulfate, the mean wear (±s.e.) was 1.7±0.7 µm, which was significantly lesser (t=3.465, p=0.00071) than the one observed for the control group (4.8±0.8 µm). The post hoc calculated statistical power was 84% for enamel and 90% for dentin.
Figure 1.
Mean wear of enamel (A) and dentin (B) blocks submitted to erosive challenge in situ by Coca-ColaTM or Coca- ColaTM supplemented with ferrous sulfate at 10 mmol/L. Means followed by distinct letters are significantly different (paired t tests, p<0.05). Bars indicate standard error of means (s.e.)
DISCUSSION
In the present in situ study, the supplementation of Coca-ColaTM with ferrous sulfate at 10 mmol/L significantly reduced the wear of both enamel and dentin. These findings confirm data obtained in vitro for enamel powder5 and enamel blocks18. The wear was reduced by approximately 2-fold in enamel and 3-fold in dentin. The higher protective effect of ferrous sulfate on dentin when compared with enamel is consistent with a previous report30, which evaluated the effect of a rinse with a solution containing ferrous sulfate at 10 mmol/L on the reduction of wear of enamel and dentin blocks in situ. This difference may be due to the different composition of enamel and dentin, which may interfere on the erosive process. In enamel, the initial stage of erosion is characterized by a softening of the surface due in part to the demineralization of the surface. At this stage of the process, repair (remineralization) is in theory still possible as the remaining tissue could act as a scaffold. In a second, more advanced stage, repair is not possible, while the remaining softened enamel beneath the lost hard tissue is remineralizable24. The mechanisms underlying the protective effect of ferrous sulfate against enamel erosion are not fully understood. It has been hypothesized that ferrous sulfate may react with phosphate dissolved from enamel after the erosive challenge. This ferric phosphate may precipitate on the enamel surface, thus reducing the surface microhardness values30, which was also observed in the present study (data not shown). The analysis of the type of compound formed on the surface of dental enamel when ferrous sulfate is used may be useful to provide new insights into its mechanisms of action on dental enamel.
Differently from enamel, erosion in dentin is first apparent at the interface between inter- and peritubular dentin, and - with increasing exposure time - results in a hollowing and funneling of the tubules. Finally, the peritubular dentin is completely dissolved. The innermost sound dentin is then followed by a zone of partly demineralized dentin until a layer of completely demineralized collagen amounting up to one third of the total etching depth is reached19,26. This causes the exposure of a completely demineralized surface layer containing only organic matrix7,19,26. Dentin contains 18-20% of organic material and 90% is type I collagen. The presence of a collagen-rich dentin surface diminishes the diffusion of acids into the tissue and also exhibits buffering properties, thus minimizing dental erosion8,20,21,27. However, this organic matrix can be mechanically or chemically degraded, which will facilitate subsequent demineralization. The chemical degradation of the organic matrix occurs by the action of MMPs from dentin and saliva6. As a result, the disruption of this collagen layer will increase the progression of dentin erosion. As it has been shown that some metallic ions can inhibit the activity of MMPs31, it could be hypothesized that ferrous sulfate added to the soft drink and/or deposited on dentin (probably as ferric phosphate, as described above for enamel) would inhibit MMPs from dentin and saliva, since ferrous sulfate has been shown to have this ability16. This would slow the rate of erosion progression by the stabilization of the collagen layer. Details regarding the role of iron on the reduction of erosive wear in dentin have been published elsewhere16. However, this was not evaluated in the present protocol and should be confirmed in further experiments. If this hypothesis is confirmed, the collagen diffusion barrier might have been responsible for the lower wear found in dentin compared with enamel in the present study.
In summary, the results of the present in situ study, which closely resembles the clinical situation, confirm previous in vitro findings that the addition of 10 mmol/L ferrous sulfate to Coca-ColaTM can reduce its erosive potential on enamel. In addition, it was also found an even higher inhibition of dentin wear when compared with enamel. In the present study, drawbacks for the volunteers, such as tooth staining, were not observed, since the contact of the supplemented beverage with their teeth was very brief. However, this side effect could be expected to occur following consumption of the supplemented beverage for a long time. Other possible adverse effects, such as modification of the taste of the beverage and toxicity, must be taken into account when beverages are intended to be supplemented with this ion. The World Health Organization34 has calculated a Provisional Maximum Tolerable Daily Intake (PMDTI) of 56 mg/day (0.8 mg/kg/day), although lower levels have been defined in some countries. Considering a beverage containing 10 mmol/L ferrous sulfate, the maximum volume to be consumed daily would be around 200 mL only. Thus, it would be interesting to test if lower ferrous sulfate concentrations would also be effective as well as the combination of lower ferrous sulfate concentrations with other ions such as, calcium, phosphate and fluoride, which have also been shown to have promising effects on enamel dissolution. Additionally, since ferrous sulfate has been shown to reduce enamel and dentin wear under acidic conditions, but toxicological issues may be a limitation to its addition to soft drinks, the incorporation of ferrous sulfate to professionally applied dental products could be a good alternative to take advantage of its beneficial properties on dental mineral loss.
