Abstract
Objectives
The Mikania laevigata extract (MLE) (popularly known in Brazil as "guaco") possesses anti-inflammatory properties. In the present study we tested the effects of MLE in a periodontitis experimental model in rats. We also investigated possible mechanisms underlying such effects.
Material and Methods
Periodontal disease was induced by a ligature placed around the mandibular first molars of each animal. Male Wistar rats were divided into 4 groups: non-ligated animals treated with vehicle; non-ligated animals treated with MLE (10 mg/kg, daily); ligature-induced animals treated with vehicle and ligature-induced animals treated with MLE (10 mg/kg, daily). Thirty days after the induction of periodontal disease, the animals were euthanized and mandibles and gingival tissues removed for further analysis.
Results
Morphometric analysis of alveolar bone loss demonstrated that MLE-treated animals presented a decreased alveolar bone loss and a lower expression of the activator of nuclear factor-κB ligand (RANKL) measured by immunohistochemistry. Moreover, gingival tissues from the MLE-treated group showed decreased neutrophil migration myeloperoxidase (MPO) assay.
Conclusions
These results indicate that MLE may be useful to control bone resorption during progression of experimental periodontitis in rats.
Keywords: Guaco, Periodontitis, Rats
INTRODUCTION
The periodontium is a topographically complex organ consisting of epithelial tissue and soft and mineralized connective tissue. The structures comprising the periodontium include gingiva, cementum, bone and periodontal ligament4. Several diseases affect the composition and integrity of periodontal structures causing the destruction of the connective tissue matrix and cells, the loss of fibrous attachment and the resorption of alveolar bone following an intense inflammatory response to bacteria7. Such diseases are highly prevalent among different world populations, being an important impact factor in health oral programs1. The main bacterial pathogens of periodontal diseases are gram-negative anaerobic species that express a number of potential virulence factors that stimulate an unbalanced production of several molecules and pro-inflammatory factors, being an important determinant in the disease outcome20.
Innumerous studies have shown that, although largely nonspecific, basic treatment consisting of oral hygiene instruction and subgingival instrumentation is able to keep the microbial load at low levels10 and, if followed by daily self-performed oral hygiene and regular professional periodontal maintenance care, leads to periodontal health in most cases 3. However, due to its unique patient and site-specific characteristics, a small proportion of the population suffers from ''refractory'' types of periodontitis, showing inadequate resolution of inflammation and failure to return tissue to homeostasis25. Since knowledge of the pathways and processes underlying resolution of periodontal tissue inflammation have grown significantly, an increased interest in substances and/or drugs that may contribute to the restoration of tissue homeostasis has occurred as a more sophisticated biological treatment modality for recurrent types of periodontal disease16.
Several biological systems are candidates to modulate the inflammatory process involved in periodontal disease. Evidence from animal experiments and short-term clinical trials demonstrate that anti-inflammatory drugs are able to decrease the rate of periodontal bone destruction by local inhibition of pro-inflammatory molecules24. In Brazil, the leaves of species of Mikania laevigata Schultz Bip. ex Baker, popularly known as "guaco", have been widely used as infusions or plasters, while the crude extract of this species is commonly commercialized as phytomedicine. Guaco is also popular in Brazil as an anti-inflammatory, antispasmodic and pain-reliever for rheumatism, arthritis, intestinal inflammation and ulcers. Among the few pharmacological and phytochemical studies published, preparations obtained from aerial parts of Mikania laevigata have been described as anti-ulcerogenic5, antimicrobial29, bronchodilatory26 and anti-inflammatory27, possibly accounted for by the presence of several chemical constituents.
Our research group has recently assessed the pharmacological properties and the underlying molecular mechanisms of Mikania laevigata, demonstrating that, in animals, its hydroalcoholic extract was able to reduce neutrophil migration and vascular permeability, and prevent the release of TNF-α and IL-1β2. Since such findings could promote a protective effect in periodontal tissues against damage, the aim of this study was to evaluate the immunomodulatory action of systemic injection of a "guaco" extract in a model of experimental periodontitis in rats.
