Abstract
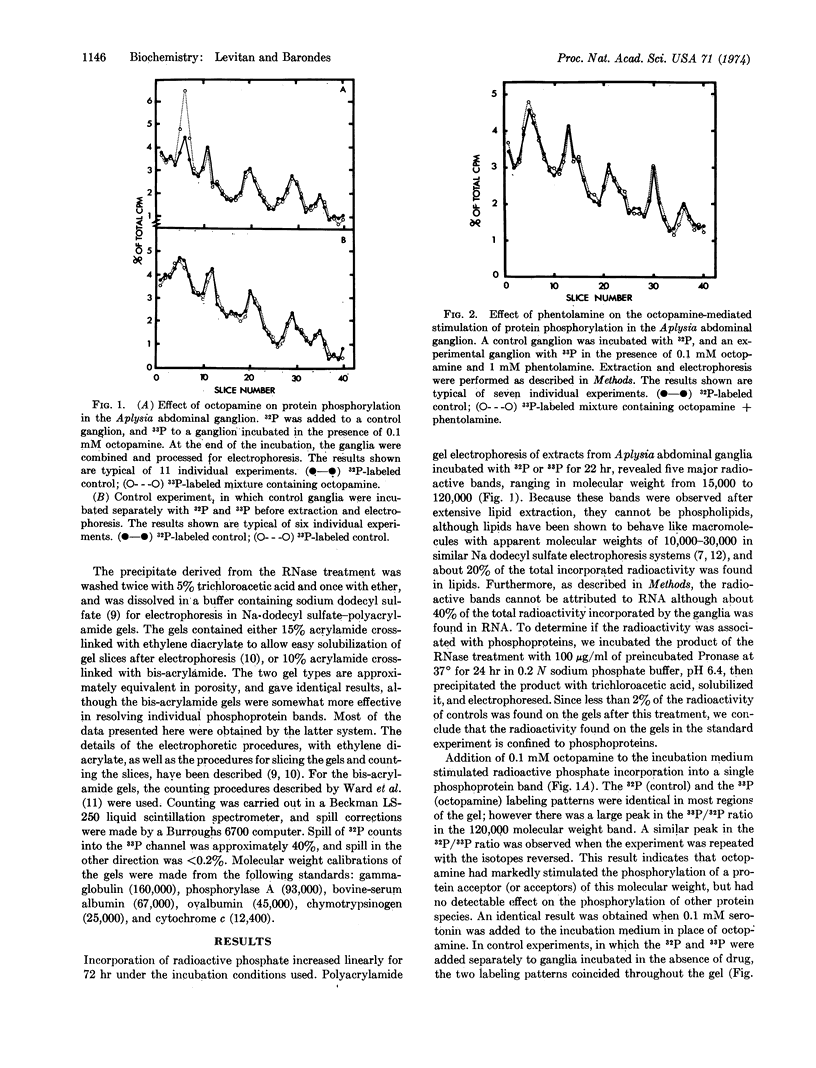
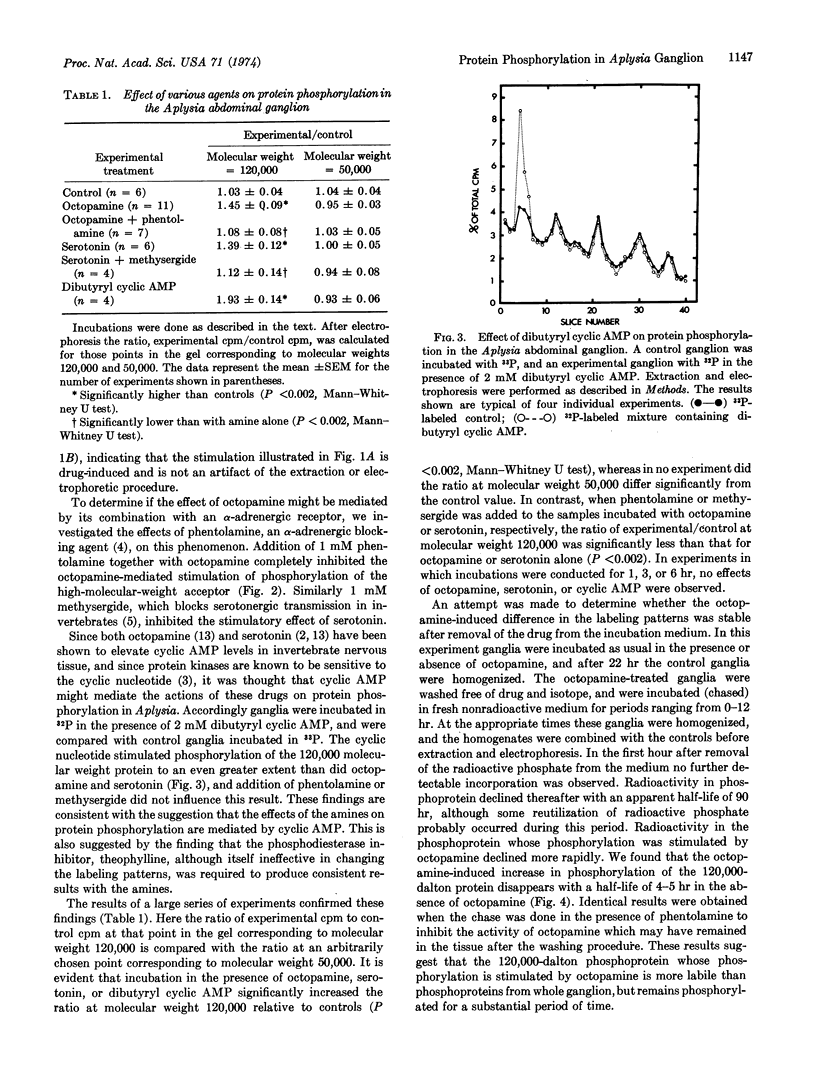
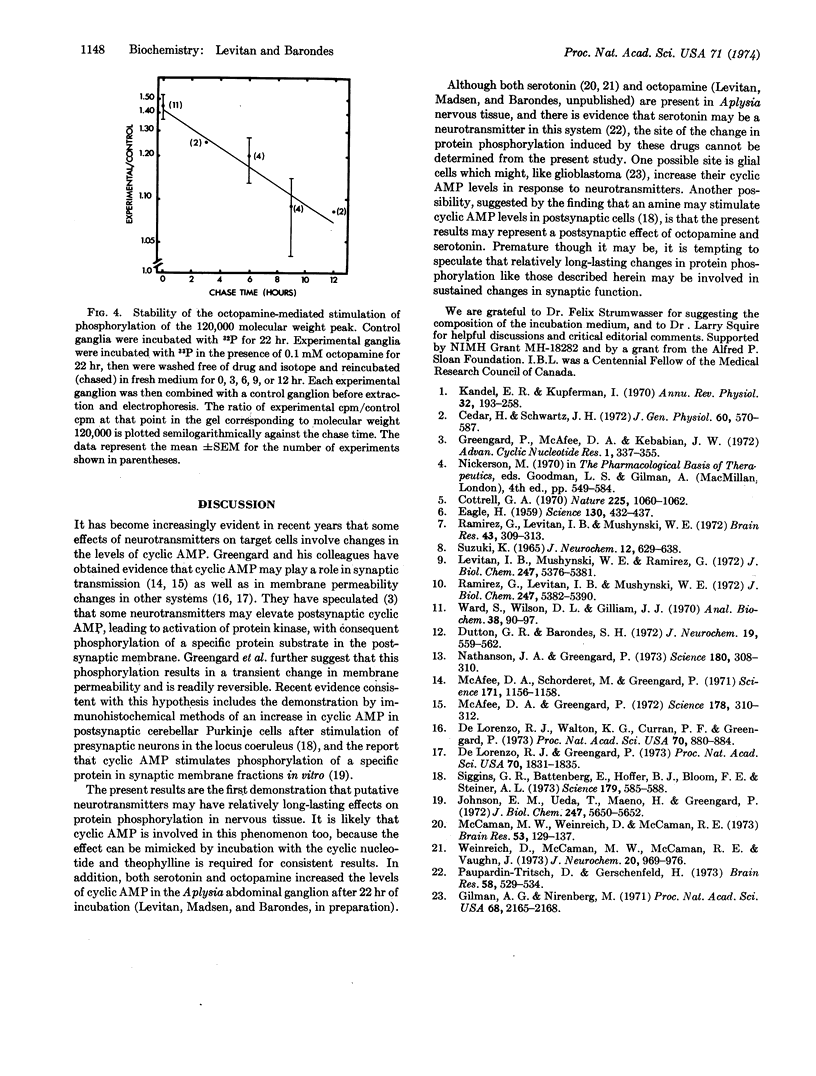
Phosphorylation of a protein (or proteins) of molecular weight 120,000 in the Aplysia abdominal ganglion, as measured by incorporation of [32P] or [33P]sodium phosphate in vitro followed by separation of the phosphoproteins on sodium dodecyl sulfate-polyacrylamide gels, was specifically stimulated by incubation in the presence of the putative neurotransmitters octopamine or serotonin. The stimulatory effect of octopamine and serotonin was inhibited by phentolamine and methysergide, respectively, and was mimicked by incubation in the presence of dibutyryl cyclic AMP. Label-chase experiments indicated that the difference between control and octopamine-treated ganglia persists for several hours after removal of the drug from the incubation medium. This result suggests that neurotransmitters may produce relatively long-lasting changes in a phosphoprotein in the ganglion, perhaps in postsynaptic cells.