CONCLUSION
The supplementation of cola drinks with ferrous sulfate can be a good alternative for the reduction of their erosive potential. Additional studies should be done to test if lower ferrous sulfate concentrations can also have a protective effect as well as the combination of ferrous sulfate with other ions.
Acknowledgments
ACKNOWLEDGEMENT
This study was supported by FAPESP (Grant n.04/12632-2).
REFERENCES
- 1.Attin T, Meyer K, Hellwig E, Buchalla W, Lennon AM. Effect of mineral supplements to citric acid on enamel erosion. Arch Oral Biol. 2003;48:753–759. doi: 10.1016/s0003-9969(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 2.Attin T, Weiss K, Becker K, Buchalla W, Wiegand A. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005;11:7–12. doi: 10.1111/j.1601-0825.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D. Intrinsic causes of erosion. Monogr Oral Sci. 2006;20:119–139. doi: 10.1159/000093359. [DOI] [PubMed] [Google Scholar]
- 4.Brookes SJ, Robinson C, Shore Rc, Kirkham J. Inhibitory effect of metal ions on acid demineralization [abstract 132] Caries Res. 2004;38(4):401–401. Presented at 51st Annual ORCA Congress; 2004 June 30-July 3; Marburg, Germany. [Google Scholar]
- 5.Buzalaf MA, Italiani FM, Kato MT, Martinhon CC, Magalhães AC. Effect of iron on inhibition of acid demineralization of bovine dental enamel in vitro. Arch Oral Biol. 2006;51:844–848. doi: 10.1016/j.archoralbio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 7.Ganss C, Klimek J, Schäffer U, Spall T. Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro. Caries Res. 2001;35:325–330. doi: 10.1159/000047470. [DOI] [PubMed] [Google Scholar]
- 8.Ganss C, Klimek J, Starck C. Quantitative analysis of the impact of the organic matrix on the fluoride effect on erosion progression in human dentine using longitudinal microradiography. Arch Oral Biol. 2004;49:931–935. doi: 10.1016/j.archoralbio.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Gregg T, Mace S, West NX, Addy M. A study in vitro of the abrasive effect of the tongue on enamel and dentine softened by acid erosion. Caries Res. 2004;38:557–560. doi: 10.1159/000080586. [DOI] [PubMed] [Google Scholar]
- 10.Hara AT, Ando M, González-Cabezas C, Cury JA, Serra MC, Zero DT. Protective effect of the dental pellicle against erosive challenges in situ. J Dent Res. 2006;85:612–616. doi: 10.1177/154405910608500706. [DOI] [PubMed] [Google Scholar]
- 11.Hughes JA, Jandt KD, Baker N, Parker D, Newcombe RG, Eisenburger M, et al. Further modification to soft drinks to minimise erosion. A study in situ. Caries Res. 2002;36:70–74. doi: 10.1159/000057594. [DOI] [PubMed] [Google Scholar]
- 12.Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink in vitro and in situ. 1. Comparison with orange juice. J Dent. 1999;27:285–289. doi: 10.1016/s0300-5712(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink. 3. Final drink and concentrate, formulae comparisons in situ and overview of the concept. J Dent. 1999;27:345–350. doi: 10.1016/s0300-5712(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 14.Hunter ML, Hughes JA, Parker DM, West NX, Newcombe RG, Addy M. Development of low erosive carbonated fruit drinks. 1. Evaluation of two experimental orange drinks in vitro and in situ. J Dent. 2003;31:253–260. doi: 10.1016/s0300-5712(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 15.Järvinen VK, Rytomaa II, Heinonen OP. Risk factors in dental erosion. J Dent Res. 1991;70:942–947. doi: 10.1177/00220345910700060601. [DOI] [PubMed] [Google Scholar]
- 16.Kato MT, Leite AL, Hannas AR, Oliveira RC, Pereira JC, Tjäderhane L, et al. Effect of iron on matrix metalloproteinase inhibition and on the prevention of dentine erosion. Caries Res. 2010;44:309–316. doi: 10.1159/000315932. [DOI] [PubMed] [Google Scholar]