MATERIAL AND METHODS
Source of Mikania laevigata and preparation of the "guaco" extract
The extract used in this study was previously obtained and tested as described elsewhere2. Briefly, the leaves of Mikania laevigata were collected at the Farm School of the University of Uberaba (Triângulo Mineiro, MG, Brazil). A voucher specimen (HUFU 54.748) has been deposited at the Herbarium of the Federal University of Uberlândia, Brazil. The samples were collected before 10:00a.m., between days 15 and 17 of November and allowed to dry at 30ºC in an oven with air renovation for 15 days. The dry plant, triturated by knife mill, was extracted by maceration with 70% ethanol:water under continuous agitation (shaker) for three times during 7 days each totalizing 21 days [the end ratio between plant and solvent was 1:8 (w/v)], obtaining a crude extract. The crude extract was dried and filtered using filter paper, concentrated in air-forced chamber at 30ºC until dry crude extract was obtained . Pharmacologic assays were carried out using dry crude extract dissolved in saline solution.
Animals
Forty male Wistar rats (250-350 g) were used in the study. The animals were kept in plastic cages with access to food and water ad libitum. Prior to the surgical procedures, all animals were allowed to acclimatize to the laboratory environment for a period of 5 days. All experiments were conducted in accordance with national health guidelines for the welfare of experimental animals and were approved by the Ethics Committee of the University of Uberaba (protocol #001/2008).
Experimental animal design
Experimental periodontitis was induced by a ligature placement. More specifically, under general anesthesia obtained by intramuscular administration of ketamine (1.0 mL/kg), ligature was placed and immobilized around both mandible first molars of each animal. The ligature was left in position for the whole experimental period so that inflammation could be constantly induced by the colonization of bacteria inside of it. One day following ligature placement, the animals were randomly assigned to one of the following groups (n=10): 1) animals without ligature placement receiving administration of empty vehicle (control); 2) animals without ligature placement receiving administration of MLE; 3) animals with ligature receiving administration of saline; 4) animals with ligature receiving administration of subcutaneous MLE (10 mg/kg/day; 200 µL. MLE or vehicle was administered daily for 30 days. Twenty-four hours after the last injection, the animals were sacrificed by anesthetic overdose. The current dose was chosen based on a previous study showing that it was effective to diminish the carrageenan-induced peritonitis15.
MPO activity assay
Neutrophil infiltration to the gingival tissues of rats was evaluated by MPO kinetic-colorimetric assay as previously described17. Samples of gingival tissue were collected in 50 mM K2HPO4 buffer (pH 6.0) containing 0.5% hexadecyl trimethylammonium bromide (HTAB) and kept at -80ºC until use. Samples were homogenized using a Polytron (PT3100) and centrifuged at 13,000 g for 4 min. The resulting supernatant devoid of debris was subjected to MPO activity assay determined by spectrophotometer at 450 nm (Spectra max®; Molecular Devices Inc., Sunnyvale, CA, USA) with three readings within 1 min. Briefly, 10 µL of sample was mixed with 200 µL of 50 mM phosphate buffer pH 6.0, containing 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The MPO activity of samples was compared with a standard curve of neutrophils. The results were presented as MPO activity (number of neutrophils X mg gingival tissue).
Histological and immunohistochemical analyses
The right and left jaws were dissected, fixed in 10% buffered neutral formalin for 48 h and decalcified in a decalcifying solution of ethylenediaminetetraacetic acid (EDTA) 10% for 3 months. After that, briefly washed in running tap water, dehydrated and embedded in paraffin wax. Each sample was sliced into 6 mm sections in sagittal directions. Sections were mounted on glass slides and stained with hematoxylin and eosin (HE) for the evaluation of bone resorption. Using an image analysis system (Image J - National Institute of Health), the area of bone loss in the furcation region was histometrically determined as previously described17.