Keywords: neurotransmitters, dibutyryl cyclic AMP
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cedar H., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J Gen Physiol. 1972 Nov;60(5):570–587. doi: 10.1085/jgp.60.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A. Direct postsynaptic responses to stimulation of serotonin-containing neurones. Nature. 1970 Mar 14;225(5237):1060–1062. doi: 10.1038/2251060a0. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo R. J., Greengard P. Activation by adenosine 3':5'-monophosphate of a membrane-bound phosphoprotein phosphatase from toad bladder. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1831–1835. doi: 10.1073/pnas.70.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. R., Barondes S. H. Macromolecular behaviour of gangliosides on electrophoresis in sodium dodecyl sulphate. J Neurochem. 1972 Feb;19(2):559–562. doi: 10.1111/j.1471-4159.1972.tb01370.x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Nirenberg M. Effect of catecholamines on the adenosine 3':5'-cyclic monophosphate concentrations of clonal satellite cells of neurons. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2165–2168. doi: 10.1073/pnas.68.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., McAfee D. A., Kebabian J. W. On the mechanism of action of cyclic AMP and its role in synaptic transmission. Adv Cyclic Nucleotide Res. 1972;1:337–355. [PubMed] [Google Scholar]
- Johnson E. M., Ueda T., Maeno H., Greengard P. Adenosine 3',5-monophosphate-dependent phosphorylation of a specific protein in synaptic membrane fractions from rat cerebrum. J Biol Chem. 1972 Sep 10;247(17):5650–5652. [PubMed] [Google Scholar]
- Kandel E. R., Kupfermann I. The functional organization of invertebrate ganglia. Annu Rev Physiol. 1970;32:193–258. doi: 10.1146/annurev.ph.32.030170.001205. [DOI] [PubMed] [Google Scholar]
- Levitan I. B., Mushynski W. E., Ramirez G. Highly purified synaptosomal membranes from rat brain. Preparation and characterization. J Biol Chem. 1972 Sep 10;247(17):5376–5381. [PubMed] [Google Scholar]
- McAfee D. A., Greengard P. Adenosine 3',5'-monophosphate: electrophysiological evidence for a role in synaptic transmission. Science. 1972 Oct;178(58):310–312. doi: 10.1126/science.178.4058.310. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Schorderet M., Greengard P. Adenosine 3',5'-monophosphate in nervous tissue: increase associated with synaptic transmission. Science. 1971 Mar 19;171(3976):1156–1158. doi: 10.1126/science.171.3976.1156. [DOI] [PubMed] [Google Scholar]
- McCaman M. W., Weinreich D., McCaman R. E. The determination of picomole levels of 5-hydroxytryptamine and dopamine in Aplysia, Tritonia and leech nervous tissues. Brain Res. 1973 Apr 13;53(1):129–137. doi: 10.1016/0006-8993(73)90772-5. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Paupardin-Tritsch D., Gerschenfeld H. M. Transmitter role of serotonin in identified synapses in Aplysia nervous system. Brain Res. 1973 Aug 30;58(2):529–534. doi: 10.1016/0006-8993(73)90027-9. [DOI] [PubMed] [Google Scholar]
- Ramirez G., Levitan I. B., Mushynski W. E. Double-isotope studies on brain protein synthesis with ( 3 H)- and ( 14 C)leucine: a warning. Brain Res. 1972 Aug 11;43(1):309–312. doi: 10.1016/0006-8993(72)90303-4. [DOI] [PubMed] [Google Scholar]
- Ramirez G., Levitan I. B., Mushynski W. E. Highly purified synaptosomal membranes from rat brain. Incorporation of amino acids into membrane proteins in vitro. J Biol Chem. 1972 Sep 10;247(17):5382–5390. [PubMed] [Google Scholar]
- Siggins G. R., Battenberg E. F., Hoffer B. J., Bloom F. E., Steiner A. L. Noradrenergic stimulation of cyclic adenosine monophosphate in rat Purkinje neurons: an immunocytochemical study. Science. 1973 Feb 9;179(4073):585–588. doi: 10.1126/science.179.4073.585. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Ward S., Wilson D. L., Gilliam J. J. Methods for fractionation and scintillation counting of radioisotope-labeled polyacrylamide gels. Anal Biochem. 1970 Nov;38(1):90–97. doi: 10.1016/0003-2697(70)90158-2. [DOI] [PubMed] [Google Scholar]
- Weinreich D., McCaman M. W., McCaman R. E., Vaughn J. E. Chemical, enzymatic and ultrastructural characterization of 5-hydroxytryptamine-containing neurons from the ganglia of Aplysia californica and Tritionia diomedia. J Neurochem. 1973 Apr;20(4):969–976. doi: 10.1111/j.1471-4159.1973.tb00067.x. [DOI] [PubMed] [Google Scholar]