- 17.Kato MT, Maria AG, Sales-Peres SHC, Buzalaf MA. Effect of iron on the dissolution of bovine enamel powder in vitro by carbonated beverages. Arch Oral Biol. 2007;52:614–617. doi: 10.1016/j.archoralbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Kato MT, Sales-Peres SHC, Buzalaf MA. Effect of iron on acid demineralization of bovine enamel blocks by a soft drink. Arch Oral Biol. 2007;52:1109–1111. doi: 10.1016/j.archoralbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Kinney JH, Balooch M, Haupt DL, Jr, Marshall SJ, Marshall GW., Jr Mineral distribution and dimensional changes in human dentin during demineralization. J Dent Res. 1995;74:1179–1184. doi: 10.1177/00220345950740050601. [DOI] [PubMed] [Google Scholar]
- 20.Kleter GA, Damen JJ, Everts V, Niehof J, Ten Cate JM. The influence of the organic matrix on demineralization of bovine root dentin in vitro. J Dent Res. 1994;73:1523–1529. doi: 10.1177/00220345940730090701. [DOI] [PubMed] [Google Scholar]
- 21.Klont B, Ten Cate JM. Remineralization of bovine incisor root lesions in vitro: the role of the collagenous matrix. Caries Res. 1991;25:39–45. doi: 10.1159/000261340. [DOI] [PubMed] [Google Scholar]
- 22.Lussi A. Erosive tooth wear - a multifactorial condition of growing concern and increasing knowledge. Monogr Oral Sci. 2006;20:1–8. doi: 10.1159/000093343. [DOI] [PubMed] [Google Scholar]
- 23.Lussi A, Jaeggi T. Chemical factors. Monogr Oral Sci. 2006;20:77–87. doi: 10.1159/000093353. [DOI] [PubMed] [Google Scholar]
- 24.Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res. 2004;38(Suppl 1):34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 25.Martinhon CC, Italiani FM, Padilha PM, Bijella MF, Delbem AC, Buzalaf MA. Effect of iron on bovine enamel and on the composition of the dental biofilm formed in situ. Arch Oral Biol. 2006;51:471–475. doi: 10.1016/j.archoralbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Meurman JH, Drysdale T, Frank RM. Experimental erosion of dentin. Scand J Dent Res. 1991;99:457–462. doi: 10.1111/j.1600-0722.1991.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 27.Ogaard B, Rolla G, Arends J. In vivo progress of enamel and root surface lesions under plaque as a function of time. Caries Res. 1988;22:302–305. doi: 10.1159/000261125. [DOI] [PubMed] [Google Scholar]
- 28.Pecharki GD, Cury JA, Paes Leme AF, Tabchoury CP, Del Bel Cury AA, Rosalen PL, et al. Effect of sucrose containing iron (II) on dental biofilm and enamel demineralization in situ. Caries Res. 2005;39:123–129. doi: 10.1159/000083157. [DOI] [PubMed] [Google Scholar]
- 29.Rios D, Honório HM, Magalhães AC, Delbem AC, Machado MA, Silva SM, et al. Effect of salivary stimulation on erosion of human and bovine enamel subjected or not to subsequent abrasion: an in situ/ex vivo study. Caries Res. 2006;40:218–223. doi: 10.1159/000092229. [DOI] [PubMed] [Google Scholar]
- 30.Sales-Peres SH, Pessan JP, Buzalaf MA. Effect of an iron mouthrinse on enamel and dentine erosion subjected or not to abrasion: an in situ/ex vivo study. Arch Oral Biol. 2007;52:128–132. doi: 10.1016/j.archoralbio.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Souza AP, Gerlach RF, Line SR. Inhibition of human gingival gelatinases (MMP-2 and MMP-9) by metal salts. Dent Mater. 2000;16:103–108. doi: 10.1016/s0109-5641(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 32.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 33.West NX, Hughes JA, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink. 2. Comparison with a conventional blackcurrant juice drink and orange juice. J Dent. 1999;27:341–344. doi: 10.1016/s0300-5712(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization - WHO . FAO/WHO Expert Committee on Food Additives Toxicological evaluation of certain food additives and contaminants. Geneva: WHO; 1983. (Series WFA 18). [Google Scholar]