Additional sections were mounted on glass slides pre-treated with 3-aminopropyltriethoxy-silane (Sigma Chemical Co., St. Louis, MO, USA) and used for immunohistochemical analysis. Sections were treated with 3% hydrogen peroxide (H2O2) for 30 min to block endogenous peroxidase. To block the nonspecific binding of antibody, the sections were treated with phosphate buffered saline (PBS)-1% bovine serum albumin (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature before incubation with the primary antibody (polyclonal antibodies against RANKL (1:50) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 24 h at 4ºC. Biotinylated secondary antibody was used (Sigma;1:100) for 60 min at room temperature. The slides were treated with Vectastain ABC-AP kit (diluted at 1:100; Vector Laboratories) for 60 min at room temperature, and the specific reaction by each antibody was visualized using 3, 3'diaminobenzidine (DAB). The slides were then counterstained with Mayer's hematoxylin, dehydrated through graded ethanol, cleared in xylene, and mounted in slides with the help of Permount mounting media (Fisher Scientific). Negative controls were obtained by omission of the primary antibodies.
At least 10 representative sections of each specimen were analyzed under a light microscope (Olympus BX50, Tokyo, Japan). Immunohistochemical analysis was performed individually by two previous calibrated examiners (JCC and VJS-F; Kappa index=0.97) that were blind to the treatment conditions. The number of stained cells was counted and normalized in the area where bone resorption was taking place.
Statistical analysis
Data were expressed as mean±SD (standard deviation). Statistical comparisons between groups were made using ANOVA analysis of variance followed by Bonferroni test. Significance was accepted when the p value was ≤0.05.
RESULTS
Effect of MLE on furcation bone loss
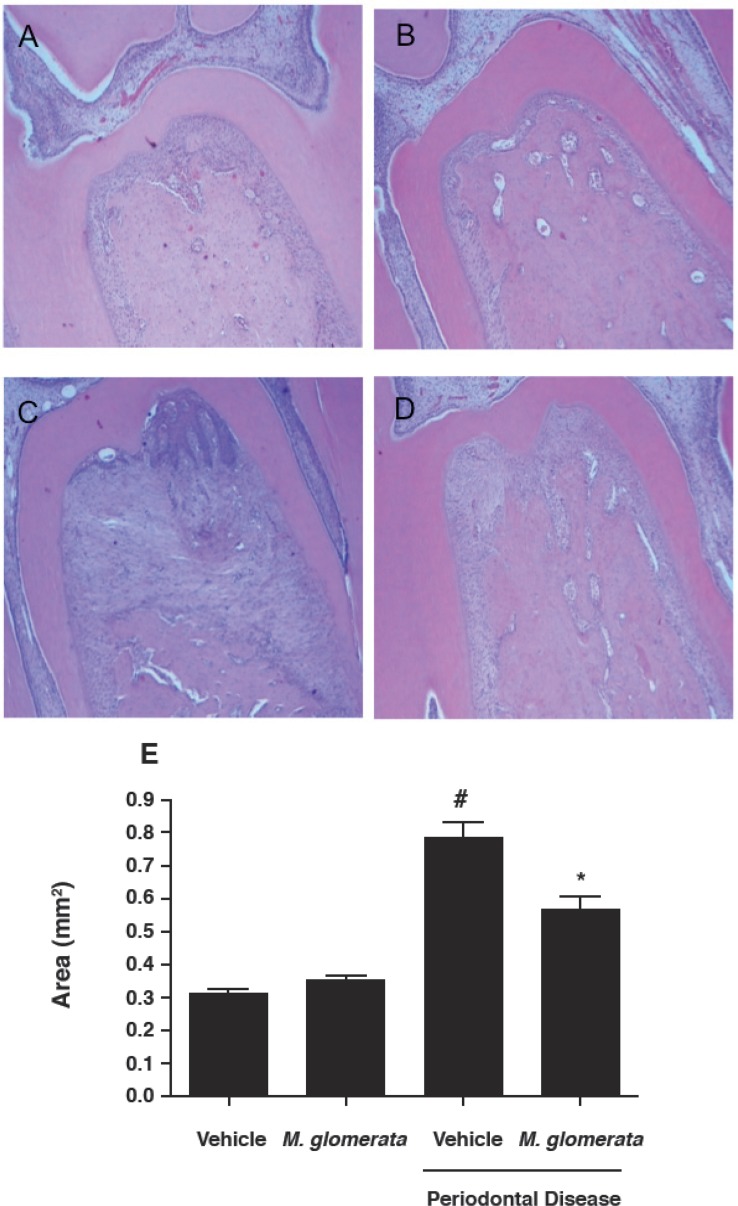
