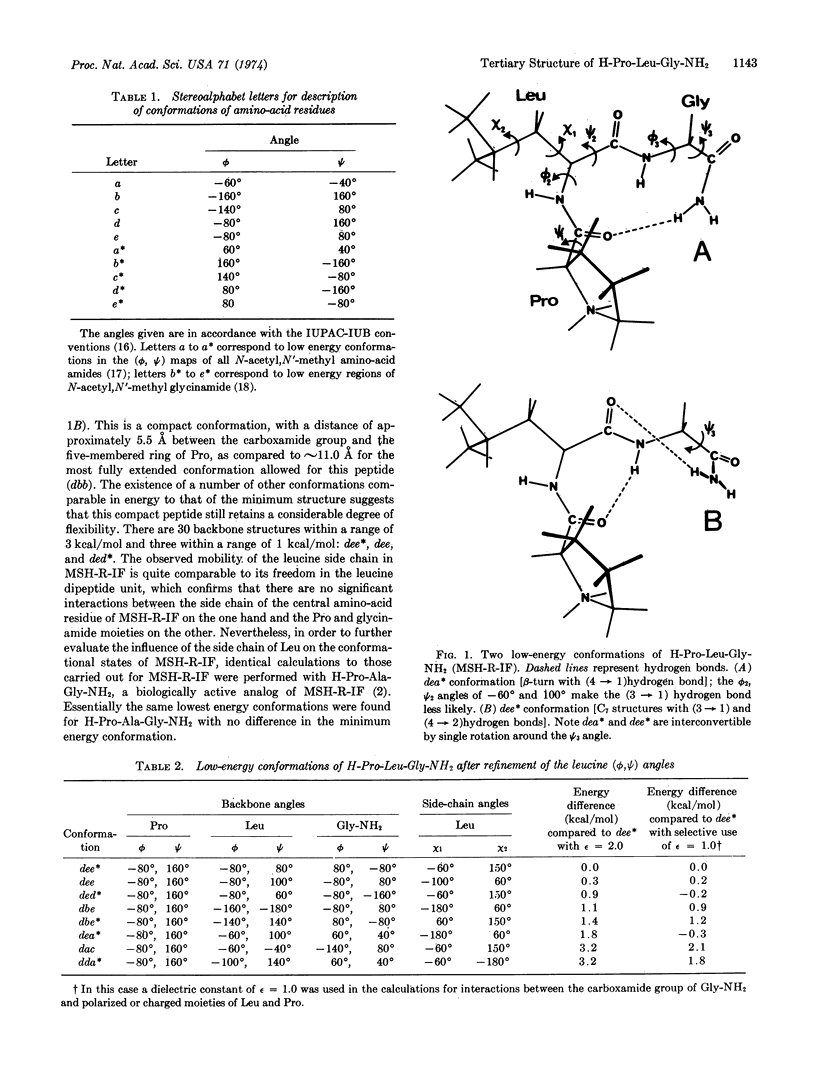
Abstract
Conformational energy calculations were carried out on H-Pro-Leu-Gly-NH2, the factor that inhibits the release of melanocyte stimulating hormone, and its biologically active analog, H-Pro-Ala-Gly-NH2. Both peptides were found to be relatively compact molecules that retain, however, some degree of flexibility. After structure refinement, H-Pro-Leu-Gly-NH2 possesses at least three preferred compact conformations. Two of these conformations occupy rather broad and flat energy troughs, while a third occupies a narrow and deep potential energy well. This third structure, which consists of a 10-membered β-turn closed by a (4 → 1) hydrogen bond between the proton of the trans carboxamide of Gly and the C=O of Pro, is the one that was proposed for H-Pro-Leu-Gly-NH2 in dimethylsulfoxide and was also found by x-ray analysis.
Keywords: conformation, conformation-activity relationship, hypothalamic factor
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celis M. E., Hase S., Walter R. Structure-activity studies of MSH-release-inhibiting hormone. FEBS Lett. 1972 Nov 1;27(2):327–330. doi: 10.1016/0014-5793(72)80651-3. [DOI] [PubMed] [Google Scholar]
- Celis M. E., Taleisnik S., Walter R. Regulation of formation and proposed structure of the factor inhibiting the release of melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1428–1433. doi: 10.1073/pnas.68.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers R., Walter R., Smith I. C. Intramolecular motion in peptide determined by 13C NMR: a spin-lattice relaxation time-study on MSH-release-inhibiting factor. FEBS Lett. 1973 Nov 15;37(1):27–32. doi: 10.1016/0014-5793(73)80419-3. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., Brewster A. I., Glasel J. A. NMR studies on the conformation of derivatives of the side chain of oxytocin: examples of cis-trans isomerism. Proc Natl Acad Sci U S A. 1971 Feb;68(2):450–453. doi: 10.1073/pnas.68.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquori A. M. The stereochemical code and the logic of a protein molecule. Q Rev Biophys. 1969 May;2(1):65–92. doi: 10.1017/s0033583500000792. [DOI] [PubMed] [Google Scholar]
- Nair R. M., Kastin A. J., Schally A. V. Isolation and structure of hypothalamic MSH release-inhibition hormone. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1376–1381. doi: 10.1016/s0006-291x(71)80026-8. [DOI] [PubMed] [Google Scholar]
- Popov E. M., Lipkind G. M. Conformational states of aminoacyl residues of proteins. Main polypeptide chain. Mol Biol. 1972 Jan;5(4):496–505. [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Reed L. L., Johnson P. L. Solid state conformation of the C-terminal tripeptide of oxytocin, L-Pro-L-Leu-Gly-NH2 0.5H2O. J Am Chem Soc. 1973 Oct 31;95(22):7523–7524. doi: 10.1021/ja00803a062. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Walter R., Griffiths E. C., Hooper K. C. Production of MSH-release-inhibiting hormone by a particulate preparation of hypothalami: mechanisms of oxytocin inactivation. Brain Res. 1973 Oct 12;60(2):449–457. doi: 10.1016/0006-8993(73)90802-0. [DOI] [PubMed] [Google Scholar]