To assess whether MLE would affect bone loss in the furcation region, HE sections were histometrically analyzed. The resorption area measured after the experimental period demonstrated that ligature induced experimental periodontitis significantly increased bone loss (p<0.05) (Figure 1C) when compared with both vehicle (0.31±0.03 mm2) and MLE (0.35±0.06 mm2) non-ligated groups, which were not different when compared with each other (p>0.05) (Figures 1A and 1B). Additionally, the results demonstrated that MLE administration (10 mg/kg, daily) was able to significantly inhibit (0.56±0.13 mm2; p<0.05) the volume of bone loss in ligated teeth (Figure 1D), however still higher when compared with the non-ligated teeth (p<0.05). The values of the resorption area of the three groups were represented in Figure 1.
Figure 1.
Mikania laevigata extract (MLE) decreases alveolar bone resorption. Histology at the furcation of first molars sampled from the rats euthanized after 30 days of experiments is shown [hematoxylin and eosin(HE) staining]; (A) non-ligated animals treated with vehicle, (B) non-ligated animals treated with (MLE) (10 mg/kg/day for 30 consecutive days), (C) ligature-induced periodontitis treated with vehicle for 30 days, (D) ligature-induced periodontitis treated with subcutaneous MLE (10 mg/kg/ day for 30 consecutive days). (E) Results are expressed as mean area (mm2) ± standard deviation of 10 animals in each group. Different symbols indicate intergroup statistical significance (ANOVA followed by Bonferroni’s test). Magnification at 40× objective (scale bar=100 μm)
Effect of MLE on neutrophil migration and RANKL expression
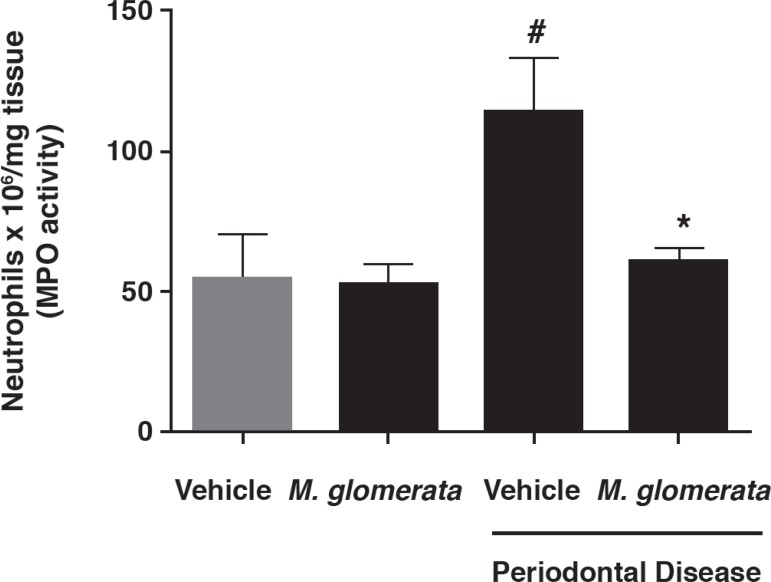
Next, the possible interference of MLE on neutrophil migration was investigated. According to MPO assay, MLE administration did not exert any significant effect on neutrophil accumulation on non-ligated teeth (55.74±25.84 versus 53.14±15.21 neutrophils x 106; p>0.05) by measuring gingival tissue MPO. On the other hand, the increased levels of neutrophil accumulation observed on ligated teeth (113.82±38.96 neutrophils x 106; p<0.05) were significantly decreased by MLE administration (61.23±10.12 neutrophils x 106; p<0.05) (Figure 2). Thus, MLE was able to reduce neutrophil accumulation and consequently inflammation in the gingival tissue.
Figure 2.
Mikania laevigata extract (MLE) decreases neutrophil migration to the gingival tissue. In order to estimate the relative numbers of infiltrating neutrophils in the gingival tissue, myeloperoxidase (MPO) activity present in the gingival tissue homogenates was measured. Results are shown as mean MPO activity ± standard deviation. # p<0.05 compared with control animals; * p<0.05 compared with ligature-induced periodontitis treated with vehicle (ANOVA followed by Bonferroni's test)
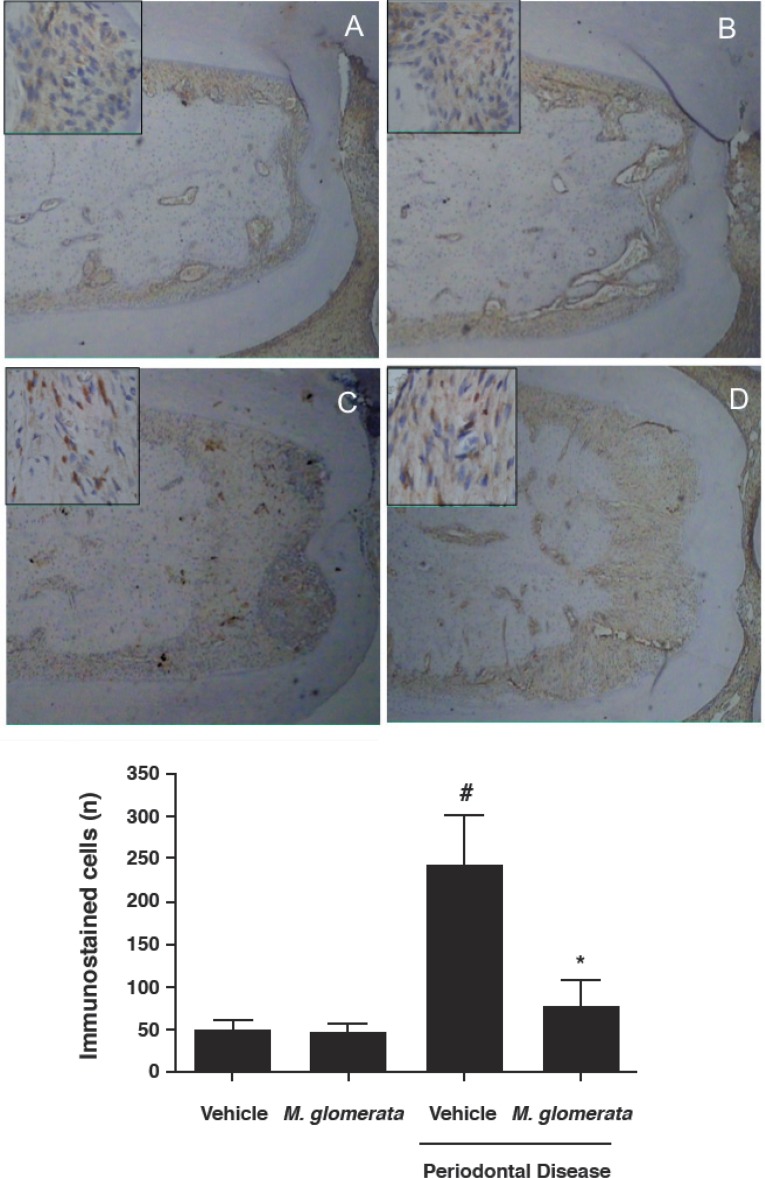
On the other hand, since a reduced bone resorption was observed in the MLE-treated group, we tested the hypothesis that MLE decreases the release of osteoclast regulatory factors. In this way, we used an immunohistochemistry assay to visualize the expression of RANKL on periodontal tissues. As shown in Figure 3, animals with ligated-induced periodontitis treated with MLE presented significantly less RANKL-immunostained cells (76.25±64.40 immunostained cells; p<0.05) in the periodontal tissue when compared with ligated-animals treated with vehicle (242.00±46.23 immunostained cells). Non-ligated animals (49.66±18.23 immunostained cells) were not affected by MLE administration (45.75±22.42 immunostained cells; p>0.05).
Figure 3.
Immunohistochemical staining for RANKL. Expression patterns of RANKL in the periodontal tissue at the furcation of first molars sampled from the rats euthanized at 30 days are shown. Low number of positive staining cells for RANKL was observed in the furcation of non-ligated animals treated with vehicle (A) as well the non-ligated animals treated with Mikania laevigata extract (MLE) (B). Increased number of RANKL positive cells were found in the furcation of the ligated-animals treated with vehicle (C). Lower number of RANKL stained cells were found in the furcation of the ligated-animals treated with MLE (10 mg/kg/day for 30 consecutive days). (E) Data are presented as median ± standard deviation of the average RANKL positive cells/per area of furcation. Negative control staining was carried out by the incubation with secondary antibody alone that showed lack of immunostaining pattern in all experimental groups (data not shown). # p<0.05 compared with control animals; * p<0.05 compared with ligature-induced periodontitis treated with vehicle (ANOVA followed by Bonferroni’s test). The figure is representative of at least 4 different sections obtained from each group. Scale bar at lower (40x) magnification=100 μm, and scale bar at higher (400x) magnification=20 μm
DISCUSSION
Oral diseases continue to be a major health problem worldwide. Tooth loss, caused by poor periodontal health (which affects up to 20% of the adult population worldwide) can lead to significant morbidity and premature death22. The economic impact of oral diseases is an important consideration with up to 10% of public health expenditure in developed countries related to curative dental care24. In cases where traditional and basic treatments are not able to arrest periodontal disease progression, the adjunct use of alternative substances aimed at modulating the host immune response is desirable. However, despite several agents being commercially available, most of these chemicals may present undesirable side effects9 and limited scientific evidence so far. It is recognized that most of the new drugs discovered in the last few decades have originated from nature17. Chemical constituents obtained from medicinal plants and other natural products have been increasingly used to treat many inflammatory diseases. In the present study, it was demonstrated that an extract obtained from Mikania laevigata, a popular medicinal plant in South American countries, was able to decrease alveolar bone loss, which may be explained, at least in part, by the lower expression of RANKL and decreased neutrophil migration observed on MLE-treated animals.
A growing body of research suggests that chronic periodontal disease involves a failure of inflammation resolution pathways to restore tissue homeostasis. Therefore, strategies to slow or arrest periodontal disease progression by the modulation of the host immune response have been investigated26. Among the commercially available substances earliest evaluated so far to modify resolution pathways, are the nonsteroidal anti-inflammatory drugs (NSAIDs). These agents antagonize proinflammatory pathways and/or signaling, preventing the production arachidonic acid metabolites that are proinflammatory mediators implicated in a variety of bone resorptive and tissue degrading processes18. Although in vitro14 and experimental studies12 have shown promising results, periodontal clinical trials have not reached consistent clinical benefits when combined with conventional therapy26 besides having undesirable side effects when used in a long-term basis. Hence, the search for alternative products continues and natural phytochemicals isolated from plants used in traditional medicine are considered as good alternatives to synthetic chemicals23.
More recently, the number of reports of the use of traditional plants and natural products for the treatment of periodontal disease has increased. In vitro studies evaluating the antimicrobial potential of substances isolated from popular plants have demonstrated significant antimicrobial properties against periodontal pathogens21. In addition, in vivo studies in animals using compounds of natural plants such as baicalin6 and cannabinoids17 observed a protective role against periodontal tissue breakdown in ligature-induced periodontitis, which was partially explained by their inhibitory effects on prostaglandins (COX-2), interleukins (IL-1β and TNF-α), nitric oxide and the bone related molecules RANK and RANKL. Our results corroborate with such previous findings, with the administration of MLE significantly decreasing alveolar bone resorption and promoting an inhibition of neutrophils influx and RANKL production.
In the progression of inflammatory diseases, the interactions of recruited neutrophils in the site of inflammation stimulate the local production of several inflammatory mediators that may further amplify the inflammatory response and injure the surrounding tissues13. In fact, our group recently assessed the pharmacological properties and the underlying molecular mechanisms of the hydroalcoholic MLE to confirm the popular wisdom as a putative anti-inflammatory drug2. We could observe that the anti-inflammatory effect of MLE was able to reduce neutrophil migration and vascular permeability in a peritonitis model. MLE additionally prevented the release of both TNF-α and IL-1β and contributed to a reduction in leukocyte adhesion and transmigration across the endothelium as observed by intravital microscopy. Taken together, the results suggest that use of the medicinal guaco extract may be able to suppress the development of inflammatory lesions, which are initiated by neutrophil recruitment including periodontal conditions.
The discovery of the RANK-RANKL-Osteoprotegerin (OPG) system has brought rapid progress in the understanding of the regulatory mechanisms of osteoclast differentiation and activation exerted by the immune system28. Recent studies suggest the involvement of RANKL and OPG in the pathogenesis of periodontitis28. Not only osteoblasts but also other resident cells, including cells of the periodontal ligament, participate in the regulation of RANKL and OPG in periodontal tissue16,19. The findings of the present study, demonstrated that the administration of MLE inhibited the expression of RANKL. To the best of our knowledge, no direct effect of MLE on the RANK/RANKL/OPG system has been addressed in the literature. However, several investigations have addressed the close relation between the production of inflammatory mediators and the modulation of RANKL/OPG balance. During the progression of periodontal disease, high levels of pro-inflammatory cytokines have been positively related to RANKL expression, suggesting that such inflammatory molecules play a major role in periodontal bone resorption11. Interestingly, a recent work demonstrated that lipopolysaccharide (LPS), a toll-like receptor 4 ligand, up-regulated the expression of membrane RANKL in human blood neutrophils. Besides, LPS-activated human and murine neutrophils, cocultured with human monocyte-derived osteoclasts and RAW 264.7 cells, respectively, stimulated bone resorption8. Therefore it is possible to suggest that the potent anti-inflammatory properties of MLE may have caused the diminished production of RANKL observed in MLE-treated animals observed in the present study and indirectly participated in the decreased volume of bone loss during ligature-induced periodontal disease progression.
CONCLUSION
There is considerable evidence that plant extracts have the potential to be developed into agents that can be used as preventative or treatment therapies for oral diseases. The results of the present investigation indicate that "guaco" extract may be useful to control bone resorption during progression of experimental periodontitis in rats in animal studies. While our results are encouraging, further controlled trial studies will be important to establish whether guaco is able to offer clinical therapeutic benefits.
Acknowledgments
ACKNOWLEDGEMENTS AND FUNDING
This work was partially supported by CAPES and PAPE-UNIUBE grant number 2007/013.
REFERENCES
- 1.Albandar JM, Goldstein H. Multi-level statistical models in studies of periodontal diseases. J Periodontol. 1992;63:690–695. doi: 10.1902/jop.1992.63.8.690. [DOI] [PubMed] [Google Scholar]
- 2.Alves CF, Alves VBF, Assis IP, Clemente-Napimoga JT, Uber-Bucek E, Dal-Secco D, et al. Anti-inflammatory activity and possible mechanism of extract from Mikania laevigata in carrageenaninduced peritonitis. J Pharm Pharmacol. 2009;61:1097–1104. doi: 10.1211/jpp/61.08.0014. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson P, Lindhe J. The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol. 1981;8:281–294. doi: 10.1111/j.1600-051x.1981.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 4.Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH., Jr Inflammatory and bone-related genes are modulated by aging in human periodontal ligament cells. Cytokine. 2009;46:176–181. doi: 10.1016/j.cyto.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Bighetti AE, Antônio MA, Kohn LK, Rehder VL, Foglio MA, Possenti A, et al. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomedicine. 2005;12:72–77. doi: 10.1016/j.phymed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Cai X, Li C, Du G, Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J Periodontal Res. 2008;43:14–21. doi: 10.1111/j.1600-0765.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 7.Callens A. Growth factors in periodontal regeneration - a review. In: Lang NP, Karring T, Lindhe J, editors. Proceedings of the 2nd European Workshop on Periodontology. Berlin: Quintessence Books; 1997. pp. 284–302. [Google Scholar]
- 8.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;8:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 9.Chung JY, Choo JH, Lee MH, Hwang JK. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006;13:261–266. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 11.Garlet GP, Martins W, Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004;31:671–679. doi: 10.1111/j.1600-051X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 12.Gurgel BC, Duarte PM, Nociti FH, Jr, Sallum EA, Casati MZ, Sallum AW, et al. Impact of an anti-inflammatory therapy and its withdrawal on the progression of experimental periodontitis in rats. J Periodontol. 2004;75:1613–1618. doi: 10.1902/jop.2004.75.12.1613. [DOI] [PubMed] [Google Scholar]
- 13.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 14.Kyrkanides S, O'Banion MK, Subtelny JD. Nonsteroidal anti-inflammatory drugs in orthodontic tooth movement: metalloproteinase activity and collagen synthesis by endothelial cells. Am J Orthod Dentofacial Orthop. 2000;118:203–209. doi: 10.1067/mod.2000.105872. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa TK, Kiji M, Yashiro R, Hormdee D, Lu H, Kunze M, et al. Roles of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontol 2000. 2007;43:65–84. doi: 10.1111/j.1600-0757.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 16.Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-Dos-Santos DR, et al. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and proinflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009;9:216–222. doi: 10.1016/j.intimp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 18.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, et al. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res. 2004;39:42–49. doi: 10.1111/j.1600-0765.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- 20.Pålsson-McDermott E, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park M, Bae J, Lee DS. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- 22.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 23.Prabu GR, Gnanamani A, Sadulla S. Guaijaverin - a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J Appl Microbiol. 2006;101:487–495. doi: 10.1111/j.1365-2672.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 25.Slots J. The search for effective, safe and affordable periodontal therapy. Periodontol 2000. 2002;28:9–11. doi: 10.1034/j.1600-0757.2002.280101.x. [DOI] [PubMed] [Google Scholar]
- 26.Soares de Moura R, Costa SS, Jansen JM, Silva CA, Lopes CS, Bernardo-Filho M, et al. Bronchodilator activity of Mikania glomerata Sprengel on human bronchi and guinea-pig trachea. J Pharm Pharmacol. 2002;54(2):249–256. doi: 10.1211/0022357021778277. [DOI] [PubMed] [Google Scholar]
- 27.Suyenaga ES, Reche E, Farias FM, Schapoval EE, Chaves CG, Henriques AT. Antiinflammatory investigation of some species of Mikania. Phytother Res. 2002;16:519–523. doi: 10.1002/ptr.908. [DOI] [PubMed] [Google Scholar]
- 28.Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Periodontal Res. 2005;40:287–293. doi: 10.1111/j.1600-0765.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 29.Yatsuda R, Rosalen PL, Cury JA, Murata RM, Rehder VL, Melo LV, et al. Effects of Mikania genus plants on growth and cell adherence of mutans streptococci. J Ethnopharmacol. 2005;97:183–189. doi: 10.1016/j.jep.2004.09.042. [DOI] [PubMed] [Google Scholar]